Copyright 2011 Cognizant Comm. Corp. E-ISSN 1555-3892 www.cognizantcommunication.com
Induced Pluripotent Stem (iPS) Cell Research Overview
Shih-Ping Liu,*† Ru-Huei Fu,*‡ Yu-Chuen Huang,§¶ Shih-Yin Chen,§¶ Ying-Jiun Chien,†
Chien Yu Hsu,† Chang-Hai Tsai,#** Woei-Cherng Shyu,*‡ and Shinn-Zong Lin*‡††
*Center for Neuropsychiatry, China Medical University and Hospital, Taichung, Taiwan †Graduate Institute of Basic Medical Science, China Medical University, Taichung, Taiwan
‡Department of Immunology, China Medical University, Taichung, Taiwan
§Genetics Center, Department of Medical Research, China Medical University Hospital, Taichung, Taiwan ¶Graduate Institute of Chinese Medical Science, College of Chinese Medicine, China Medical University, Taichung, Taiwan
#Department of Pediatrics, China Medical University Hospital, Taichung, Taiwan **Department of Healthcare Administration, Asia University, Taichung, Taiwan
††China Medical University Beigang Hospital, Yunlin, Taiwan
Stem cells are capable of self-renewal and differentiation into a wide range of cell types with multiple clinical therapeutic applications. The two most important issues associated with embryonic stem (ES) cells are immune rejection and medical ethics. In 2006, induced pluripotent (iPS) cells were generated from somatic cells via the introduction of four transcriptional factors: OCT4, SOX2, c-MYC, and KLF4. Re-searchers found that iPS cell morphology, proliferation, surface antigens, gene expression, telomerase activ-ity, and the epigenetic status of pluripotent cell-specific genes were similar to the same characteristics in ES cells. iPS cells are capable of overcoming hurdles associated with ES cells due to their generation from mature somatic cells (e.g., fibroblasts). For this reason, iPS cells are considered an increasingly important cell therapy technology. iPS cell production entails the use of retroviruses, lentiviruses, adenoviruses, plas-mid transfections, transposons, or recombinant proteins. In this article we discuss the advantages and limita-tions of each strategy and address issues associated with clinical trials, including the potential for liver tumor formation and low generation efficiency.
Key words: Induced pluripotent stem cells; Embryonic stem cells; Cell therapy
INTRODUCTION
In 2006, Takahashi and Yamanaka became the first researchers to successfully produce induced pluripotent (iPS) cells (23). They reported that both embryonic and adult mouse fibroblasts acquired capabilities similar to embryonic stem (ES) cells following treatment with four transcriptional factors (selected from 24 candidates): Oct3/4, Sox2, c-Myc, and Klf4 (23,27). The following year, two separate sets of four factors were shown to reprogram human somatic cells to pluripotency at simi-lar efficiency levels: OCT4, SOX2, C-MYC, and KLF4 by Takahashi et al. (22) and OCT4, SOX2, NANOG, and LIN28 by Yu et al. (30). iPS cell morphology, pro-liferation, surface antigens, gene expression, telomerase activity, and the epigenetic status of pluripotent cell-specific genes are similar to the same characteristics in
Online prepub date: September 30, 2010.
Address correspondence to Shinn-Zong Lin, MD., Ph.D., Center for Neuropsychiatry, China Medical University and Hospital, Taichung, Taiwan. Tel: 886-4-22052121, ext. 6034; Fax: 886-4-220806666; E-mail: shinnzong@yahoo.com.tw or Woei-Cherng Shyu, M.D., Ph.D., Center for Neuro-psychiatry, China Medical University and Hospital, Taichung, Taiwan. Tel: 886-4-22052121, ext. 7811; Fax: 886-4-22052121, ext. 7810; E-mail: shyu9423@gmail.com
1
ES cells (22,30). Also similar to ES cells, iPS cells are capable of differentiating into three germ layer cell types—ectoderm, endoderm, and mesoderm—in vitro and in teratomas (23,27). Accordingly, iPS cells hold great promise for medicine due to their potential for gen-erating patient-specific cell types for cell replacement therapy and producing in vitro disease models without embryonic tissues or oocytes. To date, at least six strate-gies for making iPS cells have been identified: retrovi-rus, lentiviretrovi-rus, adenoviretrovi-rus, plasmid transfection, transpo-son, and recombinant protein. Each strategy has advantages and limitations, which is the focus of this overview article.
