國 立 交 通 大 學
生物科技研究所
碩 士 論 文
克雷白氏肺炎桿菌 CG43 莢膜多醣體的產生和酪胺酸
磷酸化作用的研究
Capsular polysaccharide production and
protein-tyrosine phosphorylation
in Klebsiella pneumoniae CG43
研 究 生:白平輝
學 號:9128526
指導教授:彭慧玲 博士
中華民國九十三年七月
致謝
雖然我是生物背景,但是上研究所之前,除了實驗課之外,沒有真正
接觸過實驗,這一篇論文的完成,真的要感謝很多人的幫忙。最感謝
彭老師給我機會進入生科所,又讓我進入實驗室,除了提供好的環
境、研究材料,更在實驗上給我許多建議和幫助;尤其,我是一個有
點不專注、鬆散的人,老師讓我體會到一位好的研究者該有的嚴謹態
度,並給我許多空間來進行研究,更在適當時機,提醒要有專注的主
題,我才能有初步的結果,老師,非常謝謝你。感謝張晃猷老師,上
張老師的課,讓我對生物科技有較深的認識,更感謝張老是在論文和
工作上的建議。感謝邱顯泰老師,上老師的生化課,老師的認真、專
業的態度讓我收穫很多,也感謝邱老師抽空擔任我的口試主持人,更
給了我許多寶貴的建議。在實驗上,感謝靖婷學姊,在開始時,帶了
我許多的實驗,讓我可以順利進入實驗的正軌;感謝盈璁學長,在許
多實驗問題、想法上,給了許多幫助和建議;感謝怡琪學姊,提供了
我莢膜的多株抗體,讓我在實驗上有了進展;感謝丸子學姊,除了在
實驗上的建議,更感謝你分擔我情緒上的壓力;感謝小新的協助,除
了實驗室的工作上幫我很多,更在實驗方面給了我許多的意見和激
盪。感謝榕華學姊、同學珮瑄、婉君,學弟佑俊、健誠、育聖、士毅、
學妹欣穎在實驗和生活上的幫忙,謝謝智凱幫我處理畢業的事。感謝
女友佩娟幫我分擔生活、實驗的壓力,讓我可以順利完成兩年的碩士
學業。
Contents--- 2
Abstract--- 3
Introduction--- 5
Materials and Methods--- 12
Results--- 18
Discussion--- 23
References--- 26
Table--- 32
Figure--- 38
Appendix--- 55
中文摘要
蛋白質酪胺酸的磷酸化,在真核生物的生理作用中扮演重要的調控角
色,長久以來被認知。然而,在原核生物中的功能和重要性卻仍待釐
清。本實驗室的研究對象-克雷白氏肺炎桿菌,具有厚實的莢膜,為
此菌引起感染的重要因子。先前研究已發現,負責合成莢膜的基因組
中含有一個酪胺酸激酶-Yco6 和一個去磷酸酶-Yor5。本研究的目標
在釐清
Yco6 和 Yor5 的交互作用,並分析此作用如何影響克雷白氏菌
CG43 K2 血清型的莢膜生成。首先,我們分別建構了 yor5 和 yco6 的
缺損突變株;進一步利用西方轉印法分析,我們證明兩個突變株的莢
膜多醣體(CPS)皆明顯減少;此外,結合西方轉印法和阿爾新藍-銀染法的分析結果,我們發現這兩個基因的缺陷也會影響莢膜多醣體
形成時的聚合作用。同時,我們利用大腸桿菌系統來表現和純化
Yor5、Yco6 和莢膜多醣體基因組中的重組蛋白質,由硝基苯酚磷酸
鹽(pNPP)的分解作用,我們證實重組蛋白質 Yor5 的去磷酸酶活性;
而利用體外的去磷酸化實驗,我們也證明
Yor5 會調控 Yco6 的磷酸化
程度;在尋找
Yco6 目標蛋白質的磷酸化實驗中,我們初步發現位在
莢膜多醣體基因組的
Orf1, Orf3,和 Orf15 及 Ugd 不被 Yco6 所磷酸
化。更進一步,我們利用免疫沉澱法,在全菌體蛋白質中發現可受
Abstract
The phosphorylation of proteins at tyrosine residues has long been known to play a key role in the control of numerous fundamental functions in eukaryotes. In contrast, the biological significance of the protein-tyrosine phosphorylation is not clear in prokaryotes. It has been demonstrated that Yco6 is a tyrosine kinase and Yor5 is a phophatase in the capsule synthesis operon of Klebsiella pneumoniae, an opportunistic pathogen with a heavy capsule as an important virulence factor. In this study, we intend to demonstrate how the Yor5/Yco6 pair affects the capsule production in Klebsiella pneumoniae CG43 of K2 serotype. Firstly, we have constructed respectively yor5 and yco6 deletion mutants. The two mutants appeared to reduce significantly the production of capsular polysaccharides (CPS) as demonstrated by Western blot analysis with an anti-K2 antibody. Using alcian blue-silver stain to analyze the CPS, we have shown that the mutants most likely affect polymerization of the CPS. Secondly, Yor5, Yco6 and some of the genes encoding CPS operon were over-expressed respectively in E. coli and the recombinant proteins purified by a nickel-resin affinity column. The phosphatase activity of Yor5 was demonstrated by its capability to decompose p-nitrophenyl phosphate (PNPP). Using an in vitro dephosphorylation assay, we have shown also that Yor5 can regulate the phosphorlation level of Yco6. Subsequently, in vitro phosphorlation assay showed that Orf1, Orf3 and Orf15 in K2 cps gene cluster and Ugd are not the targets of Yco6. Finally, immunoprecipitation employed to search for the targets of Yco6 has identified several likely target proteins and the identities remain to be verified.
Introduction
Klebsiella pneumoniae, a member of the Enterobacteriaceae, is a rod-shaped and
highly encapsulated bacterium which is often present as a saprophyte in nasopharynx and in intestinal tract. It is also an important cause of community-acquired disease including pneumonia, bacteremia, septicemia, urinary tract and respiratory infections, occurring particularly in immunocompromised patients (Podschun et al., 1998). The current research of K. pneumoniae revealed a series of virulence factors including capsular polysaccharide, which protects the bacterium from phagocytosis and also preventing from killing by serum factors (Simoons et al., 1986);lipopolysaccharides, consisted of lipid A, core and O antigens, are essential for bacteria to resist complement-mediated killing (Alberti et al., 1996);siderophores that are capable of iron chelating (Chart et al., 1988);pili (fimbriae) which bind specifically to the receptors on cellular surface prior infection (Fader et al., 1979).
Thick capsule is a representing feature of K. pneumoniae and at least 77 distinct serotypes have been classified in the bacteria (Mizuta et al., 1984). On the basis of mouse lethality assay, the strains belonging to serotypes K1 and K2 were found to be the most virulent (Simoons et al., 1986). K. pneumoniae CG43, a clinical isolate of K2 serotype, showing a strong virulence to Balb/c mice with 50 % lethal dose as low as 10 CFU has been demonstrated in our laboratory (Chang et al., 1996). In
Escherichia coli, CPS can be classified into four groups by their chemical and
physical criteria. The group I of a high molecular mass contains uronic acid as the acidic component and is coexpressed with specific O polysaccharides (Whitfield et al., 1999). The structure of K. pneumoniae K2 CPS has been determined as [→ )
4-Glc-(1→3)-α-Glc-(1→4)-β-Man-(3←1)-α-GlcA)-(1→] n (Wacharotayankun et al.,
1992), which is made from a similar biosynthetic pathway to that of the E. coli group I CPS (Whitfield et al., 1999).
The gene cluster cps (capsular polysaccharide synthesis) region that is responsible for K2 CPS synthesis of K. pneumoniae Chedid has been determined, which contains a total of 19 open reading frames organized into 3 transcriptional units as shown in Appendix 1 (Arakawa et al., 1995). As shown in Appendix 2, orf1 is a UTP-glucose-1-phosphate uridylyltransferase homolog; orf2 is predicted as a membrane associated phospholipid phosphatase; orf3 is homologous to the gene product required for surface expression of E. coli group 1 K antigen; orf4 is a Wza homolog, which is a periplasmic protein involved in polysaccharide export; orf5, Yor5, is a low molecular weight phosphatase; orf6, Yco6, is a protein tyrosine kinase; orf7 and orf8 are glycosyltransferase homologs; orf9 is a likely mannosyltransferase; orf10 is a CapD homolog required for the biosynthesis of type 1 capsular polysaccharide (Hoskins et al., 2001); orf11 is a Wzx homolog, which is likely a flippase involved in the export of O-antigen and teichoic acid (Pausen et al., 1997); orf12 is a hypothetical protein of 65.4 kDa; orf13 is an acetyltransferase homolog; orf14, is a glycosyltransferase homolog; orf15 is a homolog of gluconate-6-phosphate dehydrogenase; orf16 is a mannose-1- phosphate guanylyl-transferase homolog; orf17 is a phosphomannonutase dehydro- genase homolog ; orf18 and orf19 are homologous to transport proteins.
