INTRODUCTION
American eels Anguilla rostrata exhibit high tempo-ral and geographic variability in many life history traits (Tesch 1977, Helfman et al. 1987, Oliveira 1999). Such plasticity in biological characteristics and environmen-tal requirements may be fundamenenvironmen-tal to the success of this panmictic and semelparous species. It spawns in the Sargasso Sea and ranges geographically from Venezuela to Greenland. The Gulf Stream distributes
the leaf-like larvae, leptocephali, along the Atlantic coast of North America. The larvae metamorphose to glass eels in continental waters and migrate inshore to coastal waters, estuaries and streams, where they be-come pigmented elvers. Most American eel elvers probably migrate into freshwater, as do Japanese and European eel elvers. A variable but unknown propor-tion of elvers may remain in coastal and estuarine waters for times ranging from 1 to several years, or for their entire juvenile life, before beginning sexual mat-uration and the spawning migration (Smith & Saunders 1955, Tsukamoto et al. 1998, Tzeng et al. 2000, in press).
© Inter-Research 2002 · www.int-res.com *E-mail: jessopb@mar.dfo-mpo.gc.ca
Migratory behaviour and habitat use by American
eels
Anguilla rostrata as revealed by otolith
microchemistry
B. M. Jessop
1,*, J.-C. Shiao
2, Y. Iizuka
3, W.-N. Tzeng
21Department of Fisheries and Oceans, Bedford Institute of Oceanography, PO Box 1006, Dartmouth, Nova Scotia
B2Y 4A2, Canada
2Department of Zoology, College of Science, National Taiwan University, Taipei, Taiwan 10617, ROC 3Institute of Earth Sciences, Academia Sinica, Nankang, Taipei, Taiwan 11529, ROC
ABSTRACT: The environmental history of American eels Anguilla rostrata from the East River, Nova Scotia, was investigated by electron microprobe analysis of the Sr:Ca ratio along transects of the eel otolith. The mean (± SD) Sr:Ca ratio in the otoliths of juvenile American eels was 5.42 ×10– 3± 1.22 ×
10– 3at the elver check and decreased to 2.38 ×10– 3± 0.99 × 10– 3at the first annulus for eels that
migrated directly into the river but increased to 7.28 ×10– 3± 1.09 ×10– 3for eels that had remained in
the estuary for 1 yr or more before entering the river. At the otolith edge, Sr:Ca ratios of 4.0 ×10– 3or
less indicated freshwater residence and ratios of 5.0 × 10– 3or more indicated estuarine residence.
Four distinct but interrelated behavioural groups were identified by the temporal changes in Sr:Ca ratios in their otoliths: (1) entrance into freshwater as an elver, (2) coastal or estuarine residence for 1 yr or more before entering freshwater, and, after entering freshwater, (3) continuous freshwater res-idence until the silver eel stage and (4) freshwater resres-idence for 1 yr or more before engaging in peri-odic, seasonal movements between estuary and freshwater until the silver eel stage. Small (< 70 mm total length), highly pigmented elvers that arrived early in the elver run were confirmed as slow growing age-1 juvenile eels. Juvenile eels that remained 1 yr or more in the estuary before entering the river contributed to the production of silver eels to a relatively greater extent than did elvers that entered the river during the year of continental arrival.
KEY WORDS: American eel · Otolith · Strontium:calcium ratio · Environmental history
Patterns in the strontium (Sr) to calcium (Ca) ratio of otoliths, in combination with age data, have been used to elucidate the environmental history of fish, particu-larly the habitat use and seasonal migration for various fishes, including Anguilla spp. (Otake et al. 1994, Tzeng & Tsai 1994, Tzeng 1996, Tzeng et al. 1997, 1999, 2000, in press, Secor 1999, Secor & Rooker 2000). The variability in migratory behaviours associated with habitat selection has recently been investigated for leptocephali metamorphosing to glass eels over the continental shelf (Cheng & Tzeng 1996, Wang & Tzeng 1998, 2000, Arai et al. 2000, Shiao et al. 2001) and for estuarine- and freshwater-resident yellow and silver eels (Tzeng et al. 1997, 2000, in press). Otolith micro-constituents are measured precisely along a radius be-tween the nucleus and edge of the otolith so as to reconstruct a chronology of environmental conditions related to age and life stage. A positive relationship exists between otolith Sr:Ca ratio and ambient salini-ties among marine, estuarine and freshwater fishes (Tzeng 1996, Kawakami et al. 1998, Secor & Rooker 2000).
Runs of juvenile eels of varying age, based upon their size composition, into and up rivers during spring are well known, but autumnal estuarine-to-freshwater migrations and spring freshwater-to-estuary migra-tions of juvenile American eels may also occur (Smith & Saunders 1955, Medcof 1969). Upstream migrations of 450 to 1200 juvenile eels of about 70 to 200 mm total length (TL) occurred concurrent with and following the annual elver run (elvers are typically 50 to 70 mm TL; Jessop 1998) into the East River, Chester, Nova Scotia, Canada, between 1996 and 2000 (e.g. Jessop 1997). The age composition and residence history of these juveniles is uncertain. We hypothesise that the eel stock within an estuary and associated river water-shed is composed of 1 or more of the following behav-ioural groups: (1) coastal or estuarine resident, (2) entrance to freshwater as an elver, (3) coastal or estu-arine resident for 1 yr or more before entering fresh-water as a juvenile eel, and, after freshfresh-water entrance, (4) continuous residence in freshwater until exiting as a silver eel and (5) freshwater resident for 1 yr or more before migrating periodically and irregularly between the river and estuary until exiting as a silver eel.
In the absence of definitive age information, size and pigmentation may not always be sufficient to distin-guish elvers from small juveniles. Jessop (1998) hypothesised that the elver-sized (less than about 70 mm), heavily pigmented eels early in the run are small juveniles rather than elvers that have pigmented particularly rapidly.
The observed annual spring migration of juvenile eels of various ages from the estuary to the river should be evident in the otolith environmental history of silver
eels from the river, consistent with the observations by Smith & Saunders (1955) and Medcof (1969) of sea-sonal migrations between river and estuary. In addi-tion, the proportion of silver eels showing a history of river entrance as an elver or as a juvenile should indi-cate the relative importance of each group’s contribu-tion to the development of the river stock. The high mortality rate (M ) of elvers during their first summer in freshwater (M > 0.99; Jessop 2000) and the size of the juvenile run lead to the hypothesis that a substantial proportion of silver eels would show evidence of a period of estuarine residence as a juvenile prior to en-tering the river.
This study examined the Sr and Ca deposition pat-terns in the otoliths of American eels in relation to their age and migratory history (upstream-migrant juve-niles, downstream-migrant silver eels) for evidence in support of hypotheses that (1) a complex variety of mi-gration and habitat residence patterns occurs; (2) el-ver-sized, heavily pigmented eels early in the run are small juveniles; and (3) the observed pattern of juve-nile eel migration is evident in the otolith environmen-tal history of silver eels.