iPS CELL IMPORTANCE AND LIMITATIONS
Both ES and iPS cells are pluripotent and capable of differentiating into three primary germ layer
deriva-tives—an important characteristic for producing healthy cells for therapeutic purposes. While there are reports of ES cell applications for therapeutic approaches in animal models (9,13,16,17,28), at least two important road-blocks to their use in humans must be overcome: post-transplantation immune rejection and ethical issues. iPS cell technology addresses both concerns: it is possible to use a patient’s own somatic cells to generate thera-peutic iPS cells (thus eliminating the potential for im-mune rejection), and they represent an acceptable alter-native to the use of human embryos for stem cell production. An added benefit is that patient-specific iPS cells can be used for drug and regenerative medical re-search.
At least three significant hurdles remain: (a) the low efficiency of primary human cell reprogramming, which makes it difficult to generate patient-specific iPS cells from small initial cell populations; (b) the integration of viral transgenes into the somatic genome, especially oncogenes such as c-MYC and KLF4 (note that c-MYC retrovirus reactivation contributes to tumor formation in chimeric mice derived from iPS cells) (27); and (c) iPS cell teratoma formation can lead to tumor formation. Be-cause even a small number of undifferentiated cells can result in teratoma formation, a key goal is inducing iPS differentiation into target cell types without producing large numbers of undifferentiated cells. Some chimeric mice success has been achieved using iPS cells, but tu-mor formation potential has not been completely elimi-nated.
VIRUS-GENERATED iPS CELLS
Currently the majority of iPS cells are generated by retrovirus transduction, through which gene expression levels are higher and silenced after cells turn into iPS cells. These characteristics are important for cell therapy applications because they indicate that reduced abnor-mal gene expression occurs during stem cell stages. When Takahashi et al. generated the first iPS cells from mouse embryonic fibroblasts, reported efficiency was 0.1% (23). The following year they used the same tech-nique to obtain human iPS cells, and efficiency de-creased to 0.01% (22). To reduce c-Myc oncogene gen-eration potential, the same group used only three factors (Oct4, Sox2, and Klf4) to generate mouse and human iPS cells, but efficiencies fell to 0.001% and 0.01%, re-spectively (Table 1) (18). iPS cell clone formation did not occur when only two transcriptional factors (Oct4 and Sox2) were used to generate mouse iPS cells (12). An alternative strategy tested by Huangfu et al. entailed using Oct4, Sox2, and valproic acid (VPA, a histone deacetylase inhibitor that supports primary human fibro-blast reprogramming with two factors) to generate hu-man iPS cells from huhu-man fibroblasts (8). Their results support the possibility of reprogramming through purely
chemical means, which would increase both cell safety and the potential for therapeutic use. However, this strat-egy still carries the risk of viruses being integrated into chromosomes and altering endogenous genome se-quences. In 2009, Kim et al. generated mouse iPS cells from neural stem cells after introducing a single factor (Oct4) (11). Although their work did not entail the use of somatic cells, their results are still considered impor-tant because they indicate that iPS cells with only one transcriptional factor are capable of generating c-MYC and KLF4 oncogenes.
Lentiviruses have also been used to generate human iPS cells from somatic cells. In 2007, Yu et al. became the first team to generate human iPS cells using a lentiv-irus (overexpressing 4 of 14 candidate genes: OCT4, SOX2, NANOG, and LIN28) (30) at an efficiency of 0.01%—the same as that reported by Takahashi et al. for OCT4, SOX2, c-MYC, and KLF4 (22). The follow-ing year Liao et al. used the transcriptional factors OCT4, SOX2, C-MYC, KLF4, NANOG, and LIN28 to generate iPS cells at an efficiency level of 0.1%—10 times higher than previously reported (14). They specu-lated that the addition of c-MYC and/or KLF4 either prevented apoptosis or regulated the cell cycle. The use of a lentivirus to introduce genes for iPS cell generation supported the integration of genes sequences into the ge-nome. Unlike retroviruses, the gene expression levels of lentivirus transduction do not shut down following trans-formation into iPS cells, meaning that iPS cells gener-ated via lentiviruses cannot be used for clinical trials. To prevent viral integration into genome sequences, Stadtfeld et al. (21) used an adenovirus carrying OCT4, SOX2, c-MYC, and KLF4 to generate mouse iPS cells from hepatocytes—the first instance of iPS cell genera-tion without virus integragenera-tion, a more acceptable method for clinical applications. However, because the effi-ciency of this strategy is very low (0.0006%), it may never be practical for generating human iPS cells.