In recent years, significant advances have been made in our understanding of the mechanisms involved in regulation, assembly and transport of capsular polysaccharide. The assembly and transport mechanisms of bacterial capsular
polysaccharide have been divided into two types: Wzy-dependent system and ATP binding cassette (ABC) transporter-dependent system (Whitfield et al., 1999). The former, originally described for biosynthesis of Salmonella serogroups B, D and E O antigens, is responsible for polymerization and transmembrane export of the group 1 and 4 capsules. Individual repeat units are assembled on a carrier lipid (undecaprenyl phosphate) by the sequential activities of serial glycosyltransferase enzymes (Clarke, 1995). The lipid-linked repeat units are then transferred across the plasma membrane by Wzx protein, using a mechanism that had yet to be established (Paulsen et al., 2003; Yoshida et al., 2003). Sequence similarity placed Wzx into a family of polysaccharide exporter proteins recently designated polysaccharide specific translocation-PST (Paulsen et al., 1997). Polymerization of the lipid-linked repeat units at the reducing terminus, one repeat unit at a time, appears to occur at the periplasmic face of the plasma membrane and is catalyzed by the polymerase enzyme, Wzy (Drummelsmith and Whitfield, 1999). In contrast, polymerization and export of the group 2 and 3 capsules by ABC transporter-dependent systems take place on the cytoplasmic face of the plasma membrane, which also involve a sequential action of glycosyltransferases to elongate the polysaccharide at the non-reducing end. The nascent polysaccharide is then transported across the plasma membrane by an ABC-2 transporter. In the case of group 2 capsules, this transporter comprises KpsM, the transmembrane component and KpsT, the ATPase component (Pavelka et al., 1994; Russo et al., 1998).
The regulatory strategy of colanic acid biosynthesis employed by E. coli usually serves as a model for group I CPS synthesis (Stout et al., 1991). It is built by the RcsC/YojN/RcsB two-component regulatory pair, which involved a multistep signaling transduction. The RcsC hybrid sensor kinase senses certain environmental
stimuli and the signal relayed to the downstream signaling component YojN (a histidine-containing phosphotransferHPt factor), which serves as an intermediate for the phosphorelay.Eventually, the RcsB response regulator acquires the phosphoryl group from YojN. The phosphorylated RcsB functions as a DNA-binding transcriptional regulator, which together with RcsA activate the transcription of the
cps genes for CPS production. In addition to RcsB regulator, the rmpA2 locus in
pLVPK (for Large Virulence Plasmid in Klebsiella) was also demonstrated as an alternative activator for CPS biosynthesis in K. pneumoniae CG43 (Lai et al., 2003). In additional, the cps gene cluster of CPS group 1 contains a highly conserved block of three genes, wza-wzb-wzc, which were originally identified in E. coli K30 (Drummelsmith et al., 1999). The three genes encode respectively an outer membrane lipoprotein (Wza), a cytoplasmic tyrosine phosphatase (Wzb) and a tyrosine kinase (Wzc). The orthologous genes have also been described in a diverse system including
E. coli K-12 (Stevenson et al., 1996), Erwinia amylovora amsH, amsI and amsA
(Bugert and Geider, 1995), K. pneumoniae orf4-yor5-yco6. The conserved tyrosine kinase in cps gene cluster suggests a regulatory role of tyrosine phosphorylation in bacterial CPS formation.
Ineukaryotes, a plethora of protein-tyrosine kinases andphosphotyrosine-protein phosphatases that catalyze the reversible phosphorylation on tyrosine residues of proteinshave been detected; theyhavebeen shown to be critical in theregulation of variousimportant biologicalfunctions, including signaltransduction,growth control, and malignanttransformation (Fantl et al., 1993). In contrast, only a few reports described the functional roles of tyrosine protein phosphorylation in prokaryotes. Phosphorylation on tyrosine of bacterial proteins has been reported for the first time
al., 1986). An autokinase activity of PTK was later demonstrated in vitro at the
expense of ATP (Vincent et al., 1999). The mechanism of autophosphorylation of Wzc, a tyrosine kinase in E. coli K-12, was also demonstrated (Grangeasse et al., 2002). The tyrosine kinase, which is a protein of 720 amino acids bound to the inner membrane of bacteria, consists of two functional domains: a C-terminal domain that contains the tyrosine kinase activity and an N-terminal transmembrane domain. Tyrosine phosphorylation occurs at the expense of ATP molecules that bind to Walker A and B motifs of the C-terminal domain. This domain contains six phosphorylation sites; five of these sites are located at the extreme C-terminus of the molecule in the form of a tyrosine cluster, and one is located further upstream, at Y569. It has been shown that Y569 can firstly autophosphorylate through an intramolecular reaction that induces an enhanced kinase activity of Wzc, which in turn, results in the phosphorylation of the five terminal tyrosine residues by an intermolecular process (Grangeasse et al., 2002). Phosphotyosine protein phosphatase, Wzb, is able to specifically dephosphorylate the cognate protein- tyrosine kinases, thus plays a possible regulatory role for tyrosine phosphorylation.
Some reports provide evidence to show that the activity of those protein tyrosine kinases is associated with virulence and production of the exopolysaccharide (Ilan et
al., 1999). The phosphorylation of E. coli tyrosine autokinase Wzc appeared to be
essential for the assembly of groupⅠcapsular polysaccharides (Wugeditsch et al., 2001). When this protein is phosphorylated, the production of the particular exopolysaccharide, colanic acid, is blocked. The production of colanic acid starts again when Wzc is dephosphorylated by its cognate phosphoprotein phosphatase, Wzb (Vincent et al., 2000). The tyrosine-cluster of Wzc also plays an important role in capsular polysaccharide production. The wzc mutant lacking the tyrosine cluster is
defective in producing capsular polysaccharide. Deletion of any two tyrosine residues of the tyrosine-cluster appeared to show the effect on capsular polysaccharide production. However, deleting each one of the tyrosine residues in the cluster revealed no obvious effect in the synthesis of capsular polysaccharide (Paiment et al., 2002). It is believed that the phosphorylation degree of the tyrosine cluster affect the production of capsular polysaccharide.
It has been demonstrated that Wzc can oligomerize to form essentiallytrimers and hexamers by in vivo cross-linking experiments. It was suggested that the oligomerized Wzc plays a role in capsular polysaccharide translocation (Doublet et al., 2002). Wza can also form a multimeric complex in the outer membrane for translocation of the group 1 capsular polysaccharide to E. coli surface. It have been demonstrated that Wza is able to form a protein complex with Wzc to help for the CPS translocation (Nesper et al., 2003). Not until very recently, UDP-glucose dehydeogenase was identified as a target of Wzc kinase using in vitro phosphorylation assay (Grangeasse et al., 2003). It was showed that Ugd could be phosphorylated by Wzc and the phosphorylation enhances its enzyme activity to affect LPS and colanic acid production.
A hypothetic model (Rahn et al., 2003) has recently been proposed to explain the fuctional roles of Wza, Wzb, Wzc, Wzx and Wzy. Step A-glycosyltransferases synthesize undecaprenol pyrophosphate (und-PP)-linked repeat units at the cyto- plasmic face of the inner membrane. Step B-the und-PP-linked repeat units are flipped across the inner membrane by a process involving Wzx. Step C-the repeat units are polymerized in a Wzy-dependent reaction. Step D-Wzc undergoes autopho- sphorylation, followed by transphosphorylation between proteins in an oligomeric
form, to enhance further polymerization. Step E-dephosphorylation of Wzc by the Wzb phosphatase is also crucial for capsule synthesis. Step F-exportation of the polymer to the surface requires the outer memebrane Wza multimeric complex, as an export channel. Step G-Wzi is required for an efficient assembly of the capsular layer.
