行政院國家科學委員會專題研究計畫 期中進度報告
乳癌之腫瘤浸潤淋巴細胞中 T 細胞之研究(1/2)
計畫類別: 個別型計畫 計畫編號: NSC92-2314-B-002-279- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院外科 計畫主持人: 張金堅 共同主持人: 果伽蘭 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 5 月 31 日
(一)中文摘要。(五百字以內)
關鍵詞: 乳癌, 腫瘤浸潤淋巴細胞(Tumor infiltrating lymphocyte, TIL), T 淋巴細胞
在一些癌症中,腫瘤組織中有淋巴細胞的浸潤証實是預後較佳的徵候1-5。而這種現 象被認為是自體針對癌細胞所產生的特異性免疫反應,而非一般的發炎狀態。浸潤的淋 巴細胞絕大多數是帶有 CD3+抗原的 T 淋巴細胞,其中包含數目不等的 CD4+及 CD8+細 胞,NK 細胞只佔少數,B 淋巴細胞則不存在6-10。 在乳癌的研究則發現乳癌的期別與組織中浸潤淋巴細胞的多寡有關:癌細胞中的 浸潤淋巴細胞較正常組織中多,而且更常見。此外,CD4+ /CD8 的比例愈高,腫瘤愈大, 淋巴腺轉移的機會也高11。在動物實驗中,TIL 殺死癌細胞的能力高達 LAK 細胞的 50-100 倍12,因此,腫瘤浸潤淋巴細胞 (TIL) 很有可能成為癌症誘發性免疫治療的明日之星。 本研究的第一年著重在了解浸潤淋巴細胞在腫瘤組織及正常乳腺的分佈情形,並與 血液中的分佈比較;第二年將會深入研究與腫瘤免疫調控相關的受體。 在浸潤淋巴球的分佈上,在 23 位個案的完整分析中:腫瘤組織較正常組織為多( 4.15 ± 3.86 x 103 vs. 2.99 ± 3.78 x 103 cell/mg, P=0.016),與文獻報告一致。在比較免疫細胞在 腫瘤細胞及血液的分配比例方面,在腫瘤組織中,NK 細胞及 B 細胞的比例遠低於血液 (P <0.001);然而 T 細胞(CD3+)的比例則較血液中為高(P <0.001);若探究 T 細胞中 CD4 及 CD8 的分佈趨勢正好相反,腫瘤組織中的 CD8+多,CD4+的比例則血液中較高,
(二)英文摘要
Key words: Breast cancer, Tumor infiltrating lymphocyte (TIL), T lymphocyte
Lymphoid infiltration in tumor tissues has been demonstrated a favorable sign for prognosis of hosts in several malignant tumors1-5. Therefore, lymphocytes’ infiltration is considered a result of tumor targeted, specific interactions rather than of an inflammatory response. Most of the infiltrating cells are CD3+ T cells with a variable number of CD4+ and CD8+. In most of the tumors, no B cells are found and natural killer cells constitute only a small minority of Tumor infiltrating lymphocytes (TIL)6-10.
In human breast cancer , there was a significant reverse correlation between the intensity of the T-cell infiltration and the clinical stages. In general, lymphocytes are found
more frequently and more abundantly in cancer than in its normal counterparts. Furthermore, it is observed an increased CD4+/CD8+ ratio correlated with tumor’s size and lymph node metastases 11. Studies in experimental animals have shown that the adoptive transfer of TIL is
50-100 times competent than LAK cell in mediating tumor regression12. Thus, TIL is a potentially promising candidate for adoptive immunotherapy. TIL from primary breast carcinomas can be propagated in large numbers in vitro with rIL2 while still retaining autologous tumor specificity and MHC-restricted CTL activity 13.
In frist year of this project, we explored the distribution of immune cells in cancer , normal tissue and peripheral blood. The amount of mononuclear cells per mg of tissue in
breast cancer was more than in its normal counterpart( 4.15 ± 3.86 x 103 vs. 2.99 ± 3.78 x 103cell/mg, P=0.016). Comparing the lymphocytes isolated from PBMCs and TILs, the median percentage on infiltrating natural killer (NK) cells and B cells was significantly lower in TILs than in PBMCs (P < 0.001 in NK cells and in B cells). We also found that the median
percentage of CD3+ T cells in TILs was higher than that in PBMCs (P <0.001). High ratio of CD8+ T cell subpopulation was noted within gated autologous CD3+ TILs than PBMCs (63.1%±14.3%, vs. 33.3%±12.6%, P <0.001). Low ratio of CD4+
T cell subpopulation was
noted within gated autologous CD3+ TILs than PBMCs (36.95%±14.29% vs. 66.68%±12.62%,
P <0.001). The CD4+/CD8+ratio were significantly reversed in TILs (0.66±0.41 vs. 2.33±0.96,
P <0.001), which was in accordance with our previous finding.