MATERIALS AND METHODS
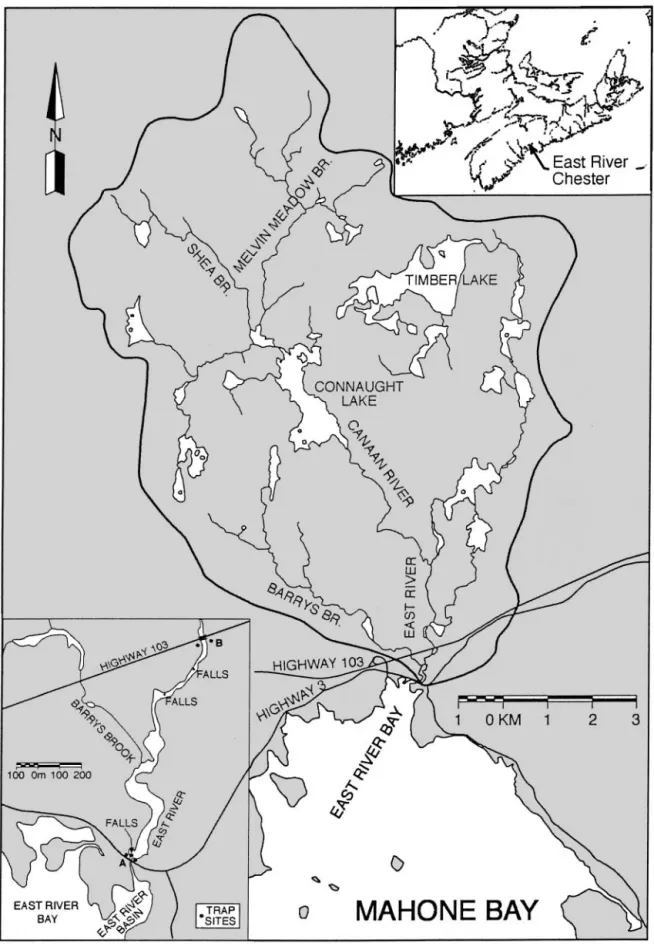
Study area.The East River (near Chester, Nova Sco-tia) has a watershed area of 134 km2 and drains into
Mahone Bay, which is located slightly south of the mid-point along the Atlantic coast of Nova Scotia (Fig. 1). A small falls (0.6 m) occurs at the outlet of the river just upstream of the high-tide mark. The river ranges in pH from 4.7 to 5.0; conductivity averages 24 µmho cm–1 (Watt et al. 1995). The Sr:Ca ratio of the river water at site B of Fig. 1 was measured as 5.6 ×10– 3in April 2001.
River water temperature ranges from about 1–2°C in the winter to 23–25°C in the summer. River discharge ranges from < 5 m3s–1during the summer to perhaps
35 m3×s–1during the spring flood.
The main branch of the East River (East Branch) was limed because it had a slightly higher natural pH (about 5.0) than did the Canaan River tributary (4.6 to 4.7) and would require less lime to attain a desired pH (Watt & White 1992, Watt et al. 1995). Four lakes (about 53% of the total watershed lake area) in the East Branch had about 350 t of powdered calcite applied annually between 1986 and 1996 (Watt & White 1992). The calcite dissolution rate during the first year follow-ing application was about 35%, and large amounts of calcite remained in the lakes that were limed. The low-ermost lake that was limed is 11.6 km from the river mouth. The Sr:Ca ratio of the water in the East River upriver of the junction with the Canaan River averaged 4.1 ×10– 3in July 2001. The Canaan River and Barrys
Fig. 1. Maps of the East River (Chester, Nova Scotia, Canada) showing location along the Atlantic coast of Nova Scotia, tributary rivers and elver trap sites at (A) river mouth and (B) Highway 103 culvert on main stem
Brook tributaries contain about 47% of the watershed lake area and were not limed. The Canaan River has low calcium concentrations (mean 930 µg l–1) and little
buffering capacity (alkalinity 0.0 ppm) with marginally better conditions in the East Branch. After 5 yr of lim-ing, pH values in the East Branch increased from about 5.5 to 6.7 but have since returned to previous levels.
The salinity in Mahone Bay about 2 km from the river mouth varies seasonally and with depth from ~27 to 31.5 ‰ (M. Dadswell, Acadia University, Wolfville, NS, pers. comm.). In general, winter surface (0 to 60 m) salinities in the coastal zone are 31 to 32 ‰ and sum-mer salinities are 30 to 31 ‰. Water temperatures in the East River estuary are about –1 to 2°C during winter and rise to about 18 to 20°C during the summer but are often in the range 12 to 17°C. The estuary is well mixed, with a maximum tidal range of ~2 m and an average range of 1.5 m.
Sample collection. Length, weight, pigmentation stage and sex (from silver eels) were obtained from elvers, juvenile or yellow eels and silver eels randomly collected from the East River (Table 1). Otoliths were removed for age determination and microchemistry analysis. Elvers are defined here as young-of-the-year (YOY) or age-0 eels with pigmentation stage from glass eel to fully pigmented (stage VIB of Elie et al. 1982 or stage 7 of Haro & Krueger 1988). Although the term juvenile eel includes elvers, the term juvenile eel in most contexts refers to larger, older (yellow) eels prior to the start of sexual maturation (silvering) and migration to the sea to spawn.
TLs were measured fresh, after anaesthetisation in MS-222, to 0.1 for eels <100 and to 1.0 mm for larger eels. Weights were measured to 0.01 g for eels less than 10 g and to 0.1 g for larger eels. Elver pigmenta-tion was classified as stages 1 to 7, following Haro & Krueger (1988). Older juvenile eels, often readily iden-tified as yellow eels, were classified as stage 8, an extension of the system used by Haro & Krueger (1988)
and equivalent to stage VII of Elie et al. (1982). Elvers and juvenile eels were then preserved in 95% ethanol. The length distributions of juvenile eels selected for otolith extraction from the different sample groups were matched with the length distributions of those groups when freshly measured. The matched cases were used to develop a linear conversion equation for application to other cases: PL = 3.681 + 0.908 FL, where PL is preserved length and FL is fresh length in mm over the range from 60 to 180 mm (n = 36, r2= 0.998,
p < 0.001).
The silver eels were frozen fresh and stored in a sealed bag, then thawed prior to length measure-ments. American eels of about 400 mm length shrink by about 1.6% after freezing and thawing (W. Morri-son, Chesapeake Bay Biological Laboratory, Solomons, MD, USA, pers. comm.), while European eels of similar size shrink about 2.5% in length and 2.7% in weight (Wickstrom 1986). The sex of the silver eels was evalu-ated macroscopically and later compared with the gen-eralisation that male silver eels are less than 400 mm long (Krueger & Oliveira 1997). The heads were then removed and preserved in 95% ethanol for later removal of the otoliths. Proctor & Thresher (1998) found little difference between preservation methods such as freezing and ethanol immersion on otolith Ca concentration, and variability in Sr:Ca ratio was due largely to variability in Sr concentration.