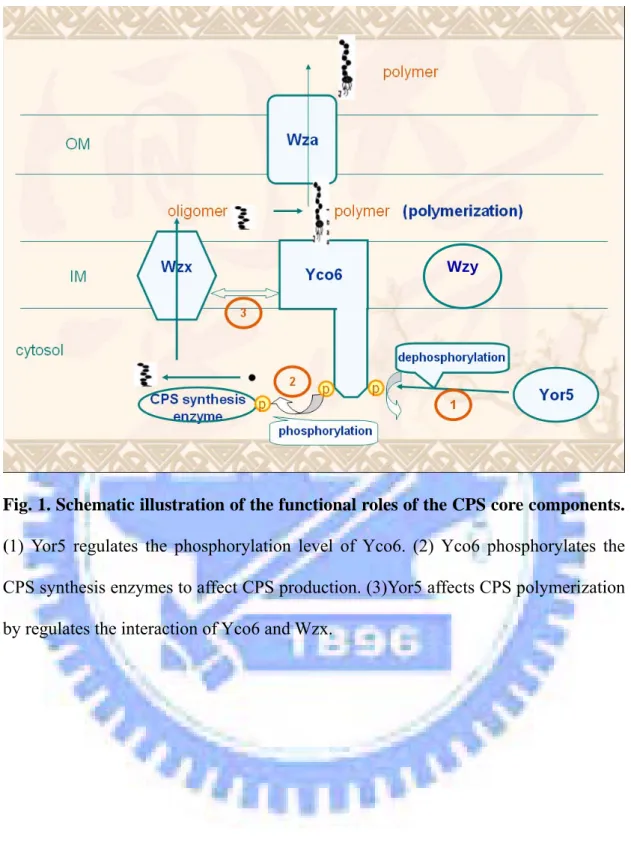
Accordingly, we propose in Fig. 1 that: (1) Yor5 regulates the Yco6 phosphorylation level without alternation of the kinase activity; (2) Yco6 phosphorylates the CPS synthesis enzymes and hence affects CPS production; (3) Yor5 affects CPS polymerization by regulates the interaction of Yco6 and Wzx. In the study, we aim to validate the hypothesis, and the proposed experiments are as following:
1. Construction of K. pneumoniae CG43 yor5, yco6 and wzx mutants and the capsular polysaccharide production of the mutants are compared.
2. Using in vitro dephosphorylation assay to confirm the interaction between Yor5 and Yco6.
3. Identification of the target proteins phosphorylated by Yco6 with anti-phospho- tyrosine monoclonal antibody.
4. Using in vitro phosphorylation assay to demonstrate if Ugd or any one of the cps gene products is the target of Yco6.
Materials and methods
Plasmids, bacterial strains, and growth conditions. The bacterial strains and
plasmids used in this study are listed in Table 1 and Table 2 and K. pneumoniae CG43 is a clinical isolate recovered from a patient in the Chang Gung Memorial Hospital, Linkou. The bacteria were propagated at 37 ° C in Luria-Bertani (LB) broth or the medium supplemented with appropriate antibiotics which include kanamycin (25 µg/ml), ampicillin (100 µg/ml) and tetracycline (20 µg/ml).
Nucleotide sequence analysis. Homology search analysis and alignment were
performed with BlastP analysis in NCBI (http://www.ncbi.nlm.nih.gov). Signal sequences in the predicted amino acid sequenceswere found by SignalP 3.0 (Jannick
et al.,2004) and LipoP 1.0 (Juncker et al.,2003). Transmembrane regions in the
predicted amino acid sequences were predicted by TopPred 2 (Gunnar von Heijn, 1992).
Construction of yor5, yco6, wza and wzx delection mutants. K. pneumoniae CG43
mutants disrupted specifically at yor5, yco6, wza, or wzx genes were constructed by the allelic exchange strategy. The primer sets used for PCR amplification of the DNA fragments flanking sequence are listed in Table 3. The generated DNA fragments were cloned into pKAS46, a suicide vector (a generous gift of K. Skorupski, University of New Hampshire), and the resulting plasmids were then mobilized to K.
pneumoniae CG43S3 through conjugation from E. coli S17-1 λ pir. The trans-
conjugants were selected with kanamycin on minimal medium. Some of the kanamycin-resistant transconjugants was picked and then spread onto a LB plate supplemented with streptomycin (500 µg/ml). After the occurrence of a double cross-
over, the streptomycin-resistant and kanamycin-sensitive colonies were isolated, and the deletions of yor5, yco6, or wzx were verified by PCR and by Southern blot analysis with a gene specific probe.
Quantification the glucuronic acid content. The CPS was extracted by using the
method described previously (Domenico et al., 1989). Briefly, bacteria were collected from 500 µl of culture and mixed with 100 µl of 1% Zwittergent 3-14 detergent (Sigma-Aldrich, Milwaukee, WI) in 100 mM citric acid (pH2.0). The mixture was incubated at 50oC for 20 min. After centrifugation, 250 µl of the supernatant was transferred to a new tube and CPS was precipitated with 1 ml of absolute ethanol. The pellet was then dissolved in 200 µl distilled water and a 1,200 µl of 12.5 mM borax (Sigma-Aldrich, Milwaukee, WI) in H2SO4 was added. The mixture was
vigorously vortexed, boiled for 5 min, cooled, and then 20 µl of 0.15% 3-hydroxydiphenol (Sigma-Aldrich, Milwaukee, WI) was added and the absorbance at 520 nm was measured. The uronic acid content was determined from a standard curve of glucuronic acid (Sigma-Aldrich, Milwaukee, WI) and expressed as µg per 109 CFU.
The purification of capsular polysaccharides. Capsular polysaccharides were
purified as described previously (Whitfield et al., 1992). Briefly, cells were harvested from LB broth grown for 16 h. The cells were pelleted at 13,200 rpm for 10min and the supernatant was removed. The pellet was resuspended with 500 µl PBS, extracted with hot-phenol and then dialyzed against water over night. The crude extract are treated with DNase (4 mg/ml), RNase (0.2 mg/ml) and proteinase K (4 mg/ml) and dialyzed against water again.
Analysis of capsular polysaccharide. The amount of CPS was determined by dot
blot assay. A two fold serial dilution of the CPS extracted from wild and the mutants were immobilized on a polyvinylidene difluoride (PVDF) membrane. The CPS was detected with an anti-K2 polyclonal antiserum and an alkaline phosphatase- conjugated anti-mouse antibody. The intensity was estimated by relative densito- metric analysis of the reaction with the ScanAlyze analysis system which was developed by Michael Eisen of Stanford University.
The CPS were then analyzed by electrophoresis on 5% or 10% DOC-PAGE and then transferred to a PVDF membrane for immunoblotting and the polysaccharides detected likewise. The CPS pattern wsa also analyzed with alcian blue-silver stain as described (Reuhs et al., 1998). After electrophoresis, the gel was immediately immersed in 100 ml of alcian blue solution (0.005% alcian blue in 40% ethanol-5% and gently rocked for 30 min. This was followed by a change to 100 ml of fresh solution and overnight rocking. The gel was rinsed for 5 min in dH2O, oxidized in 100
ml of 0.7% sodium metaperiodate (in dH2O) for 10 min, and washed five times in 200
ml of dH2O for 5 min each time. One milliliter of the final wash was added to 1 ml of
the 10% silver solution (BIO-RAD 161-0444). The gel was washed two more times if a precipitate formed. Then, the gel was stained in 100 ml of silver solution (10% Bio-Rad silver concentrate) for 10 min and rinsed in dH2O for 5 min. The gel was
rinsed again in 100 ml of Bio-Rad developer (3.0 g/200 ml of dH2O) with agitation until dark precipitate formed and immediately drained to remove all precipitate. Finally, the color was developed with the remaining developer for 5 min. The development was stopped in 100 ml of 5% acetic acid for10 min followed by a 200 ml dH2O.
Expression and purification of the recombinant Yor5, Yco6E22, Yco6E23, UGD,
ORF1 and ORF15. The coding region of yor5 was PCR amplified with primers Yor3
and Yor4 (Table 3) and the product cloned into EcoRV/SacI fragment of pET30b (Novagen, Madison, Wis.). The resulting plasmid pET-Yor5 allows inframe fusion of the yor5 coding region to six histidine codons at the N terminus and the transcription from a T7 promoter. Cells were grown to mid-exponential growth; expression of Yor5 was induced by addition of 1 mM isopropyl-1-thio- -D-galactopyranoside (IPTG).