There is no definitive answer to the question of how many transcriptional factors are required to generate iPS cells, but generating them from somatic cells treated with only one factor is an important research goal. The most important factor appears to be OCT4, which by itself is sufficient for generating iPS cells from neural stem cells (11). An interesting possibility is using a sin-gle transcriptional factor to induce OCT4, SOX2, C-MYC, and KLF4; that is, having it serve as a substitute for those four factors. If successful, this factor will both simplify the generation process for iPS cells and support their use in clinical trials.
GENERATING iPS CELLS WITH PLASMID TRANSFECTIONS
In 2008, Okita et al. (the same Japanese group that generated the first iPS cells) reported that they had
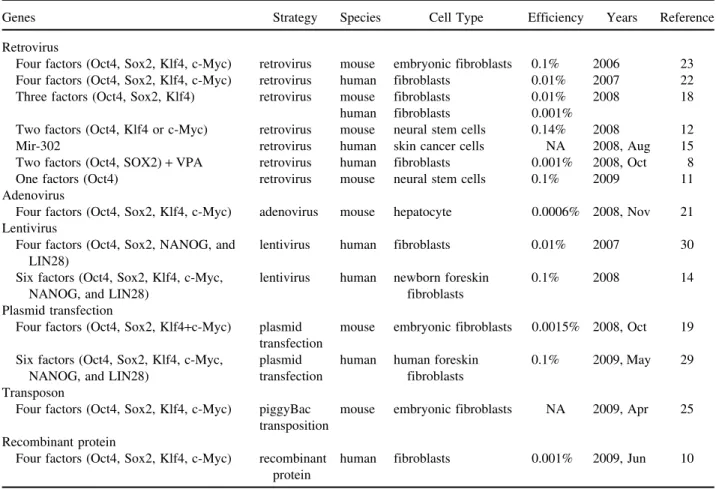
suc-Table 1. The Many Ways of Reprogramming Methods to Make iPS Cells
Genes Strategy Species Cell Type Efficiency Years Reference Retrovirus
Four factors (Oct4, Sox2, Klf4, c-Myc) retrovirus mouse embryonic fibroblasts 0.1% 2006 23 Four factors (Oct4, Sox2, Klf4, c-Myc) retrovirus human fibroblasts 0.01% 2007 22 Three factors (Oct4, Sox2, Klf4) retrovirus mouse fibroblasts 0.01% 2008 18
human fibroblasts 0.001%
Two factors (Oct4, Klf4 or c-Myc) retrovirus mouse neural stem cells 0.14% 2008 12 Mir-302 retrovirus human skin cancer cells NA 2008, Aug 15 Two factors (Oct4, SOX2)+ VPA retrovirus human fibroblasts 0.001% 2008, Oct 8 One factors (Oct4) retrovirus mouse neural stem cells 0.1% 2009 11 Adenovirus
Four factors (Oct4, Sox2, Klf4, c-Myc) adenovirus mouse hepatocyte 0.0006% 2008, Nov 21 Lentivirus
Four factors (Oct4, Sox2, NANOG, and lentivirus human fibroblasts 0.01% 2007 30 LIN28)
Six factors (Oct4, Sox2, Klf4, c-Myc, lentivirus human newborn foreskin 0.1% 2008 14
NANOG, and LIN28) fibroblasts
Plasmid transfection
Four factors (Oct4, Sox2, Klf4+c-Myc) plasmid mouse embryonic fibroblasts 0.0015% 2008, Oct 19 transfection
Six factors (Oct4, Sox2, Klf4, c-Myc, plasmid human human foreskin 0.1% 2009, May 29 NANOG, and LIN28) transfection fibroblasts
Transposon
Four factors (Oct4, Sox2, Klf4, c-Myc) piggyBac mouse embryonic fibroblasts NA 2009, Apr 25 transposition
Recombinant protein
Four factors (Oct4, Sox2, Klf4, c-Myc) recombinant human fibroblasts 0.001% 2009, Jun 10 protein
cessfully generated mouse iPS cells without viral vectors (19). The repeated transfection of two expression plas-mids (one containing the cDNAs of Oct3/4, Sox2, and Klf4 and the other containing c-Myc cDNA) into mouse embryonic fibroblasts resulted in iPS cells with no indi-cations of plasmid integration. When transplanted into mice, these cells produced teratomas and contributed to adult chimeras (19). This production of virus-free iPS cells addressed a critical safety concern for their use in regenerative medicine. Still, this method is very ineffi-cient (0.0015%); therefore, it remains to be seen whether it can be used to generate human iPS cells from human fibroblasts.