The distribution of natural killer (NK) cells and B cells was similar in NILs and TILs.
High ratio of CD8+ T cell (66.70%±14.19%, vs. 50.16%±14.02%, P =0.002) and low ratio of CD4+ T cell (33.30%±14.19% vs. 49.84%±14.01%, P=0.002) was also noted within gated autologous CD3+ TILs than NILs. The CD4/CD8 ratio were also significantly reversed in TILs (0.55±0.33 vs. 1.22±0.52, P <0.001), which was in accordance with our previous finding in cervical cancer.
(三) Background and significance
Key words: Breast cancer, Tumor infiltrating lymphocyte (TIL), T lymphocyte
Lymphoid infiltration in tumor tissues has been demonstrated a favorable sign for prognosis of hosts in several malignant tumors1-5. Therefore, lymphocytes’ infiltration is considered a result of tumor targeted, specific interactions rather than of an inflammatory response. Most of the infiltrating cells are CD3+ T cells with a variable number of CD4+ and CD8+. In most of the tumors, no B cells are found and natural killer cells constitute only a small minority of Tumor infiltrating lymphocytes (TIL)6-10.
In human breast cancer , there was a significant reverse correlation between the intensity of the T-cell infiltration and the clinical stages. In general, lymphocytes are found
more frequently and more abundantly in cancer than in its normal counterparts. Furthermore, it is observed an increased CD4+/CD8+ ratio correlated with tumor’s size and lymph node metastases 11. Studies in experimental animals have shown that the adoptive transfer of TIL is
50-100 times competent than LAK cell in mediating tumor regression12. Thus, TIL is a potentially promising candidate for adoptive immunotherapy. TIL from primary breast carcinomas can be propagated in large numbers in vitro with rIL2 while still retaining autologous tumor specificity and MHC-restricted CTL activity 13.
How cancer cells can escape immune surveillance is an important topic in cancer immunology. Natural killer (NK) cells have been shown to express inhibitory receptors specific for major histocompatibility complex (MHC) class I antigens (natural killer receptors (NKRs)) 14, 15. The NKR–MHC class I interaction leads to inhibition of NK-mediated lysis of MHC class I target cells 14-16. A subset of T cells also expresses inhibitory receptors, but the functional significance of these receptors on T cells is unclear.
Human NK and T cells express two families of MHC class I reactive inhibitory receptors: irnmunoglobulin-like receptors (for example, KIR) directly interact with various MHC class I molecules (human Leukocyte antigen (HLA)-A.-B,-C and -G)17, and lectinlike receptors (CD94/NKG2), which interact with HLA-E-presenting signal-sequence peptides derived from other M H C class I molecules.
It has been reported that inhibitory NK receptors expressed by CD8+ were able to counterbalance TCR-mediated activation by inhibitory signals that are transduced on specific binding to HLA-I molecules. The expression of inhibitory NKRs that counteract the function of cytotoxic T lymphocytes (CTLs) in cancer milieu is unclear, It has been proposed that NKR-expressing CTLs is potentially harmful to the host, as suggested by recent data from HIV infections 18or from patients with melanoma19. Therefore, it is important to explore the possible expression of NKRs in TILs and define the mechanisms leading to the expression of inhibitory NKR by T lymphocytes
MATERIALS AND METHODS
Patient Recruitment
A total of 23 patients with Stage Ia–IIIa breast carcinoma who were admitted for surgery were enrolled prospectively in this study. A complete history was obtained for each patient. The inclusion criteria were as follows: 1) tissue-proven breast carcinoma, 2) no apparent mastitis, 3) not immuno-compromised, 4) no previous therapy or surgical procedure for breast lesions, and 5) non-pregnant. All patients under investigation were free of concomitant
illnesses, particularly infectious diseases. There also was no evidence of human immunodeficiency virus infection in any patient. Informed consent was obtained for collecting the materials in this study. After staging operation, the surgical specimens were examined carefully by experienced pathologists to exclude the possibility of coexisting malignancy. Breast tissues from normal part of the same patients were collected as normal controls. Each case of breast carcinoma was evaluated for clinical parameters including grade, lymphatic or vascular permeation, lymph node metastatic status, and surgical stage.