Four groups of eels were examined (Table 1): • Group 1 — upstream-migrant juvenile eels collected
from elver traps situated just upstream of the head of tide between May 5 and June 19, 2000 (Site A, Fig. 1). Up to 6 eels, as available, were collected for each 5 mm length interval between 70 and 150 mm or more. These eels were fully pigmented (stage 8), with the larger eels appearing as yellow eels. • Group 2 — upstream-migrant juvenile (pigment stage
8) eels collected by elver trap just upstream of the head of tide (Site A, Fig. 1) between May 5 and 22
Group Sampling Site Stage Sex n Age (yr) Length (mm) Weight (g)
date mm/dd/yy Mean ± SD Range Mean ± SD Range
1 05/05/00– River Juvenile Undiff. 29 1–4 101.9 ± 25.84 72.4–180.1 1.42 ± 1.650 0.29–8.2 06/19/00 mouth
2 05/05/00– River Juvenile Undiff. 8 1 66.0 ± 2.94 62.0–69.8 0.25 ± 0.068 0.14–0.32 05/22/00 mouth 3 06/28/00 1.3 km Elver/ Undiff. 19 1–2 70.9 ± 6.05 63.0–84.0 0.33 ± 0.108 0.19–0.58 upriver Juvenile 4 09/28/98– 1.3 km Silvera m 35 10–25 355 ± 18.0 326–412 78.9 ± 10.04 62.7–115.2 09/30/98 upriver f 27 11–29 468 ± 86.6 378–740 207.6 ± 176.81 92.6–882.2
aOtoliths from 2 silver eels were unmatched with biological data due to data processing error
Table 1. Sample data for microchemistry analysis of otoliths of American eels collected from the East River, Nova Scotia. Undiff.: undifferentiated gonads
•2000, that were < 70 mm long, i.e. the size of larger •elvers (Jessop 1998).
• Group 3 — upstream-migrant juvenile eels collected on June 28, 2000, from elver traps located 1.3 km upstream from the river mouth (Site B, Fig. 1). • Group 4 — downstream-migrant silver eels collected
from a weir located 1.3 km upstream from the river mouth (Site B, Fig. 1) during September 28 to 30, 1998. Microchemical analysis. The otoliths were prepared for electron probe microanalysis of the weight ratio of Sr:Ca by the methods described in Tzeng et al. (1997). The Sr and Ca concentration from the primordium to the otolith edge was measured by an electron probe microanalyser (EPMA; JXA-8800R, JEOL) with a wavelength dispersive spectrometer (WDS). The electron beam was defocused at intervals of approximately 10 µm on an area of about 5 µm diameter by beam conditions of 15 keV and 3 nA. The wavelength dispersive spectrum was measured for 120 s at peak positions and 20 s at background positions for Sr Lαand at 20 s and 10 s for Ca Kα. The beam power density of 2.3 W m2was within the range of 0.5 to 3 W m2 sug-gested by Gunn et al. (1992), and the counting time was sufficient to ensure effective measure-ment. Calcite (CaCO3; NMNH 136321) and
strontiantite (SrCO3; NMNH R10065) were used
as standards. The weight ratio of Sr:Ca was cal-culated after correction with the ZAF method (Z, atomic number effect; A, absorption of X-rays within the specimen; F, fluorescence effects; Goldstein et al. 1984). After microprobe analysis, the otoliths were repolished and etched with 5% EDTA to enhance the annuli (Tzeng et al. 1994). All of the eel otoliths analysed for Sr:Ca ratio were aged and the distances were measured (µm) from the core to various life stage markers: metamorphosis check at the transition from lep-tocephalus larvae to glass eel, elver check (nom-inally the freshwater check) at the transition from glass eel to elver and the annuli marking age in years. Mean (± SD) otolith Sr:Ca ratios were calculated at each of the various life stage markers for the juvenile eels of Groups 1 to 3 and the silver eels of Group 4. The Sr:Ca ratio at the otolith edge was assumed to reflect the recent environmental history of the eel and the salinity of its place of capture. The environmental history of each eel was interpreted by examining the temporal pattern of Sr:Ca ratios along the otolith transect with respect to the estuarine-freshwater decision criteria (see ‘Results’), the location of otolith checks representing life history transi-tions such as the metamorphosis from lepto-cephalus to glass eel, age as determined from
otolith annuli (Jessop 1987, Oliveira 1996), and the location and timing of capture.
RESULTS
Discrimination of estuarine and freshwater eels by Sr:Ca ratios
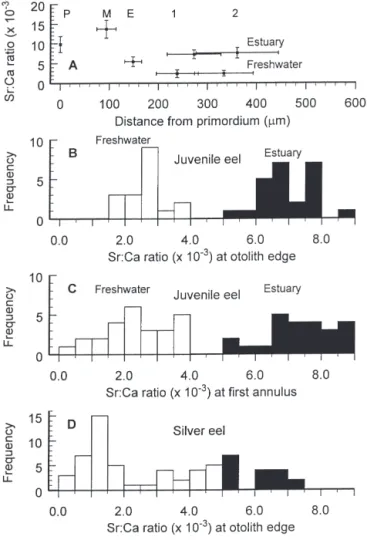
Otolith Sr:Ca ratios varied consistently at various life history stages and in response to different environ-mental salinities (Fig. 2A). For 56 juveniles of Groups 1 to 3, the Sr:Ca ratio averaged 9.82 ± 2.01 ×10– 3at the
Fig. 2. Anguilla rostrata. (A) Mean Sr:Ca ratios and distances (± SD)
along the otolith radius from the primordium (P), metamorphosis check (M), elver check (E), and the first (1) and second (2) annuli for juvenile American eels entering freshwater as an elver and after a period of estuarine residence; (B) frequency distribution of the mean of the final 3 Sr:Ca ratios at the otolith edge for freshwater- (n = 18) and estuarine- (n = 24) resident juvenile eels; (C) frequency distribu-tion of Sr:Ca ratios at the first otolith annulus for freshwater- (n = 26) and estuarine- (n = 25) resident eels; and (D) frequency distribution of the mean of the final 6 Sr:Ca ratios at the otolith edge for
primordium, 13.63 ×10– 3± 2.29 ×10– 3at the
metamor-phosis check and 5.42 ×10– 3± 1.22 ×10– 3at the elver
check (P, M and E, respectively, in Fig. 2A). The Sr:Ca ratios of these life history stages did not differ signifi-cantly between those eels that entered freshwater as elvers and those that did not (all p > 0.16). Thus, these eels experienced a similar environmental history before the elver stage. At the first annulus, elvers that migrated directly into the river had significantly lower
Sr:Ca ratios (2.38 ×10– 3± 0.99 ×10– 3) than those that
remained in the estuary for a year (7.28 ×10– 3± 1.09 ×
10– 3) (t = 17.0, df = 1, 50, p < 0.001). The mean distance from the core of the otolith to the elver check was 148.4 ± 4.5 µm. At age-1, elvers that migrated to the river grew less than those that remained in the estuary, as measured by otolith transect distances (238.4 vs 272.6 µm; t = 2.5, df = 1, 50, p = 0.015). At age-2, the growth of the eels in freshwater did not differ from that in the estuary (333.4 vs 360.6 µm; t = 0.7, df = 12, p = 0.50), mainly because of the high variability in otolith growth rate at this age and the small sample size.
The grand mean of the means of the final 3 Sr:Ca ratios at the otolith edge was 6.98 ×10– 3(n = 24, range 5.38 ×10– 3to 8.73 ×10– 3) for upstream-migrant
juve-nile eels (Group 1, Site A, Fig. 1) and 2.65 ×10– 3(n =
18, range 1.74 ×10– 3to 3.82 ×10– 3) for juveniles caught
upstream later in the year (Group 3, Site B, Fig. 1). For estuarine and freshwater eels, the means of the Sr:Ca ratios differed significantly (t = 19.7, df = 1, 40, p < 0.001) and their ranges did not overlap (Fig. 2C). How-ever, 5 eels from Group 1 were excluded on the basis of patterns in their Sr:Ca ratios between the freshwater check and otolith edge that were more consistent with eels that had spent the past year in the river rather than in the estuary (see following section). One eel from Group 3 was excluded on the basis that its otolith his-tory was consistent with residence in the estuary dur-ing the previous year. The Sr:Ca ratios at the first annulus also comprised 2 groups, ranging from 0.5 × 10– 3 to 3.8 × 10– 3 and from 5.0 × 10– 3 to 8.8 × 10– 3
(Fig. 2B). On the basis of the Sr:Ca ratios at the otolith edge and the distribution of values at the first annulus, we considered Sr:Ca ratios less than 4.0 ×10– 3to indi-cate freshwater residence and values greater than 5.0×10– 3to indicate estuarine residence.