In 2009, Yu et al. used the same nonviral vector transfection strategy to generate human iPS cells, utiliz-ing three plasmids containutiliz-ing six transcriptional factors (OCT4, SOX2, C-MYC, KLF4, NANOG, and LIN28); this method achieved a much higher efficiency level of ⬃0.1% (29). They also determined that the generated human iPS cells were (a) completely free of vector and transgene sequences, and (b) similar to human ES cells in terms of proliferative and developmental potential. Their results demonstrate that human somatic cell repro-gramming does not require genomic integration or the
continued presence of exogenous reprogramming fac-tors, and thus removes one obstacle to clinical applica-tions of human iPS cells. If this strategy does not trigger the c-MYC and KLF4 oncogenes while still achieving higher efficiencies, it may emerge as the best method for generating iPS cells.
GENERATING iPS CELLS BY MICRO-RNA
To prevent the generation of oncogenes such as c-MYC and KLF4, researchers are searching for alterna-tive approaches to generating iPS cells by looking at factors that are abundant in stem cells and lacking in somatic cells. In 2008, Lin et al. (15) reported that mir-302 reprograms human skin cancer cells into a pluripo-tent ES cell-like state. The mir-302 micro-RNA family (referred to as mir-302s) is expressed most abundantly in slow-growing human ES cells and quickly decreases after cell differentiation and proliferation (20). In addi-tion to reprogramming cancer cells into an induced plur-ipotent state, mir-302s maintain this state under a feeder-free cultural condition that may offer opportunities for therapeutic intervention, making them a focus of interest as a potential key factor in ES cell renewal and pluripo-tency maintenance (15). Lin et al. were the first to use
micro-RNA to generate iPS cells, but they used a human skin cancer cell line instead of human somatic cells such as fibroblasts. If micro-RNAs can be used to generate iPS cells from human somatic cells, it will help prevent the use of oncogenes such as c-MYC and KLF4.
GENERATING iPS CELLS BY TRANSPOSONS
In 2008, a new approach to generating iPS cells with-out vector integration was reported by Woltjen et al., who used piggyBac (PB) transposition to insert OCT4, SOX2, C-MYC, and KLF4 into mouse embryonic fibro-blasts in order to generate iPS cells (25). Several re-searchers have demonstrated the functionality of PB transposition (which is host factor independent) in vari-ous human and mvari-ouse cell lines (2,3,24,26). PB transpo-son/transposase technology only requires (a) the in-verted terminal repeats that flank the targeted transgene, and (b) the transient expression of the transposase en-zyme to catalyze insertion or excision events (7). Indi-vidual PB insertions can be removed from established iPS cells in order to prevent gene insertions and onco-gene overexpression. Although this technique does not utilize the virus system, it still carries the risk of trans-genes remaining in the genome.
GENERATING iPS CELLS BY RECOMBINANT PROTEINS
In 2009, Kim et al. (10) generated stable DNA-free iPS cells from human fibroblasts by directly delivering four reprogramming proteins (Oct4, Sox2, Klf4, and c-Myc) and fusing them with a cell-penetrating peptide (CPP). A major challenge to the intracellular delivery of proteins and other macromolecules is their limited abil-ity to cross cellular membranes (1). In 1988, Frankel and Pabo found that the HIV transactivator of transcription (HIV-TAT) has a short basic segment residing at amino acids 48–60 that supports its penetration into cell mem-branes and subsequent activation of HIV-specific genes (5,6). This and other naturally occurring CPPs that are capable of overcoming cell membrane barriers contain high proportions of basic amino acids (e.g., arginine or lysine) (4,31). Kim et al.’s DNA-free iPS cells (protein-based iPS) are similar to ES cells in terms of morphol-ogy, proliferation, surface antigens, gene expression, telomerase activity, and the epigenetic status of pluripo-tent cell-specific genes (10). They are capable of differ-entiating into ectoderm, endoderm, and mesoderm layer cell types in vitro and in teratomas. Protein-based iPS technology represents a new and potentially safe method for generating patient-specific stem cells, one that does not require ex utero embryos. However, at present DNA-free iPS cells generation efficiency is significantly lower compared to virus-based protocols (approximately 0.001% vs. 0.01% of input cells) (10).