The overexpressed His-Yor5 protein was then purified from the soluble fraction of E.
coli NovaBlue (DE3) carrying pET-Yor5 cell lysate by affinity chromatography with
His-Bind resin (Novagen). The purified Yor5 was then concentrated and dialyzed against A buffer (50 mM Tris-HCl, pH 7.4 , 100 mM NaCl, 1 mM EDTA), and the purity was determined by SDS-PAGE analysis. Yco6E22, Yco6E23, Ugd, Orf1 and Orf15 were prepared and purified likewise. Their primers, cloning sites and expression vector were shown at Table. 2. The purified Yco6E22 and Yco6E23 were concentrated and dialyzed against the buffer (10 mM sodium phosphate pH7.4, 150 mM NaCl, 1 mM EDTA, 10% glycerol). Whereas, the purified UGD, ORF1 and ORF15 were concentrated and dialyzed against PBS.
Phosphatase activity assay. The acidic phosphatase activity to detect the formation
of p-nitrophenol from p-nitrophenyl phosphate (PNPP) was monitored at 37°C by using a method as descirbed (Preneta et al., 2002) The dephosphorylation rates were determined at 405 nm in a reaction buffer containing 100 mM sodium citrate (pH 6.5), 1 mM EDTA and 10 mM PNPP. The amount of p-nitrophenol released was estimated by using a molar extinction coefficient e405 of 18,000 M-1 cm-1 (Cirri et al., 1993).
In vitro phosphorylation assay. In vitro phosphorylation using about 3 mg of the
purified His-Yco6E22 and His-Yco6E23 protein were performed at 37°C in 200 µl of a buffer containing 25 mM Tris-HCl (pH 7.0), 1 mM DTT, 5 mM MgCl2, 1 mM
EDTA and 5 µCi of [γ-p32] ATP. After 30 min of incubation, the reaction was stopped by addition of an equal volume of 2Х sample buffer, and the mixture was heated at 100°C for 5 min. One-dimensional gel electrophoresis was performed using 12.5% SDS polyacrylamide gel and the gels were stained with Coomassie blue while the gels were detected by InstantImagerTM (Packard Instrument Company). In vitro phospho- rylation assay was also used to identify if Orf1, Orf3, Orf15 and Ugd are the targets of Yco6. About 10 mg of Orf1, Orf3, Orf15 and Ugd respectively was incubated with Yco6E23 for 30 min. The reaction was stopped and detected likewise.
Dephosphorylation assay. Dephosphorylation using about 0.1 mg of purified
Yco6E22 and Yco6E23 protein was performed as the above described. After 10 min of incubation, dephosphorylation assay of Yco6E23 was carried out with 0.2 mg of purified Yor5 at 37 °C for 2 to 30 min in 30 µl of buffer consisting of 100 mM sodium citrate (pH6.5) and 1 mM EDTA. The reaction was stopped by addition of an equal volume of 2Х sample buffer, and the mixture was heated at 100 °C for 5 min. The Yco6E22 and Yco6E23 protein was separated by gel electrophoresis and analyzed by autoradiography. The radioactive bands were excised, and their radioactivities were detected by InstantImagerTM (Packard Instrument Company).
Immunoblot analysis of the phosphotyrosine proteins. The whole cell protein lysates was analyzed using 12.5% SDS PAGE gels and the resolved proteins were transferred to a polyvinylidene difluoride (PVDF) membrane electrophoretically. The
transfer buffer contains 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM
KH2PO4 and 20% methanol. The phosphotyrosine proteins were detected using
anti-phosphotyrosine monoclonal antibody with an anti-mouse AP conjugate (Sigma P3300) as the secondary antibody. The bound antibody was detected by using nitro blue tetrazolium chloride/5- bromo-4-chloro-3- indolyl phosphate.
Immunoprecipitation. Cells were harvested from 3 ml LB broth grown for 16 h. The
cells were pelleted at 13,200 rpm for 10min and the supernatant was removed. The pellet was resuspended with 500 µl IP buffer containing 50 mM Tris-HCl (pH7.5), 150 mM NaCl and 1% Triton-X100 to sonicate. The total proteins of the samples collected from the soluble fraction were regulated to equal, and then add 15 µl protein A/G (Santa Cruz Biotechnology sc2003) to precleaning at 4 °C for 4 h. The mixes were centrifuged at 10,000 rpm for 5 min. Add 4 µl anti-phosphotyrosine antibody-conjugated agarose (Sigma PT66) to be mixed at 4 °C overnight. Then 50 µl protein A-G was added to be mixed for 4 h to catch the target proteins-antibody conjugants. The proteins were pelleted at 10,000 rpm for 5 min and then 40 µl sample buffer was added to heat 100 °C for 10min. The proteins were resolved by 12.5% SDS-PAGE.
Preparation of anti-Yor5, Yco6 and Wza polyclonal antibody. BALB/c mice were
immunized using Yor5, Yco6 and Wza. Aliquots of this material containing 100 µg of these proteins were emulsified 1:1 in Freund’s complete adjuvant. After ten days, the material of equal proteins emulsified 1:1 in Freund’s incomplete adjuvant was injected into the mouse again. Serum samples showed a strong anti-Yor5, Yco6E23 and Wza, as assessed by ELISA.
Result
Comparative analysis of the K. pneumoniae cps gene clusters. The K2 cps gene
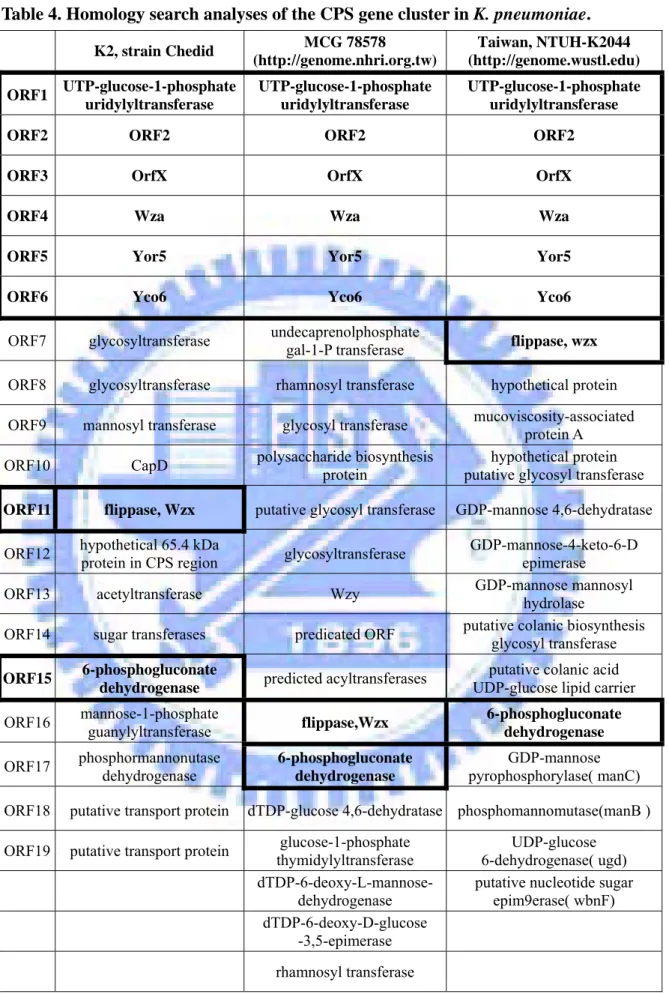
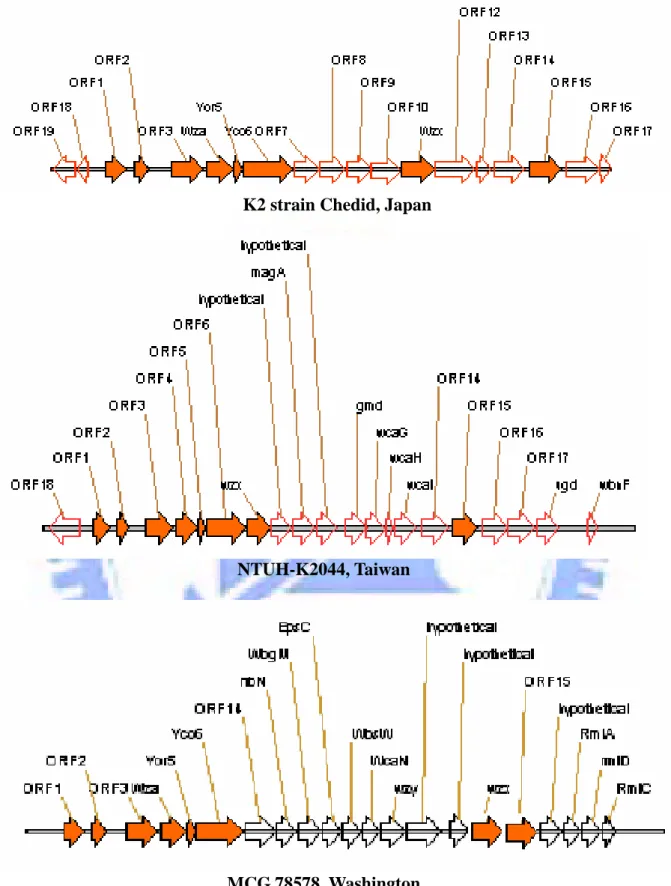
cluster of K. pneumoniae Chedid has been sequence determined, which is consisted of 19 open reading frames organized into 3 transcriptional units has been identified (Appendix 1). K. pneumoniae CG43, as well as K. pneumoniae Chedid, produce capsular polysaccharide of K2 serotype (Chang et al., 1996). Comparative analysis of the cps gene cluster of Chedid, NTUH-K2044 with K1 serotype, and WCG78578 with unknown serotype used BLASTP program revealed eight core components shown in Fig. 2 and Table 4. The orf18 and orf19 in Chedid cps gene cluster appeared to be the orf18 in that of NTUH-K2044. We also have found the eight conserved genes which encode respectively UTP-glucose-1-phosphate uridylyltransferase, ORF2, ORFX, Wza, Yor5, Yco6, Wzx and 6-phosphogluconate dehydrogenase while using a Blastp search , homology search allowed us assign the functions of the cps genes (Table 4). The features of the eighteen ORF were described as shown in Table 5.