The mean Sr:Ca ratios in the final 50 µm nearest the edge of otoliths from 64 silver eels ranged from 0.38 × 10– 3to 7.46 ×10– 3, with modes at 1.0 ×10– 3to 1.5 ×10– 3
and 5.0 ×10– 3to 5.5 ×10– 3(Fig. 2D). Of the 9 eels with
Sr:Ca ratios between 4.01 ×10– 3and 4.99 × 10– 3, the
Sr:Ca ratios declined sharply in 5 eels as the otolith edge was approached.
Four of 14 putative freshwater-resident juvenile eels had 1 probe spot with an Sr:Ca ratio greater than 4.0 × 10– 3and less than 5.0 ×10– 3out of 78 to 124 spots along
the transect from the first annulus to the otolith edge, while 2 eels had 2 disjunct spots. The transition in sil-ver eel otoliths of Sr:Ca ratios to below 4.0 × 10– 3 to
above 5.0 ×10– 3was not always sharp, and runs of 2 to
5 intermediate values sometimes occurred. The possi-ble meaning of single spots is discussed later, but 2 or more contiguous spots greater than 4.0 ×10– 3or less
than 5.0 ×10– 3were considered to represent a change in habitat, with intermediate values representing tran-Fig. 3. Anguilla rostrata. Sr:Ca ratio transects along the otolith
radius from core to edge of juvenile American eels longer than 70 mm caught at the mouth of the East River and illus-trating various patterns of movement between estuarine and freshwaters: (A) rapid entrance to the river and continuous residence until briefly flushed to tidal waters during the spring freshet; (B) rapid or delayed entrance to the river and return to the estuary; (C) estuarine residence for 1 yr or more before entering the river; (D,E) estuarine residence and movement between low and high salinity areas. The arrows mark the elver check and subsequent annuli. Annuli were not
sitional conditions. The context of particular Sr:Ca ratios relative to associated values must be considered. Although the residence classification criteria for Sr:Ca ratios is disjunct, the incorporation in the otolith of evi-dence of a habitat shift is a continuous process. The potential benefits in interpreting the trend of smooth-ing the data by a movsmooth-ing average with, perhaps, a win-dow width of 3 and the possibility of obscuring impor-tant variability were not examined.
Sr:Ca ratios in otoliths of juvenile eels of Groups 1 and 2 at the river mouth
Group 1
Upstream-migrant juvenile eels of Group 1 had a mean length of 101.9 mm (range 72.4 to 180.1 mm) and ranged in age from 1 to 4 yr or more (Table 1). Juvenile eel lengths increased with age, but length-at-age was highly variable. The otoliths from 29 eels of Group 1 showed 4 main types of habitat residence and move-ment patterns: (1) entrance to freshwater as an elver (Fig. 3A, Table 2), either rapidly (Fig. 3B, B2-12) or delayed (Fig. 3B, B1-5); (2) coastal or estuarine resi-dence for 1 yr or more before entering freshwater as a juvenile (Fig. 3C,D,E); and, after river entrance, (3) continuous freshwater residence (Fig. 3A) or (4) a return to the estuary (Fig. 3B). During estuarine resi-dence, juvenile eels may move periodically between zones of higher and lower salinity in the estuary or river mouth (Fig. 3D,E).
Twelve eels (41%) entered the river directly from the estuary as elvers, of which 5 eels (17%) remained in freshwater, as inferred from the pattern of Sr:Ca ratios in their otoliths (Table 2). After the eels entered fresh-water, as determined by the position of the elver check, the Sr:Ca ratio averaged 2.02 × 10– 3 ± 0.51 ×
10– 3(range 0.4 ×10– 3to 3.4 ×10– 3) for eel B1-9 and 1.42
×10– 3± 0.31 ×10– 3(range 0.0 to 2.8 ×10– 3) for eel
B2-14 (Fig. 3A). These eels had briefly exited the river for the upper estuary during the spring prior to being caught on the return migration into the river because no evidence of an extended residence in higher salin-ity water was observed in the Sr:Ca ratio at the otolith edge following capture.
After entering the upper estuary or river, 7 eels (24%) returned to the estuary within a year, where they re-mained for 1 or 2 yr before re-entering the river (Table 2). For example, the Sr:Ca ratio for eel B2-12 (Fig. 3B) was 0.02 ×10– 3at the freshwater check, 6.6 ×10– 3at the
first annulus and 6.2 ×10– 3at the otolith edge.
Seventeen eels (59%) remained in the estuary until entering the river from 1 to 4 yr later (Table 2). Elvers that remained in the estuary following continental arrival, such as eel B1-1 (Fig. 3C), had mean Sr:Ca ratios after the elver check of 6.97 ×10– 3± 0.41 ×10– 3
(range 5.8 ×10– 3to 8.4 ×10– 3) and, for eel B2-3, 7.45 ×
10– 3± 0.37 × 10– 3(range 5.1 × 10– 3to 9.3 ×10– 3). The
estuary-resident group showed evidence of movement between areas of high and low salinity (Fig. 3D). For example, fish B2-11 had a Sr:Ca ratio change from 8.5
× 10– 3to 13.6 × 10– 3to 8.0 × 10– 3during the summer between ages 2 and 3.
Seven eels (41%) of the estuarine-resident group made periodic, sometimes seasonal, movements between the estuary and river mouth or river prior to entering the river at the time of capture. Brief excur-sions from high salinity waters to freshwater and return (Fig. 3E) are represented by the change in Sr:Ca ratio of fish B2-13 from 9.0 ×10– 3to 3.8 ×10– 3to 10.4 ×
10– 3in the autumn between ages 1 and 2, and by the
change from 9.1 ×10– 3to 4.3 ×10– 3to 8.6 ×10– 3during
the spring at age-3.
About half (52%) of the juvenile eels of Group 1 had otoliths that had not yet acquired the annulus for the current year. Most (77%) age-1 eels showed plus growth or an annulus at the otolith edge while most (75%) eels age-2 or older did not. For age-1 juvenile eels with an annulus evident, the mean otolith growth
Behaviour pattern Juvenile eels (%) Silver eels (%)
Group 1 Group 2 Group 3 Group 4
(n = 29) (n = 8) (n = 19) (n = 64)
Enter river as elvers 41 13 89 75
Continuous freshwater residence 17 89 28
Irregular movement(s) between river and estuary 24 47
Enter river as juveniles 59 87 11 25
Continuous freshwater residence 11 8
Irregular movement(s) between river and estuary 17
Table 2. Anguilla rostrata. Life history behaviour patterns of juvenile and silver American eels as interpreted from the Sr:Ca ratios
along a transect from otolith nucleus to edge and the location and timing of capture. The decision criterion for freshwater resi-dence was an Sr:Ca ratio ≤4.0 ×10– 3; for estuarine residence it was ≥5.0 ×10– 3
between the elver check and annulus was 124.4 µm (n = 9, SD = 41.26, range 60 to 179 µm).