CONCLUSION AND RESEARCH DIRECTIONS
Still in its infancy (27), iPS technology should even-tually make cell transplantation therapies possible for a wide variety of diseases and injuries, while circumvent-ing ethical issues and immune rejection challenges. Re-searchers must evaluate different types of original cells and induction methods to determine the best combina-tion for generating the safest iPS cells. At minimum, researchers need to focus on four limitations to using iPS cells in clinical applications: (a) unacceptably low efficiency; (b) complexity, with the ideal process re-duced to a single step; (c) safety in light of the potential for cancer formation, with all iPS cells generated by any method from any cell source submitted to vigorous ex-amination prior to use in clinical applications (27); and (d) the use of animal feeders, noting that iPS cell cultur-ing technology currently uses mouse embryonic fibro-blasts as the feeder layer, meaning there is potential for the secretion of factors that might change iPS cell char-acteristics.
ACKNOWLEDGMENTS: This study is supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH99-TD-B-111-004), Taiwan De-partment of Health Cancer Research Center of Excellence (DOH99-TD-C-111-005), and the China Medical University topnotch plan (CMU98-CT-30). We also thank Mr. Jon Linde-mann for his editing of the manuscript.
REFERENCES
1. Belting, M.; Sandgren, S.; Wittrup, A. Nuclear delivery of macromolecules: Barriers and carriers. Adv. Drug Deliv. Rev. 57(4):505–527; 2005.
2. Cadinanos, J.; Bradley, A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 35(12):e87; 2007.
3. Ding, S.; Wu, X.; Li, G.; Han, M.; Zhuang, Y.; Xu, T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122(3):473–483; 2005. 4. El-Sayed, A.; Futaki, S.; Harashima, H. Delivery of
mac-romolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 11(1): 13–22; 2009.
5. Frankel, A. D.; Bredt, D. S.; Pabo, C. O. Tat protein from human immunodeficiency virus forms a metal-linked di-mer. Science 240(4848):70–73; 1988.
6. Frankel, A. D.; Pabo, C. O. Cellular uptake of the tat pro-tein from human immunodeficiency virus. Cell 55(6): 1189–1193; 1988.
7. Fraser, M. J.; Ciszczon, T.; Elick, T.; Bauser, C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovi-rus genome in cell lines from two species of Lepidoptera. Insect Mol. Biol. 5(2):141–151; 1996.
8. Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelen-boom, A.; Chen, S.; Muhlestein, W.; Melton, D. A. Induc-tion of pluripotent stem cells from primary human fibro-blasts with only Oct4 and Sox2. Nat. Biotechnol. 26(11): 1269–1275; 2008.
9. Keirstead, H. S.; Nistor, G.; Bernal, G.; Totoiu, M.; Clout-ier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants
remyelinate and restore locomotion after spinal cord in-jury. J. Neurosci. 25(19):4694–4705; 2005.
10. Kim, D.; Kim, C. H.; Moon, J. I.; Chung, Y. G.; Chang, M. Y.; Han, B. S.; Ko, S.; Yang, E.; Cha, K. Y.; Lanza, R.; Kim, K. S. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4(6):472–476; 2009.
11. Kim, J. B.; Sebastiano, V.; Wu, G.; Arauzo-Bravo, M. J.; Sasse, P.; Gentile, L.; Ko, K.; Ruau, D.; Ehrich, M.; van den Boom, D.; Meyer, J.; Hubner, K.; Bernemann, C.; Ortmeier, C.; Zenke, M.; Fleischmann, B. K.; Zaehres, H.; Scholer, H. R. Oct4-induced pluripotency in adult neural stem cells. Cell 136(3):411–419; 2009.