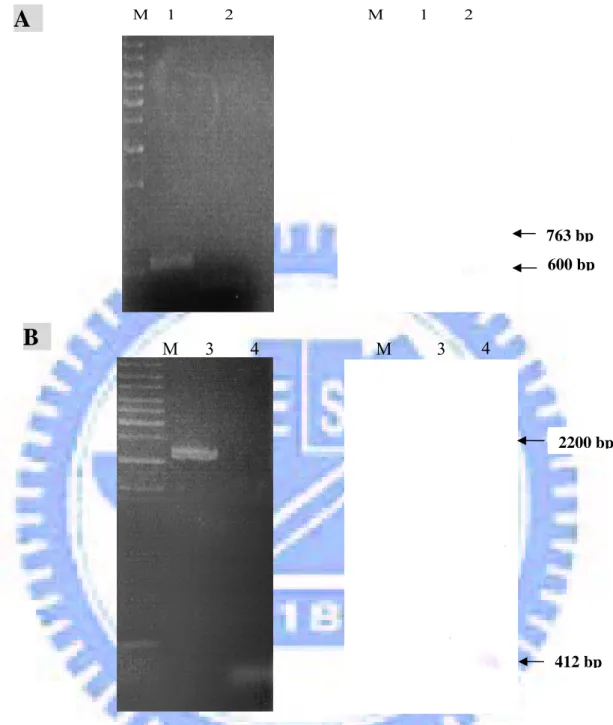
Construction of yor5 and yco6 mutants. To demonstrate that the functional roles of
Yor5 and Yco6, we have constructed the yor5 and yco6 deletion mutants. As shown in Fig. 3A, 650-bp signal was obtained in the mutant while using Yor5 gene as the probe, indicated an expected deletion of yor5. PCR using the primer pair Yco6E1 and Yco6E4 amplified a 2200-bp product in wild type strain and a 400-bp fragment in CG43-S3-yco6-. The yco6 deletion in the mutant strain was further demonstrated by
Southern blot analysis using the probe encoding the Yco6 (Fig. 3B).
Characterization of the yor5 and Yco6 deletion mutants. To investigate the role of
Yor5 and Yco6 in K2 CPS biosynthesis, the K. pneumoniae yor5 and yco6 deletion mutants were generated. We have found that the mutant strains displayed a smaller
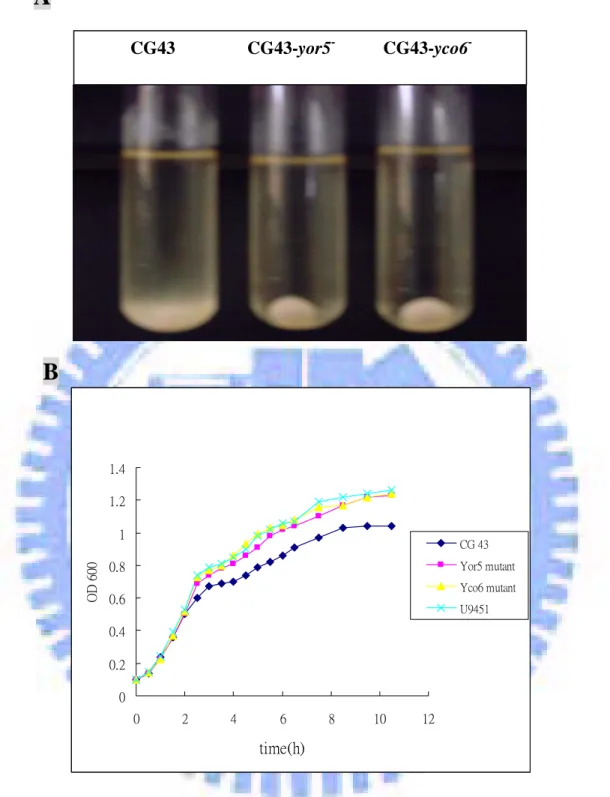
colony morphology on LB agar compared to the wild type strain. The mucoidy of the mutants appeared to be significantly reduced as determined by string formation. The loss of mucoidy was also evident when the bacterial cultures were subjected to low-speed centrifugation. As shown in Fig. 4A, both CG43S3-yor5- and CG43S3-yco6- cells were precipitated much faster than the strain CG43S3. Besides the mucoidy phenotypes, both mutants showed a higher growth rate than wild type in LB broth (Fig. 4B).
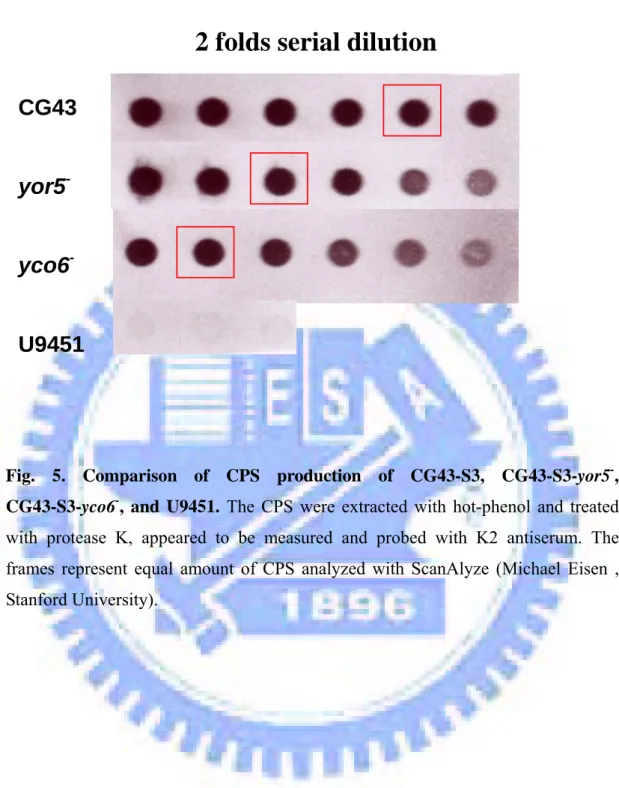
Yor5 and Yco6 are involved in the regulation of K2 capsule. To compare the CPS
production in CG43S3, CG43S3-yor5-, CG43S3-yco6- and U9451, dot blot probed with anti-K2 polycolonal antibody showed that the CPS amount of CG43S3 is about four-time neither eight-time than that of CG43S3-yor5- and CG43S3-yco6-, respectively ( Fig. 5). The result indicated that both yor5 and yco6 affect the bacterial CPS production. To further analyze the mutation effects, the amount of CPS in CG43S3, CG43S3-yor5- and CG43S3-yco6- were determinated by measuring the content of glucuronic acid, which serves as an indicator of K2 CPS (Vodonik SA and Gray GR, 1988). As shown in Fig. 6, the glucuronic acid amounts of that CG43S3-yor5- and CG43S3-yco6- appeared to reduce approximately three fold comparing to CG43S3. The mutations of yor5 and yco6, as demonstrated in Fig. 7, also affect CPS pattern. In either CG43S3-yor5- or CG43S3-yco6- , the high molecular weight fraction of CPS disappeared in CG43S3 as demonstrated by immune blot analysis using anti-K2 antiserum (Fig. 7A) The changes from polymeric form to oligomeric form in CG43S3-yor5- and CG43S3-yco6- were also confirmed using alcian blue-silver staining of the CPS (Fig. 7B). In order to further analyze the mutant effects of these mutations on oligomeric CPS, the CPS samples were applied to 10%
DOC PAGE. As shown in Fig. 8, wild-type and two mutants revealed a similar CPS ladder pattern indicating that the mutation at either yor5 or yco6 does affect the synthesis of CPS oligomer in K. pneumoniae.