Group 2
The small (< 70 mm), highly pigmented, early arriv-ing juvenile eels of Group 2 had a mean length of 66.0 mm (range 62.0 to 69.8 mm) and all were age-1 (Tables 1 & 2). Six of the 8 juveniles examined had Sr:Ca ratios that averaged 2.22 ± 1.15 × 10– 3 (range
0.5 to 3.7 ×10– 3) at the position of the annulus,
indi-cating freshwater residence (eel B4-1, Fig. 4A). Two juveniles had overwintered in the estuary, as indi-cated by Sr:Ca ratios at the annulus of 6.9 ×10– 3and
8.4 × 10– 3(eel B4-10, Fig. 4A). When aged, eel B4-7
(Fig. 4B) showed very little growth after the elver check and no annulus; all other aged juveniles of this group showed slight plus growth beyond the annu-lus. The mean otolith growth between the elver check and first annulus was 68.7 µm (n = 7, SD = 22.4, range 49 to 110 µm) and was significantly
smaller than for the age-1 juveniles longer than 70 mm (t = –3.21, df = 1,14, p = 0.006).
Sr:Ca ratios in otoliths of juvenile eels of Group 3 from upstream
Otoliths were examined from 19 juvenile eels of Group 3 (Tables 1 & 2). All were age-1 or age-2 and ranged in length from 63.5 to 84.0 mm with a mean of 70.9 mm. For 18 eels, the mean Sr:Ca ratio at the first annulus was 2.51 ×10– 3± 0.46 ×10– 3(range 0.7 ×10– 3
to 3.8 ×10– 3), indicating that they had remained 1 yr in
the lower river before migrating further upstream e.g. eel B3-18 (Fig. 4C). Eel B3-1 had an Sr:Ca ratio at the first annulus of 7.7 ×10– 3, indicating estuary residence
for a year before entering the river and migrating upstream (Fig. 4C). Growth after the first annulus was evident in this eel, but there was no indication of a decline in Sr:Ca ratio indicative of freshwater resi-dence. Assuming that this juvenile entered the river at the peak of the juvenile run on May 22, then a change in environmental salinity may take more than 31 d to register in the otolith at mean daily water temperatures that increased from 13.8 to 23.0°C over the period of upstream migration. All (n = 14) age-1 juvenile eels of this group possessed an annulus or plus growth, as did 80% of age-2 juveniles.
Sr:Ca ratios in otoliths of silver eels of Group 4 from upstream
Otoliths of the 64 silver eels of Group 4 showed the same variety of temporal, life history patterns in the Sr:Ca ratio as the juvenile eels (Table 2, Fig. 5). The Sr:Ca ratio means at the primordium, metamorphosis check and elver check did not differ significantly from those for juvenile eels (all p > 0.01). Forty-eight of 64 eels (75%) showed otolith Sr:Ca ratios declining to ≤4 ×10– 3
during the year of continental arrival, suggesting direct entry as elvers into the river (Table 2). For example, Sr:Ca ratios for eel Z87 (Fig. 5A) averaged 4.15 ×10– 3± 0.42 ×10– 3(range 3.1 ×10– 3to 5.4 ×10– 3) between the
elver check and the first annulus and 1.70 ×10– 3± 0.17 ×
10– 3(range 0.0 to 3.5 ×10– 3) after the first annulus.
Com-parable values for eel Z68 were 5.57 ×10– 3± 0.94 ×10– 3
(range 2.9 ×10– 3to 7.0 ×10– 3) and 2.27 ×10– 3± 0.21 ×
10– 3(range 0.0 to 4.8 ×10– 3, with 1 of 96 values
exceed-ing 4.0).
Sixteen silver eels (25%) had otolith Sr:Ca ratios that remained above 4 ×10– 3for the first 1 or 2 yr (rarely
more), indicating a period of residence in the estuary and delayed entry into the river (Table 2, Fig. 5A,D). The mean Sr:Ca ratios between the elver check and Fig. 4. Anguilla rostrata. Sr:Ca ratio transects along the otolith
radius from core to edge of juvenile American eels less than 70 mm long and of pigmentation stage 8 caught at the mouth of the East River and illustrating various patterns of move-ment between estuarine and freshwaters: (A) entrance to the river as an elver and residence for 1 yr before being flushed to tidal waters during the spring freshet and estuarine residence for 1 yr before entering the river; (B) highly pigmented elver; and (C) juvenile eels caught 1.3 km upriver and showing estu-arine residence and delayed entry for 1 yr and rapid entrance and residence in the lower river for 1 yr before further upstream migration. The arrows mark the elver check and
first annulus of juvenile eels that remained at least a year in the estuary before entering the river were slightly higher but not significantly different (t = 0.6, df = 1,18, p = 0.55) from those of silver eels that behaved similarly (juvenile mean 6.41 × 10– 3, range
5.20 ×10– 3to 7.46 ×10– 3, n = 13; silver eel mean 6.11 ×
10– 3, range 4.15 ×10– 3to 9.28 ×10– 3, n = 7).
After the initial entry to low salinity or freshwater, 2 additional behavioural groups were evident from the otoliths of the 64 silver eels examined: (1) continuous residence in freshwater (Table 2, Fig. 5A) and (2) irreg-ular movements of variable duration (1 to 10 yr) between fresh and estuarine water of varying salinities (Table 2, Fig. 5B–E). Of the 48 eels that entered the river as elvers, 30 (63%) showed Sr:Ca ratios greater than 5 after 1 yr or more in the river, suggesting a return to estuarine waters. Of the 23 eels (36% of the total number of silver eels) that evidently remained in freshwater until silvering and migrating downstream, 18 (78%) entered the river as elvers and 5 (12%) entered as juveniles. Forty-one (64% of the total) silver eels showed a pattern of irregular movement between low and high salinity waters, of which 14 (34%) returned to freshwater from 1 to 11 yr before silvering. The seasonal timing and direction of such movements could be interpreted as ranging from spring to fall or even winter, and upstream and downstream. In the final 50 µm of the Sr:Ca transect, 26 (41% of the total and 60% of the migratory group) of silver eel otoliths had mean Sr:Ca ratios exceeding 4.5 × 10– 3, often
greater than 6 ×10– 3(Fig. 4D).
Male eels ranged in length between 326 and 412 mm, and female eels ranged between 378 and 740 mm. After adjusting for 2.0% shrinkage in length due to freezing and thawing, all female eels but one exceeded 400 mm, the length that typically separates female from male American eels in this region (Krueger & Oliveira 1997). Of the 64 silver eels whose otoliths were examined, 35 were male, 27 were female and 2 were of unknown (due to a data processing error) sex. The sex ratios of all behavioural groups were similar.
Relative origin of silver eels
Although most (75%) silver eels from the East River derived from elvers that entered the river from the estuary shortly after continental arrival, 25% derived from juvenile eels that spent 1 yr or more in the estuary before entering the river. The relative contribution to silver eel production by elvers and estuary-derived juvenile eels was estimated with the following assump-tions: an annual elver run to the river of 850 000 elvers (1996 to 2000 mean of 845 000 elvers; Jessop in press), an average run of 750 juvenile eels from the estuary
(1996 to 2000 mean of 750 juveniles) and an annual mortality rate of elvers in the river of 99.5% (Jessop 2000). The size of the annual run of silver eels is unknown. If 850 000 elvers produce 75% of the silver eel run and 750 juveniles produce 25% of the run, then the relative production of silver eels was 380 times higher for juvenile eels that delayed entrance to the river than for elvers that entered the river directly.