Histologic grades of breast carcinoma included grade I, grade II, and grade III. Surgical
staging of each patient was defined according to the TNM staging system of breast carcinoma.
Collection of Tumor Tissue and Peripheral Blood
For separating breast cancer cells, normal breast-infiltrating lymphocytes (NILs), and TILs, tissue specimens were aseptically excised immediately after operation from at least four different tumor sites and two sites of normal breast. Fragments of tissue are carefully washed with phosphate-buffered saline (PBS) for removal of contaminated blood. Tissue specimens are cut, minced, and pressed gently through a 380-µm sieve and then a 45.7-µm sieve with RPMI-1640 medium (Gibco, Life Technologies, Grand Island, NY, USA). The filtered solution is centrifuged, then layered over a Percoll discontinuous gradient (30%, 55%, and
100%) and centrifuged at 800 x g for 30 minutes. The enriched mononuclear cell suspension is collected from the interface of the 55% and 100% Percoll solutions and then washed twice with RPMI 1640 medium. The recovered cells are checked for viability with the Trypan Blue staining method and counted. Normal breast cells are separated by the same procedure as mentioned above. Venous blood of each patient is obtained before operation and transferred to test tubes containing heparin. Peripheral blood mononuclear cells (PBMCs) are isolated by Ficoll hypaque (1.077 density). The PBMCs of patients with breast carcinoma are
resuspended at 1x106 cells/mL in RPMI medium.
Immunophenotyping Analysis by Flow Cytometry
Monoclonal antibodies labeled with FITC, PE, and Per-CP (Becton-Dickinson Immuno-cytometry System; Beckton-Dickinson Inc., San Jose, CA, USA) will be used for three-color flow cytometry. The following matchings are arranged: anti-CD45-FITC + anti-CD14-PE, anti-CD3-FITC + anti-CD19-PE, anti-CD3-FITC + anti-CD4-PE, anti-CD3-FITC + anti-CD8-PE (Becton-Dickinson Immunocytometry Systems,
Becton-Dickinson, San Jose, CA); a mixture of PE-coupled NKR-specific mAbs: anti-CD94 (Immunotech, Marsseille, France), anti-NKG2A (Immunotech), anti-CD158a (EB6,
Immunotech), anti-CD158b (GL183, Immunotech), anti-NKB1 (NKB1, BD
Immunocytometry Systems); anti-CD8-PerCP, and anti-CD3-PerCP. A Simultest control (mouse IgG1-FITC + IgG2a-PE) is used as background control. Three-color flow cytometry is performed on a FACScalibur (Beckton-Dickinson Inc., San Jose, CA, USA) utilizing an argon ion laser at 15 milliwatts with an excitation wavelength of 488 nm and a 633nm HeNe diode
thousand events acquired for lymphocytes were measured in each cell suspension. The
leukogate was set around the lymphocytes (CD45+CD14-) to exclude other cells from analysis. The regional gate was set on FL1 (anti-CD3-FITC) to measure the proportion of lymphocytes in the sample being studied. Data was acquired with CellQuest software (BD Biosciences) and analyzed with CellQuest software.
Statistical Analysis
Data were expressed as the mean ±srandard deviation (SD) unless otherwise indicated.
One-way ANOVA were used in this study. The post Hoc test (Bonferroni T test) was used for comparing the subpopulations of immunocytes between TILs, NILs, and PBMCs in individual groups. Statistical significance was defined as a P < 0.05.
RESULTS & DISCUSSIONS
Weight and Cell Yields of Breast Carcinoma and Normal Breast Specimens
Histologic examination of the resected specimens revealed that there were 23 breast carcinoma and 23 autologous normal breast tissue. Table 1 lists the average weight of tissue specimens and the yield of cells from breast carcinoma and normal breast counterpart. The amount of mononuclear cells per mg of tissue in breast cancer was more than in its normal
counterpart( 4.15 ± 3.86 x 103 vs. 2.99 ± 3.78 x 103cell/mg, P=0.016),. The cell viability was around 90-95% at the completion of the isolation procedure as determined by the Trypan Blue staining method. There was no obvious cell loss when dispersing different tissue specimens by the mechanical dispersal methods.