In vitro autophosphorylation of Yco6 and its dephosphorylation by Yor5
To demonstrate the interaction between Yor5 and Yco6, we have overexpressed both genes in E. coli and purified the recombinant Yor5 and Yco6E23. Yco6E23 contains only kinase domain of Yco6, is soluble for purification. As showed in Fig. 9, the protein resolved by SDS-PAGE is of more than 90% purity as determinated by Coomassie Blue staining. To assess phosphatase activity of the recombinant His6-Yor5, we carried out a
p-nitrophenyl phosphate (pNPP) decomposition assay, which was monitored at 37°C by
detecting the formation of p-nitrophenol from PNPP (Fig. 10A). The dephosphorylation rate of His6-Yor5 calculated is 3.2×10-7 moles/min/mg determined at 405 nm (Fig. 10B).
On the basis of Coomassie Blue-staining, the recombinant Yco6E23 appeared to be not soluble (Fig. 11A). We then combine with sorbitol-betaine induction at low temperate and the concentration of imidazole in the wash step to increase solubility of recombinant His6-Yco6E23. As shown in Fig. 11B, the protein purity reached more
than 80%. The purified Yco6E23 showed an autokinase activity by incorporating the [γ-p32] ATP after 30 min incubation and then the phosphorylated Yco6E23 appeared to be dephosphorylated by Yor5 in a time-dependent manner (Fig. 12).
Yco6E23 is able to form a dimerized complex.
As shown in Fig. 12, a high-molecular band was found which likely represents the hetero-complex between Yor5 and Yco6E23 or an Yco6E23 homodimer. The strength of complex formation appeared to be high, which is able to resist SDS treatment. We
then performed a Western blot with anti-phosphotyrosine monoclonal antibody and anti-His.tag monoclonal antibody to investigate the possibility. We have proved that Yco6E23 is able to form a SDS-resistant dimmer which also showed a certain degree of heat-resistant (Fig. 13).
Identification of the Yco6 Target proteins in K. pneumoniae CG43
The above mentioned experiments suggest that Yco6 is able to regulate CPS synthesis enzymes through phosphorylation. Western bolt of whole cell lysate probed with anti-phosphotyrosine monoclonal antibody (Sigma P3300) was therefore used for the target protein identification. The phosphorylated Yco6, which of the predicted molecule weight is approximately 80 kDa disappeared in CG43S3-yco6- as shown in Fig. 14. In addition, five phosphorylated proteins were found to be missing in CG43S3-yco6-. In order to resolve better for isolation of the target proteins, immunopreciptation was used with anti-phosphotyrosine monoclonal antibody (Sigma PT 66), which is suitable for immunopreciptation. The resolution again appeared to be poor; however, several missing bands were still been identified.
Identification of the likely targets of Yco6 in the cps gene cluster.
We intended to overexpress the entire cps gene cluster in E. coli and purified the recombinant proteins of the following. However, we have only successfully overexpress Orf1, Orf3 and Orf15 in E coli as shown in Fig. 16, in addition to Ugd, which have been reported recently that Wzc can phosphorylate Ugd to increase its enzyme activity (Grangeasse, 2003). His6-ORF1 appeared to be insoluble protein, the
soluble proteins; His6-ORF1, His6-ORF15 and His6-Ugd were subsequently purified
Disscusion
Comparative analysis of the cps gene clusters of K. pneumoniae Chedid, MCG 78578 and NTUH-K2044 revealed at eight conserved genes including orf1, orf2, orfX,
wza, yor5, yco6, wzx and orf15 (Table 4 and 5 ). Four of them, OrfX, Wza, Yor5 and
Yco6 have also been identified in many serotypes of E. coli (Rahn et al. 1999). The conserved orfX-yco6 region represents the feature of group 1 capsule clusters and distinguishes all of them from the gene clusters of group 4 capsules and O antigens (Whitfield and Roberts, 1999). Another important feature of group 1 capsule is the Wzy-depnedent system, which includes the basic components, Wzx (flippase) and Wzy (polymerase). It is likely that Wzy is a highly diversed protein because we were unable to assign a Wzy homolog in either of three K. pneumoniae strains by using a sequence similarity search. The conserved orf1 encoding UTP-glucose-1-phosphate uridylyl- transferase, orf2 and orf15 encoding 6-phosphogluconate dehydrogenaseare are likely playing similar role in affecting the CPS synthesis in the three strains. The genes located downstream of Yco6 are highly variable, which encode respectively glycosyl- transferase and sugar nucleotide synthetic enzymes. The variable genes are most possible the key determinants of the capsule serotype.
In this study, the functional roles of Yor5 and Yco6 in capsule formation of capsular polysaccharide synthesis in K. pneumoniae CG43 were investigated. We have demonstrated that the phosphatase-Yor5 and autokinase-Yco6 are both involved in K.
pneumoniae CG43. Yor5 and Yco6 encoding genes located within the cps gene cluster
in K. pneumoniae appeared to affect both synthesis and polymerization of the CPS. The yor5 and yco6 deletion mutants both showed less colony mucoidy and a higher growth rate, which is likely due to switching off the nutrient-demanding process for
CPS synthesis. Further analysis of the CPS content showed that the production of CPS in wild type is much higher than that of the mutants. We have demonstrated that Yor5 is capable of dephosphorylating Yco6, which supports the model that Yor5 plays a regulatory role in the phosphorylation level of Yco6. The dephosphorylated Yco6 can again be phosphorylated by its autokinase activity. Theconserved features in Yco6 homologues suggest a shared and widespreadrole in prokaryotes (Appendix 2). Since many of the kinase-containing bacteria require capsular or extracellular polysaccharides as essentialvirulence determinants, Yco6 homologues play a crucial role in pathogenesis of these organisms. Regulation by a post-translational phosphorylation event represents a new dimension in the assemblyof bacterial cpaular polysaccharides. Thus, the interaction between Yor5 and Yco6 might be cyclic in order to control translocation or assembly of CPS.
Interestingly, the two mutants not only showed a significant reduction of CPS, but also decreased the formation of high molecule weight CPS. In E. coli group 1 K30 serotype, Wzb and Wzc have also been demonstrated to affect CPS assembly (Drummelsmith and Whitfield, 1999). However, the mechanism remains to be investigated. Many studies of O-antigen revealed that many bacterial strains can efficiently translocate both full-length and truncated LPS molecules, which leads to heterogeneity of LPS (Amor and Whitfield, 1997). Translocation of CPS is likely using a different mechanism. We also predicted amino acid sequences by SignalP 3.0 and LipoP 1.0. Transmembrane regions in the predicted amino acid sequences were predicted by TopPred 2. Based on our analysis, most of enzymes involved in CPS synthesis were predicted to locate in cytosol, therefore, we suggest that the CPS repeat units were produced before being translocated to periplasmic space. It is
block the polymerization possibly by interacting with Wzx. Yor5 controls the phosphorylation level of Yco6, possibly altering its ability to interact with Wzx, which needs further investigation. Proper alterations between activation and inactivation of Yco6 by Yor5 may thus form a cycle for regulation of CPS translocation.
The protein-tyrosine kinase Wzc of E. coli was recently demonstrated to phosphorylate an endogenous enzyme, UDP-glucose dehydrogenase (Ugd), which participatesin the synthesis of the exopolysaccharide colanic acid (Grangeasse et al., 2003). Thephosphorylation of Ugd was demonstrated to actually occur ontyrosine residue, resulting in a significant increase of its dehydrogenaseactivity. In our study, several possible targets of Yco6 were examined by Western blotting and immunoprecipitation. However, these possible targets of Yco6 that disappeared in
yco6 mutant may be the result of degradation of auto-phosphorylated Yco6. To
identify these proteins, we will try to increase the concentration of the target proteins or using silver stain method to improve resolution. The candidates will then be isolated and sequence identified. We will also overexpress other genes of cps gene cluster to identify other Yco6 target proteins.