DISCUSSION
Migration patterns as inferred from otolith Sr:Ca ratios
Juvenile American eels migrate seasonally between estuary and river (Smith & Saunders 1955, Medcof Fig. 5. Anguilla rostrata. Sr:Ca ratio transects along the otolith
radius from core to edge of silver American eels from the East River and illustrating various patterns of movement: (A) estu-arine residence for 1 yr before entering and remaining in freshwater; (B) and (E) entering the river as an elver, then returning to the estuary for a period of residence before re-entering the river; (C) and (D) varying periods of estuarine residence and return to freshwater or movement between areas of varying salinity. Note the high Sr:Ca ratios at the otolith edge in some fish. The arrows mark the elver check
1969), but detailed validation and quantitative studies are lacking. Temporal patterns in the Sr:Ca ratio along a radius in the otoliths of juvenile American eels mi-grating into and silver eels mimi-grating from the East River were consistent with a variety of movement pat-terns among habitats of varying salinity (Secor et al. 1995, Tzeng 1996, Tzeng et al. 1997, Campana 1999, Secor & Rooker 2000). The Sr:Ca ratios less than 4.0 × 10– 3were consistent with residence in freshwater, and
those greater than 5.0 × 10– 3indicated estuarine
resi-dence. Occasional single values of the Sr:Ca ratio be-tween 4.0 ×10– 3and 5.0 ×10– 3in a sequence of smaller
or larger values perhaps resulted from minor analytical errors due to variation in the otolith crystalline struc-ture, in surface preparation or in growth rate (Cam-pana 1999), rather than from a change in habitat. In freshwater, reported Sr:Ca ratios have varied from 3 × 10– 3to 4 ×10– 3(Tzeng & Tsai 1994) and 4 ×10– 3(range
2 ×10– 3to 7 ×10– 3) (Tzeng 1996) for Japanese eels to 3
×10– 3for European eel (Tzeng et al. 1997). In estuarine
waters, Sr:Ca ratios have varied from 7 ×10– 3(range 4
× 10– 3 to 9 × 10– 3) at 25 ‰ salinity for Japanese eel
(Tzeng 1996) to 6 ×10– 3for European eel in 23 to 25 ‰ salinity (Tzeng et al. 1997). A similar study by Kawakami et al. (1998) also reported Sr:Ca ratios in Japanese eel elvers that averaged about 4.5 × 10– 3in
freshwater and 8.2 ×10– 3in full seawater. The minor
differences among studies may result from differences in analytical methods, sampling sites and species.
The temporal patterns in movement comprised, in varying degree of frequency of occurrence, 4 of 5 hypothesised behavioural groups: (1) entrance to freshwater as an elver, (2) coastal or estuarine resi-dence for 1 yr or more before entering freshwater as a juvenile, (3) continuous residence in freshwater until exiting as a silver eel, and (4) residence in freshwater for 1 yr or more before migrating periodically and irregularly between river and estuary until exiting the river as a silver eel. Most (64%) eels in the East River evidently engaged in periodic movement of varying duration between river and estuary. The proximate causes of such movements and their timing can only be speculated about. The sampling program was not designed to observe coastal or estuarine residents. Some elvers probably settled and remained in coastal waters or the estuary until sexual maturation. Such behaviour has been reported for European and Japan-ese eels (Tzeng et al. 2000, in press).
Not all elvers that enter the river migrate rapidly upriver. Age-1 and age-2 juvenile eels with Sr:Ca ratios indicative of residence in the lower 1.3 km of the river prior to further upstream migration were abun-dant in 2000. About 1200 juvenile eels were caught at the river mouth, yet 12 200 juvenile eels were counted at the upriver site. The year 2000 was unusual in this
respect; previous years had roughly similar numbers of juveniles at both river mouth and upriver sites. About 95% of the juvenile eels at both river mouth and upriver sites were less than 85 mm long, and 95% of the juvenile eels caught upriver were age-1 and had spent the previous year in the lower river (as repre-sented by the Group 3 eel sample). Small numbers of elvers may, after entering the river, drop back to the estuary, where they may remain from days (Jessop 2000) to a year or more (this study). Some juvenile eels resident in the lower river may also briefly drop back to the upper estuary, most likely flushed out during the spring freshet, but others may remain there for an extended time.
The small (< 70 mm TL), highly pigmented, early arriving eels were confirmed as slow growing age-1 juveniles, as had previously been hypothesised (Jessop 1998). These small juveniles were probably late arrivals during the preceding year because the mean length of elvers decreases over the run, and small quantities of elvers may continue to arrive in the estu-ary at least as late as mid-August (Jessop 1998). Of the 8 elver-sized juveniles examined, 75% had entered the river as elvers. Their capture below the falls at the river mouth indicates that they had been flushed down-stream to the upper estuary during the spring runoff. One eel was highly pigmented but showed little growth and no annulus. It may have been a heavily pigmented elver, indicating that it may sometimes be difficult to separate elvers of pigment stage 7 from juveniles of pigment stage 8, or more likely a very slow growing juvenile that had arrived in the estuary very late in the previous year.
Annulus formation in the otoliths of juvenile eels is a progressive process that evidently begins before early May and is completed more quickly in faster growing age-1 eels than in slower growing age-2 eels, for which annulus completion may take until mid-June or later. Similarly, a change in the otolith Sr:Ca ratio in response to a change in habitat salinity may take over 30 d to become evident, even in relatively fast growing young eels and perhaps much longer in slower grow-ing, older eels such as silver eels.
Hypotheses to explain the Sr:Ca ratio variability in silver eel otoliths
Once in the river, about 36% of eels remained there until migrating downstream as silver eels. Although most (64%) silver eels appeared to have made irregu-lar movements of variable duration (1 to 10 yr) be-tween fresh and estuarine waters, about 34% of these freshwater-estuarine migrants evidently returned to the river several years prior to silvering. Sharp changes
in Sr:Ca ratios to below 4.0 ×10– 3or above 5.0 ×10– 3
were readily interpreted, but extended fluctuations about these values were problematic. They perhaps reflected a series of quick movements between estuary and river or residence in the river-influenced upper estuary, in conjunction with the physiological lag in incorporating into the otolith the evidence of a habitat shift. However, it is puzzling why 41% of the silver eel total had Sr:Ca ratios greater than 4.5 × 10– 3 at the
otolith edge, which suggests estuarine residence, when they were collected in freshwater 1.3 km up-stream from the river mouth and migrating down-stream. Several hypotheses can be proposed: (1) the results are an artefact of otolith preparation and analy-sis; (2) shortly, perhaps a year, before silvering, estuar-ine-resident eels re-entered the river for a brief (no evidence of freshwater residence was yet evident in the otoliths) period of freshwater residence before migrating downstream as silver eels; and (3) once in the river, the eels had remained there and the variation in Sr:Ca ratio was due to varying environmental condi-tions in the river, such as low pH, the annual liming of one tributary of the river between 1986 and 1996 or variable annual growth rates.