Differences in Subpopulations of PBMCs and TILs of Breast Carcinoma Group. High Ratio of CD8+ T Lymphocytes Constituted Gated CD3+ TILs
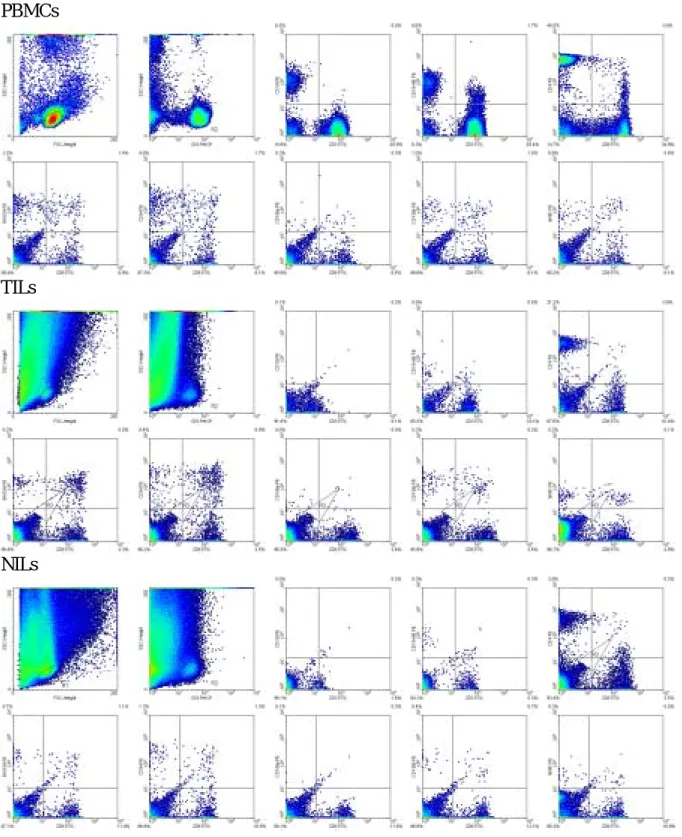
Differences in subpopulations of PBMCs and TILs are shown in Table 2. Figure 1 shows the representative data of flowcytometric analyses in selected cases. Comparing the lymphocytes isolated from PBMCs and TILs, the median percentage on infiltrating natural killer (NK) cells and B cells was significantly lower in TILs than in PBMCs (P < 0.001 in NK cells and in B cells). We also found that the median percentage of CD3+ T cells in TILs was higher than that in PBMCs (P <0.001). High ratio of CD8+ T cell subpopulation was noted within gated autologous CD3+ TILs than PBMCs (63.1%±14.3%, vs. 33.3%±12.6%, P <0.001). Low ratio of CD4+ T cell subpopulation was noted within gated autologous CD3+ TILs than PBMCs
Similar differences in Subpopulations of NILs and TILs of Breast Carcinoma Group. High Ratio of CD8+ T Lymphocytes Constituted Gated CD3+ TILs
Differences in subpopulations of NILs and TILs are shown in Table 2 as well. Comparing the lymphocytes isolated from NILs and TILs, the median percentage on infiltrating natural killer (NK) cells and B cells was similar in PBMCs (P = 0.298. in NK cells and P= 0.684 in B cells). We also found that the median percentage of CD3+ T cells in TILs was higher than that in NILs (P =0.001). High ratio of CD8+ T cell subpopulation was noted within gated
autologous CD3+ TILs than NILs (66.70%±14.19%, vs. 50.16%±14.02%, P =0.002). Low ratio of CD4+ T cell subpopulation was noted within gated autologous CD3+ TILs than NILs (33.30%±14.19% vs. 49.84%±14.01%, P=0.002). The CD4/CD8 ratio were also significantly reversed in TILs (0.55±0.33 vs. 1.22±0.52, P <0.001), which was in accordance with our previous finding in cervical cancer.
Table 1. Average weights of tissue specimens and yields of mononuclear cells from breast cancer and normal breast tissue.
Breast cancer (n = 23) Normal breast tissue (n = 23) P*
Tissue weight (mg) 945.5 1977.1 -
Yield of cells (x106) 3.90 4.41
Yield of cells per mg 4.15 ± 3.86 x 103 2.99 ± 3.78 x 103 0.016 Data are expressed as mean ± SD.
*NS: no statistical significance by One-way ANOVA.