Refernece
Alberti S, Alvarez D, Merino S, Casado MT, Vivanco F, Tomas JM, Benedi VJ.
1996. Analysis of complement C3 deposition and degradation on Klebsiella
pneumoniae. Infect Immun. 64(11):4726-32.
Amor PA, Whitfield C. 1997. Molecular and functional analysis of genes required
for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene clusters for multiple cell-surface polysaccharides. Mol Microbiol. 26(1):145-61.
Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. 1995.
Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 177(7):1788-96.
Bugert P and Geider K. 1995. Molecular analysis of the ams operon required for
exopolysaccharide synthesis of Erwinia amylovora. Mol Microbiol. 15(5):917-33. Chang HY, Lee JH, Deng WL, Fu TF, Peng HL. 1996. Virulence and outer
membrane properties of a galU mutant of Klebsiella pneumoniae CG43. Microb Pathog. 20(5):255-61.
Chart H, Stevenson P, Griffiths E. 1988. Iron-regulated outer-membrane proteins of
Escherichia coli strains associated with enteric or extraintestinal diseases of man and
animals. J Gen Microbiol. 134 Pt 6:1549-59.
Clarke BR, Bronner D, Keenleyside WJ, Severn WB, Richards JC, Whitfield C.
1995. Role of Rfe and RfbF in the initiation of biosynthesis of D-galactan I, the lipopolysaccharide O antigen from Klebsiella pneumoniae serotype O1. J Bacteriol. 177(19):5411-8.
Cortay JC, Duclos B, Cozzone AJ. 1986. Phosphorylation of an Escherichia coli
protein at tyrosine. J Mol Biol. 187(2):305-8.
Cirri P, Chiarugi P, Camici G, Manao G, Raugei G, Cappugi G, Ramponi G. 1993
The role of Cys12, Cys17 and Arg18 in the catalytic mechanism of low-M(r) cytosolic phosphotyrosine protein phosphatase. Eur J Biochem. 214(3):647-57.
Domenico P, Schwartz S, Cunha BA. 1989. Reduction of capsular polysaccharide
production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 57(12): 3778-82.
Doublet P, Grangeasse C, Obadia B, Vaganay E, Cozzone AJ. 2002. Structural
organization of the protein-tyrosine autokinase Wzc within Escherichia coli cells. J Biol Chem. 277(40):37339-48.
Drummelsmith J, Whitfield C. 1999. Gene products required for surface expression
of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol Microbiol. 31(5):1321-32.
Fader RC, Avots-Avotins AE, Davis CP. 1979. Evidence for pili-mediated adherence
of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 25(2):729-37.
Fantl WJ, Johnson DE, Williams LT. 1993. Signalling by receptor tyrosine kinases.
Annu Rev Biochem. 62:453-81.
Gottesman, S., and V. Stout. 1991. Regulation of capsular polysaccharide synthesis
in Escherichia coli K-12. Mol. Microbiol. 5:1599-1606
Grangeasse C, Doublet P, Cozzone AJ. 2002. Tyrosine phosphorylation of protein
kinase Wzc from Escherichia coli K12 occurs through a two-step process. J Biol Chem. 277(9):7127-35.
Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J Biol Chem. 278(41):39323 -9.
Hoskins J, Alborn WE Jr, Arnold J, Blaszczak LC, Burgett S, DeHoff BS, Estrem
ST, Fritz L, Fu DJ, Fuller W, Geringer C, Gilmour R, Glass JS, Khoja H, Kraft
AR, Lagace RE, LeBlanc DJ, Lee LN, Lefkowitz EJ, Lu J, Matsushima P,
McAhren SM, McHenney M, McLeaster K, Mundy CW, Nicas TI, Norris FH,
O'Gara M, Peery RB, Robertson GT, Rockey P, Sun PM, Winkler ME, Yang Y,
Young-Bellido M, Zhao G, Zook CA, Baltz RH, Jaskunas SR, Rosteck PR Jr,
Skatrud PL, Glass JI. 2001. Genome of the bacterium Streptococcus pneumoniae
strain R6. J Bacteriol. 183(19):5709-17.
Ilan O, Bloch Y, Frankel G, Ullrich H, Geider K, Rosenshine I. 1999. Protein
tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 18(12):3241-8.
Jayaratne P, Keenleyside WJ, MacLachlan PR, Dodgson C, Whitfield C. 1993.
Characterization of rcsB and rcsC from Escherichia coli O9:K30:H12 and examination of the role of the rcs regulatory system in expression of group I capsular polysaccharides. J Bacteriol. 175(17):5384-94.
Lai YC, Peng HL, Chang HY. 2003. RmpA2, an activator of capsule biosynthesis in
Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional
level. J Bacteriol. 185(3):788-800.
Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, Kato N. 1983. Virulence
for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 40(1):56-61.
Translocation of group 1 capsular polysaccharide in Escherichia coli serotype K30. Structural and functional analysis of the outer membrane lipoprotein Wza. J Biol Chem. 278(50):49763-72.
Paiment A, Hocking J, Whitfield C. 2002. Impact of phosphorylation of specific
residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J Bacteriol. 184(23):6437-47.
Paulsen, I.T., Beness, A.M., Saier, M.H. 1997. Computer-based analyses of the
protein constituents of transport systems catalysing export of complex carbohydrates in bacteria. Microbiology 143: 2685-2699 Clin Microbiol Rev. 11(4):589-603
Pavelka MS Jr, Hayes SF, Silver RP. 1994 Characterization of KpsT, the
ATP-binding component of the ABC-transporter involved with the export of capsular polysialic acid in Escherichia coli K1. J Biol Chem. 269(31):20149-58.
Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens:
epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 11(4):589-603.
Preneta R, Jarraud S, Vincent C, Doublet P, Duclos B, Etienne J, Cozzone AJ.
2002. Isolation and characterization of a protein-tyrosine kinase and a phosphotyrosine- protein phosphatase from Klebsiella pneumoniae. Comparative Biochemistry and Physiology Part B 131103-112
Rahn A, Beis K, Naismith JH, Whitfield C. 2003. A novel outer membrane protein,
Wzi, is involved in surface assembly of the Escherichia coli K30 group 1 capsule. J Bacteriol. 185(19):5882-90.
Reuhs BL, Geller DP, Kim JS, Fox JE, Kolli VS, Pueppke SG. 1998. Sinorhizobium
fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides
Russo TA, Wenderoth S, Carlino UB, Merrick JM, Lesse AJ. 1998. Identification,
genomic organization, and analysis of the group III capsular polysaccharide genes kpsD,
kpsM, kpsT, and kpsE from an extraintestinal isolate of Escherichia coli (CP9,
O4/K54/H5). J Bacteriol. 180(2):338-49.
Simoons-Smit AM, Verweij-van Vught AM, MacLaren DM. 1986. The role of K
antigens as virulence factors in Klebsiella. J Med Microbiol. Mar;21(2):133-7.
Stevenson G, Andrianopoulos K, Hobbs M, Reeves PR. 1996. Organization of the
Escherichia coli K-12 gene cluster responsible for production of the extracellular
polysaccharide colanic acid. J Bacteriol. 178(16):4885-93.
Stout V, Torres-Cabassa A, Maurizi MR, Gutnick D, Gottesman S. 1991. RcsA, an
unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 173(5):1738-47.
Stevenson, G., K. Andrianopoulos, M. Hobbs, and P. R. Reeves. 1996.
Organization of the Escherichia coli K-12 gene cluster responsible for production of the extracellular polysaccharide colanic acid. J. Bacteriol. 178:4885-4893
Wacharotayankun R, Arakawa Y, Ohta M, Hasegawa T, Mori M, Horii T, Kato
N. 1992. Involvement of rcsB in Klebsiella K2 capsule synthesis in Escherichia coli
K-12. J Bacteriol. 174(3):1063-7.
Whitfield C, Perry MB, MacLean LL, Yu SH. 1992. Structural analysis of the
O-antigen side chain polysaccharides in the lipopolysaccharides of Klebsiella serotypes O2(2a), O2(2a,2b), and O2(2a,2c). J Bacteriol. 174(15):4913-9.