The hypothesis that the high Sr:Ca ratios at the edge of some silver eel otoliths is an artefact of otolith prepa-ration and analysis rather than a reflection of the most recent environmental history of the eel is perhaps the most plausible. The transect of Sr:Ca ratios along the otolith radius may not always reach the exact edge of the otolith, the radius analysed may not be the longest one, with consequent compression of the temporal pat-tern of otolith composition at the edge, and the pol-ished plane of the otolith may be slightly rounded at the edge. If the final Sr:Ca ratio is not exactly at the otolith edge, the evidence of recent habitat change may be absent. Given that otolith growth is much reduced at older ages, the time to incorporate Sr and Ca may be increased and slower growth may magnify Sr levels (Kalish 1989). If it takes about a month to incorporate evidence of habitat change in the otoliths of fast growing juvenile eels, it may take much longer in slow growing silver eels. If Sr persists in the blood-stream for a while after a move from estuary to river, Sr:Ca ratios intermediate between those in freshwater and estuary may arise (Howland et al. 2001). The sharp decline in otolith Sr:Ca ratio spot values near the otolith edge in most silver eels with mean ratios between 4.50 ×10– 3and 4.99 ×10– 3supports the
inter-pretation of a lag in the manifestation of habitat change within the otolith. The time period represented in the otolith chronology by a microprobe spot of a given size also tends to increase as the otolith edge is approached, blurring the determination of the time at which a habitat shift occurred. Consequently, the final
readings along the outermost edge of the Sr:Ca ratio transect may not reflect the environmental history of the eel at the time of capture, particularly for those with a history of recent migration between river and estuary. Perhaps as much as a year or two may be ob-scured in this manner.
There is no obvious biological reason for estuarine-resident maturing eels to enter freshwater for a rela-tively brief period prior to silvering and beginning the spawning migration. Although the timing of such a return to the river is uncertain due to the potential problems associated with estimating the Sr:Ca ratio near the otolith edge, even if periods up to 1 or even 2 yr are obscured, the question remains unanswered as to why some estuarine-resident eels return to the river a relatively short time prior to silvering. There is no evidence that estuarine-resident eels such as those in the Baltic Sea or those in Mikawa Bay, Japan, enter freshwater just before migration (Tzeng et al. 2000, in press). Whether some river-to-estuary migrants remain in the estuary during silvering is unknown.
The hypothesis that the silver eels remained in the river as juveniles and that freshwater environmental conditions, such as low pH and the liming of one tribu-tary of the river, may account for the varying annual patterns of low and high Sr:Ca ratio depends on the Sr and Ca concentrations in the calcite, their availability relative to background environmental levels, and the effect of low or varying pH on element uptake. The ef-fect of low or changing pH on the uptake of Sr and Ca from the environment is poorly understood (Campana 1999). Ambient concentrations of Sr and Ca are re-flected in otolith composition, with the molar ratio of Sr:Ca more relevant to relative rates of uptake in fish than are the absolute concentrations (Campana 1999). Although the concentration of Sr is about 100 times higher in salt water than in freshwater, the molar ratios differ by about 4.8 times (8.6 ×10– 3vs 1.8 ×10– 3). The
molar ratio of Sr:Ca in the calcite was 0.05 × 10– 3(T.
Goodwin, Nova Scotia Department of Natural Re-sources, Halifax, pers. comm.). Thus, liming of the East Branch greatly increased the available Ca but not Sr. The increased availability of Ca in the limed area may also have had little effect because otolith Ca does not, under normal conditions, respond to variability in wa-ter concentrations (Campana 1999). The high Sr and low Ca concentrations in the river water reflect the rel-atively high Sr (till sample means of 69 to 81 ppm and bedrock means of 22 to 104 ppm) and low Ca concen-trations in the granite bedrock and overlying tills of the watershed (Graves et al. 1988). Five years after the lim-ing ceased, the molar ratio of Sr:Ca in the water near the river mouth was 2.5 × 10– 3or 53 times that in the
calcite, and the Ca concentration was similar to that in the unlimed Canaan River (mean Ca of 930 µg l–1in the
Canaan River versus 900 µg l–1 at the river mouth).
Thus, liming of the East River is unlikely to account for the variable Sr:Ca ratios observed in some silver eels or for the high Sr:Ca ratios at the otolith edge in others.
The timing of the observed movement of small, young juvenile eels into the East River during spring was readily interpretable from the Sr:Ca ratio patterns along a transect from nucleus to edge in their otoliths. The silver eel otolith data indicated that larger, older juvenile eels move between estuary and river, as was noted by Smith & Saunders (1955) and Medcoff (1969), who reported movements downstream during the spring and upstream during the autumn. However, the timing of such movements, as inter-preted from the silver eel otoliths, is more variable than previously reported. A temporal lag in incorpo-rating evidence of habitat change in the otolith, slow growth in older eels or inaccuracies in determining annulus position relative to Sr:Ca ratios may affect the reliability of interpretations of silver eel movements from otoliths.
Relative origin of silver eels
Groups of fish exhibiting different migration behav-iours or habitat use within a genetic population have been termed contingents (Clark 1968, Secor 1999). Contingent behaviours may result from early life deci-sions about the energetic trade-offs between mainte-nance and growth in relation to mortality. Diverse con-tingent migratory tactics reflect population-specific reaction norms in relation to ontogeny, population density and habitat distribution. The variety of migra-tory behaviours observed in American, European and Japanese eels is consistent with contingent theory. Such contingents likely reflect phenotypic plasticity in eel behaviour and habitat selection (Helfman et al. 1987, Vøllestad 1992) in response to the wide variety of habitats encountered by eel species throughout their wide geographic ranges.
A significant contribution to the production of silver eels in the river by juvenile eels that migrated into the river after 1 to 4 yr in the estuary emphasises the importance of different contingent migratory behav-iours in the adaptation by American eels to the variety of environments that they inhabit. A production of sil-ver eels by juvenile eels that had entered the risil-ver after 1 yr or more in the estuary that was about 380 times higher than for elvers that had entered directly into the river reflects the high mortality rate of elvers in the river (Jessop 2000). Although most elvers are believed to enter the river rather than remain in the estuary, the mortality rate of elvers remaining in the estuary may be lower than in the river.
This study has illustrated a variety of migratory behaviours by American eels, behaviours that require consideration when estimating and utilising data on population size and structure and incorporating such data into models of eel life history, mortality and pro-duction.
Acknowledgements. We thank K. Marshall, J. Orser, D.
Schnare, D. Sutherland and S. Ratelle for their assistance in various aspects of the collection and processing of the eel samples. The National Science Council, Republic of China, provided financial support for the otolith analysis through Grant Number NSC-89-2313-B002-077.