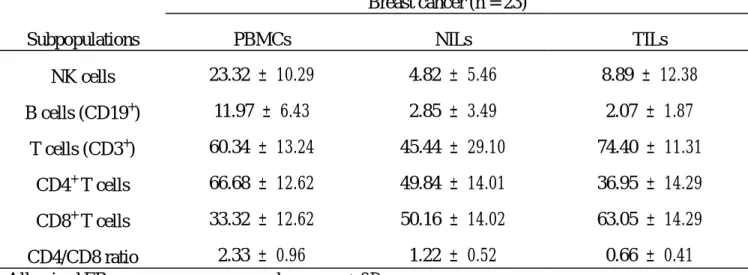
Table 2. Subpopulations of immunocytes in peripheral blood and tumor tissue or normal breast tissue of breast cancer patients.
Breast cancer (n = 23)
Subpopulations PBMCs NILs TILs
NK cells 23.32 ± 10.29 4.82 ± 5.46 8.89 ± 12.38 B cells (CD19+) 11.97 ± 6.43 2.85 ± 3.49 2.07 ± 1.87 T cells (CD3+) 60.34 ± 13.24 45.44 ± 29.10 74.40 ± 11.31 CD4+ T cells 66.68 ± 12.62 49.84 ± 14.01 36.95 ± 14.29 CD8+ T cells 33.32 ± 12.62 50.16 ± 14.02 63.05 ± 14.29 CD4/CD8 ratio 2.33 ± 0.96 1.22 ± 0.52 0.66 ± 0.41
All paired ER measures are expressed as mean ± SD.
PBMCs, peripheral blood mononuclear cells; TILs, tumor-infiltrating lymphocytes; NILs, normal breast parenchyma-infiltrating lymphocytes.
Figure 1 PBMCs
TILs
NILs
Reference
1. Yoshino, T. Yano, M. Murata, et al. Tumor-reactive T-Cells Accumulate in Lung Cancer Tissues but Fail to Respond Due to Tumor Cell-derived Factor. Cancer Research 1992;52:775-81.
2. Vose BM, Moore M. Suppressor cell activity of lymphocytes infiltrating human lung and breast turnors. Int J Cancer 1979;24:579-85.
3. Vose BM, Moore M. Human tumor-infiltrating lymphocytes: a marker of host response. Semin Hematol 22: 27-40, 1985.
4. Fiocchi C and Finke JH: Tumor-infiltrating lymphocytes: New therapy new hopes. Gastroenterology 98: 531-534, 1990.
5. Hamlin IM. Possible host resistance in carcinoma of the breast: a histological study. Br J Cancer 1968;22: 383
6. Y. Chin, J. janseens, J. Vandepitte et al. Phenotypic Analysis of Tumor-Infiltrating Lymphocytes from Human Breast Cancer. Anticancer Research 1992;12:1463-6. 7. Finke JH, Raymond P, Alexander J, et al. Vharacterization of the cytolytic activity of
CD4+ and CD8+ tumor-infiltrating lymphocytes in human renal cell carcinoma. Cancer Research 50: 2363-2370, 1990.
8. S. Von Kleist, J. Berling, W. Bohle et al. Immunohistological analysis Of lymphocyte subpopulations infiltrating breast carcinomas and benign lesions. Int. J. Cancer; 1987: 40:18.
9. T. U. An, T. Sood, G. Pietru et al. In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Am J. Pathol. 1987;128:52.
10. H. G. Gottlinger, P. Richer, J. M. Gokel et al. Infiltrating mononuelear cells in human breast carcinoma: predominance of T4+ in the tumor stroma. Int. J. Cancer 1985;35:199. 11. Y. Chin, J. janseens, J. Vandepitte et al. Phenotypic Analysis of Tumor-Infiltrating
Lymphocytes from Human Breast Cancer. Anticancer Research 1992;12:1463-6.
12. Rosenberg SA, Spiess P, Lafrenicre R: A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233: 1318-1321, 1986.
17. Coaman DN, Franger L, Borges M, et al. A novel immunoglobin superfamily receptor for cellular and viral MHC calss I molecules. Immunity. 1997; 186:1809-18.
18. De Maria. A, Ferraris, A, Guasrella, M,et al. Proc. Nat!. Acad. Sci. USA 1997; 94: 10285-10288. 19. Ikeda H, Leche B, Lehmann F, et al. Immunity 1997; 6: 199-208.