12. Kim, J. B.; Zaehres, H.; Wu, G.; Gentile, L.; Ko, K.; Sebastiano, V.; Arauzo-Bravo, M. J.; Ruau, D.; Han, D. W.; Zenke, M.; Scholer, H. R. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454(7204):646–650; 2008. 13. Lamba, D. A.; Gust, J.; Reh, T. A. Transplantation of
hu-man embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4(1):73–79; 2009.
14. Liao, J.; Wu, Z.; Wang, Y.; Cheng, L.; Cui, C.; Gao, Y.; Chen, T.; Rao, L.; Chen, S.; Jia, N.; Dai, H.; Xin, S.; Kang, J.; Pei, G.; Xiao, L. Enhanced efficiency of generat-ing induced pluripotent stem (iPS) cells from human so-matic cells by a combination of six transcription factors. Cell Res. 18(5):600–603; 2008.
15. Lin, S. L.; Chang, D. C.; Chang-Lin, S.; Lin, C. H.; Wu, D. T.; Chen, D. T.; Ying, S. Y. Mir-302 reprograms hu-man skin cancer cells into a pluripotent ES-cell-like state. RNA 14(10):2115–2124; 2008.
16. Mandai, M.; Ikeda, H.; Jin, Z. B.; Iseki, K.; Ishigami, C.; Takahashi, M. Use of lectins to enrich mouse ES-derived retinal progenitor cells for the purpose of transplantation therapy. Cell Transplant. 19(1):9–19; 2010.
17. Matsuda, R.; Yoshikawa, M.; Kimura, H.; Ouji, Y.; Nakase, H.; Nishimura, F.; Nonaka, J.; Toriumi, H.; Yamada, S.; Nishiofuku, M.; Moriya, K.; Ishizaka, S.; Nakamura, M.; Sakaki, T. Cotransplantation of mouse em-bryonic stem cells and bone marrow stromal cells follow-ing spinal cord injury suppresses tumor development. Cell Transplant. 18(1):39–54; 2009.
18. Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26(1):101–106; 2008.
19. Okita, K.; Nakagawa, M.; Hyenjong, H.; Ichisaka, T.; Yamanaka, S. Generation of mouse induced pluripotent
stem cells without viral vectors. Science 322(5903):949– 953; 2008.
20. Reya, T.; Morrison, S. J.; Clarke, M. F.; Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414(6859):105–111; 2001.
21. Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hoched-linger, K. Induced pluripotent stem cells generated with-out viral integration. Science 322(5903):945–949; 2008. 22. Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.;
Ichi-saka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripo-tent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872; 2007.
23. Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cul-tures by defined factors. Cell 126(4):663–676; 2006. 24. Wang, W.; Lin, C.; Lu, D.; Ning, Z.; Cox, T.; Melvin, D.;
Wang, X.; Bradley, A.; Liu, P. Chromosomal transposi-tion of PiggyBac in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 105(27):9290–9295; 2008. 25. Woltjen, K.; Michael, I. P.; Mohseni, P.; Desai, R.;
Milei-kovsky, M.; Hamalainen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; Kaji, K.; Sung, H. K.; Nagy, A. pig-gyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458(7239):766–770; 2009. 26. Wu, S. C.; Meir, Y. J.; Coates, C. J.; Handler, A. M.;
Pelczar, P.; Moisyadi, S.; Kaminski, J. M. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc. Natl. Acad. Sci. USA 103(41):15008–15013; 2006. 27. Yamanaka, S. A fresh look at iPS cells. Cell 137(1):13–
17; 2009.
28. Yang, D.; Zhang, Z. J.; Oldenburg, M.; Ayala, M.; Zhang, S. C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 26(1):55–63; 2008.
29. Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I. I.; Thomson, J. A. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324(5928):797–801; 2009.
30. Yu, J.; Vodyanik, M. A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J. L.; Tian, S.; Nie, J.; Jonsdottir, G. A.; Ruotti, V.; Stewart, R.; Slukvin, I. I.; Thomson, J. A. Induced pluripotent stem cell lines derived from hu-man somatic cells. Science 318(5858):1917–1920; 2007. 31. Ziegler, A.; Nervi, P.; Durrenberger, M.; Seelig, J. The
cationic cell-penetrating peptide CPP(TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: Optical, biophysical, and metabolic evidence. Biochemistry 44(1):138–148; 2005.