LITERATURE CITED
Arai T, Otake T, Tsukamoto K (2000) Timing of metamorpho-sis and larval segregation of the Atlantic eels Anguilla ros-trata and A. Anguilla, as revealed by otolith
microstruc-ture and microchemistry. Mar Biol 137:39–45
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Cheng PW, Tzeng WN (1996) Timing of metamorphosis and estuarine arrival across the dispersal range of the Japan-ese eel Anguilla japonica. Mar Ecol Prog Ser 131:87–96
Clark J (1968) Seasonal movements of striped bass contin-gents of Long Island Sound and the New York Bight. Trans Am Fish Soc 123:950–963
Elie P, Lecomte-Finiger R, Cantrelle I, Charon N (1982) Défi-nition des limites des différentes stades pigmentaires durant la phase civelle d’Anguilla anguilla L. (Poisson
téléostéen anguilliforme). Vie Milieu 32:149–157
Goldstein JI, Newbury DE, Echlin P, Joy DC, Fiori C, Lifshin E (1984) Scanning electron microscopy and x-ray micro-analysis — a text for biologists, materials scientists, and geologists. Plenum Press, New York
Graves RM, MacDonald MA, Finck PW, Boner FJ (1988) A comparison of the clast composition and geochemistry of granite tills to underlying bedrock in the Halifax pluton, central Nova Scotia. In: Prospecting in areas of glaciated terrain — 1988. Canadian Institute of Mining and Metal-lurgy, Montreal, p 21–39
Gunn JS, Harrowfield IR, Proctor CH, Thresher RE (1992) Electron microprobe analysis of fish otoliths — evaluation of techniques for studying age and stock discrimination. J Exp Mar Biol Ecol 158:1–36
Haro AJ, Krueger WH (1988) Pigmentation, size and migra-tion of elvers (Anguilla rostrata (LeSueur)) in a coastal
Rhode Island stream. Can J Zool 66:2528–2533
Helfman GS, Facey DE, Hales LS Jr, Bozeman EL Jr (1987) Reproductive ecology of the American eel. Am Fish Soc Symp 1:42–56
Howland KL, Tonn WM, Babaluk JA, Tallman RF (2001) Iden-tification of freshwater and anadromous inconnu in the Mackenzie River system by analysis of otolith strontium. Trans Am Fish Soc 130:725–741
Jessop BM (1987) Migrating American eels in Nova Scotia. Trans Am Fish Soc 116:161–170
Jessop BM (1997) The biological characteristics of, and effi-ciency of dip-net fishing for, American eel elvers in the East River, Chester, Nova Scotia, Doc 97–01. Department of Fisheries and Oceans, Halifax
Jessop BM (1998) Geographic and seasonal variation in bio-logical characteristics of American eel elvers in the Bay of Fundy area and on the Atlantic coast of Nova Scotia. Can J Zool 76:2172–2185
Jessop BM (2000) Estimates of population size and instream mortality rate of American eel elvers in a Nova Scotia river. Trans Am Fish Soc 129:514–526
Jessop BM (in press) Annual and seasonal variability in the size and biological characteristics of the runs of American eel elvers to two Nova Scotia rivers. In: Dixon DA (ed) Biology, management, and protection for catadromous eels. Am Fish Soc Symp 33
Kalish JM (1989) Otolith microchemistry: validation of the effects of physiology, age and environment on otolith com-position. J Exp Mar Biol Ecol 132:151–178
Kawakami Y and 6 others (1998) Factors influencing otolith strontium/calcium ratios in Anguilla japonica elvers.
Env-iron Biol Fish 52:299–303
Krueger WH, Oliveira K (1997) Sex, size, and gonad morphol-ogy of silver American eels Anguilla rostrata. Copeia
1997:415–420
Medcof JC (1969) Fishermen’s reports of freshwater and salt-water migrations of Nova Scotia eels (Anguilla rostrata).
Can Field Nat 83:132–138
Oliveira K (1996) Field validation of annular growth rings in the American eel, Anguilla rostrata, using
tetracycline-marked otoliths. Fish Bull 94:186–189
Oliveira K (1999) Life history characteristics and strategies of the American eel, Anguilla rostrata. Can J Fish Aquat Sci
56:795–802
Otake T, Ishii T, Nakahara M, Nakamura R (1994) Drastic changes in otolith strontium/calcium ratios in leptocephali and glass eels of Japanese eel Anguilla japonica. Mar Ecol
Prog Ser 112:189–193
Proctor CH, Thresher RE (1998) Effects of specimen handling and otolith preparation on concentration of elements in fish otoliths. Mar Biol 131:681–694
Secor DH (1999) Specifying divergent migrations in the con-cept of stock: the contingent hypothesis. Fish Res 43: 13–34
Secor DH, Rooker JR (2000) Is otolith strontium a useful scalar of life cycles in estuarine fishes? Fish Res 46:359–371 Secor DH, Henderson-Arzapalo A, Piccoli PM (1995) Can
otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes. J Exp Mar Biol Ecol 192:15–33
Shiao JC, Tzeng WN, Collins A, Jellyman DJ (2001) Dispersal pattern of glass eel stage of Anguilla australis revealed by
otolith growth increments. Mar Ecol Prog Ser 219:241–250 Smith WM, Saunders JW (1955) The American eel in certain fresh waters of the Maritime provinces of Canada. J Fish Res Board Can 12:238–269
Tesch FW (1977) The eel: biology and management of anguil-lid eels. Chapman & Hall, London
Tsukamoto K, Nakai I, Tesch WV (1998) Do all freshwater eels migrate? Nature 396:635–636
Tzeng WN (1996) Effects of salinity and ontogenetic move-ments on strontium:calcium ratios in the otoliths of the Japanese eel, Anguilla japonica Temminck and Schlegel.
J Exp Mar Biol Ecol 199:111–122
Tzeng WN, Tsai YC (1994) Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its
migra-tion from the ocean to the rivers of Taiwan. J Fish Biol 45: 671–683
Tzeng WN, Wu HF, Wickström H (1994) Scanning electron microscope analysis of annulus microstructure in otolith of European eel, Anguilla anguilla. J Fish Biol 45:479–492
Tzeng WN, Severin KP, Wickström H (1997) Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Mar Ecol Prog Ser 149:
73–81
Tzeng WN, Severin KP, Wickström H, Wang CH (1999) Stron-tium bands in relation to age marks in otoliths of European eel Anguilla anguilla. Zool Stud 38:452–457
Tzeng WN, Wang CH, Wickström H, Reizenstein M (2000) Occurrence of the semi-catadromous European eel An-guilla anAn-guilla in the Baltic Sea. Mar Biol 137:93–98
Tzeng WN, Shiao JC, Yamada Y, Oka HP (in press) Life his-tory patterns of Japanese eel Anguilla japonica in Mikawa
Bay, Japan. In: Dixon DA (ed) Biology, management, and protection for catadromous eels. Am Fish Soc Symp 33 Vøllestad LA (1992) Geographic variation in age and length at
metamorphosis of maturing European eel: environmental effects and phenotypic plasticity. J Anim Ecol 61:41–48 Wang CH, Tzeng WN (1998) Interpretation of geographic
variation in size of American eel Anguilla rostrata elvers
on the Atlantic coast of North America using their life his-tory and otolith ageing. Mar Ecol Prog Ser 168:35–43 Wang CH, Tzeng WN (2000) The timing of metamorphosis
and growth rates of American and European leptocephali: a mechanism of larval segregative migration. Fish Res 46: 191–205
Watt WD, White WJ (1992) Creating a de-acidified Atlantic salmon refuge in the East River, Nova Scotia. In: Beattie BL (ed) Report for 1991 from the Atlantic Region Monitor-ing and Effects WorkMonitor-ing Group. Environment Canada, Bedford, p 148–164
Watt WD, Scott CD, Mandell P (1995) Water chemistry data from a monitoring program designed to detect changes in the long range transport of acidic pollutants into Nova Scotia’s acidified Atlantic salmon rivers. Can Data Rep Fish Aquat Sci 972
Wickstrom H (1986) Studies on the European eel by the Insti-tute of Freshwater Research 1977–85. Inf Sötvattenslab 13
Editorial responsibility: Kenneth Sherman (Contributing Editor), Narragansett, Rhode Island, USA
Submitted: April 20, 2001; Accepted: November 29, 2001 Proofs received from author(s): April 24, 2002