Whitfield C, Roberts IS. 1999. Structure, assembly and regulation of expression of
capsules in Escherichia coli. Mol Microbiol. 31(5):1307-19.
Wugeditsch T, Paiment A, Hocking J, Drummelsmith J, Forrester C, Whitfield C.
group 1 capsular polysaccharides in Escherichia coli. J Biol Chem. 276(4):2361-71. Vincent C, Doublet P, Grangeasse C, Vaganay E, Cozzone AJ, Duclos B. 2000.
Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J Bacteriol. 181(11):3472-7.
Vodonik SA, Gray GR. 1988. Analysis of linkage positions in a polysaccharide
containing nonreducing, terminal alpha-D-glucopyranosyluronic groups by the reductive-cleavage method. Carbohydr Res. 172(2):255-66.
Yoshida T, Ayabe Y, Yasunaga M, Usami Y, Habe H, Nojiri H, Omori T. 2003.
Genes involved in the synthesis of the exopolysaccharide methanolan by the obligate methylotroph Methylobacillus sp strain 12S. Microbiology. 149(Part 2):431-44.
Table 1. Bacterial strains used and constructed in this study
Strain Genotype or relevant property Reference or source
E. coli:
XL1-blue(DE3) recA endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F, pro AB lacqZ M15:: Tn△ 10](DE3);Tetr
Stratagene
NovaBlue(DE3) endA1 hsdR17(rk12-mk12+) supE44 thi-1 recA1 gyrA96
relA1 lac[F,pro AB lacqZ M15:: Tn△ 10](DE3);Tetr
Novagen
JM109 RecA1 supE44 endA1 hsdR17 gyrA96 rolA1 thi △ (lac-proAB)
Laboratory stock BL21-RIL F- ompT hsdSB(rB-mB-)gal dcm(DE3) Laboratory stock
S17-1λpir Tpr Smr recA, thi, pro, hsdR- M+
[RP4-2-Tc::Mu:KmrTn7] (pir)
De Lorenzo et al., 1994
ICC188λpir △(ara-leu) araD △lac×74 galE galK phoA20 Taylor et al.,1989 K. pneumoniae:
CG43 Clinical isolate of K2 serotype Laboratory stock CG43-S3 rspl mutant, Strepr Laboratory stock
CG43-S3-U9451 galU deletion mutant Laboratory stock CG43-S3-yor5
-yor5 deletion mutant in CG43-S3 This study
CG43-S3-yco6
-yco6 deletion mutant in CG43-S3 This study
CG43-S3-wzx
-wzx deletion mutant in CG43-S3 This study
CG43-S3-wza
Table 2. Plasmids used and constructed in this study
Plasmids Relevant characteristic Source or reference
pGEM-Teasy PCR cloning vector, Apr Promega
YT&A vector PCR cloning vector, Apr Yeastern Biotech
pET-30a Expression vector, Kmr Novagene
pET-30b Expression vector, Kmr Novagene
pET-30c Expression vector, Kmr Novagene
pKAS46 Suicide vector, Apr Kmr Laboratory stock
pRK415 Shuttle vector, mob+, Tcr This study
pYor5 A 0.8-kb fragment of yor5 cloned into pGEM-T vector, Apr This study
pYco6E22 A 0.8-kb fragment of C-terminal yco6 (Yco6435-707)cloned into pGEM-T vector, Apr This study
pYco6E23 A 1.0-kb fragment of C-terminal yco6 (Yco6435-722)cloned into pGEM-T vector, Apr This study
pWzx A 1.5-kb fragment of wzx cloned into Y&T vector, Apr This study
pWza A 1.1-kb fragment of wza cloned into Y&T vector, Apr This study
pORF1 A 0.9-kb fragment of orf1 cloned into Y&T vector, Apr This study
pORF3 A 1.4-kb fragment of orf3 cloned into Y&T vector, Apr This study
pORF15 A 1.4-kb fragment of orf15 cloned into Y&T vector, Apr This study
pUGD A 1.2-kb fragment of ugd cloned into Y&T vector, Apr This study
pET-Yor5 pET30b-derivative expressing Yor5-His6-tag fusion: Yor5 was cloned on a EcoRⅤ/Sac fragmentⅠ , Kmr This study
pET-Yco6E22 pET30b-derivative expressing Yco6435-707 -His6-tag fusion into EcoRⅤ/SacⅠsite This study
pET-Yco6E23 pET30b-derivative expressing Yco6435-722 -His
6-tag fusion into EcoRⅤ/SacⅠsite This study
pET-ORF1 pET30c-derivative expressing ORF1-His6-tag fusion: ORF1 was cloned on a BamHⅠ/Hind fragmentⅢ ,
Kmr
This study
pET-ORF3 pET100-derivative expressing ORF3-His6-tag fusion This study
pET-ORF4 pET30c-derivative expressing ORF4-His6-tag fusion cloned into a BamHⅠ/Hind Ⅲsite This study
pET-ORF15 pET30a-derivative expressing ORF15-His6-tag fusion cloned into a BamHⅠ/ SacⅠsite This study
Deletion clones
pASm5 A 2.0-kb fragment containing a 150-bp deletion in yor5 locus cloned into pKAS46, Apr Kmr This study
pASm6 A 2.0-kb fragment containing a 1800-bp deletion in yoc6 locus cloned into pKAS46, Apr Kmr This study pASma A 2.0-kb fragment containing a 500-bp deletion in wza locus cloned into pKAS46, Apr Kmr This study pASmw A 2.0-kb fragment containing a 600-bp deletion in wzx locus cloned into pKAS46, Apr Kmr This study
Table 3. Primers used in this study Primer Sequence Yor4 5’-CTCCATTGGTTCGTTGGAAT-3’ Yor3 5’-GCATTCGCTTGTTTCTGTTC-3’ Y5D1 5’-GGTTGGCGATATCTTAATGG-3’ Y5D2 5’-GCTGATTGGTGAGCTCCATG-3’ Y5D3 5’-CGAAGCACGAGCTCAGACA-3’ Y5D4 5’-AATCGCTCCGGACCATTGGC-3’ Y6E1 5’-AGAAATTCAGGATATCATGCATG-3’ Y6E2 5’-TGGCCTTGTGATATCAGTAAGCC-3’ Y6E3 5’-CACACCATTCAGAAAACATCC-3’ Y6E4 5’-AAGGGGATTCTTCGTCCCCT-3’ Y6D1 5’-AGAGGGATATCAAATAAGCGTGT-3’ Y6D2 5’-CCACACCATTCAGGATCCATCCT-3’ Y6D3 5’-ATAGGATCCTCAAGAAGGGGACG-3’ Y6D4 5’-TATTAGATGCGAGCTCACGGC-3’ WxE1 5’-GATGAGTTTAAAATTAAAAACGATAA-3’ WxE2 5’-GAATGATTTGGCAGCATATTTA-3’ WxD1 5’-TCGATATCGCTATATTGACAACC-3’ WxD2 5’-AGAAAGCTTAATCTATCCAGCC-3’ WxD3 5’-CTGCAAAGCTTGGTTTATGCA-3’ WxD4 5’-CTGATAGATGAGCTCTGGAATG-3’
WaE1 5’-CACC AAGAAAAAAATTGTTAGATTTTCG-3’ WaE2 5’-CTAAACATATTATGGCCAATCC-3’ WaD1 5’-TTTCTATGGGCAGATGGTTG-3’ WaD2 5’-CAGAATTCACCCAGTTACCG-3’ WaD3 5’-CCGCAGTGGTATGACAATTG-3’ WaD4 5’-GCTGACTATCGGGAAGCATC-3’ ORF1E1 5’-TTGCAACCAAACAGGTGAAG-3’ ORF1E2 5’-GGTTTCAGGCTGGCGCTCA-3’ ORF3E1 5’-CACC ATAAAAATTGCGCGCATTG-3’ ORF3E2 5’-CCCTTATTTTCAGCATTCAGC-3’ ORF15E1 5’-GACCACACCAGACAGGAGCAAGT-3’ ORF15E2 5’-CTCGGGCGGCATATAAAGA-3’
UGDE1 5’-CGAATGAAAATTACTATTTCCGG-3’ UGDE2 5’-CCAGTGTCAGACAGGCAGAA-3’
Table 4. Homology search analyses of the CPS gene cluster in K. pneumoniae. K2, strain Chedid MCG 78578 (http://genome.nhri.org.tw) Taiwan, NTUH-K2044 (http://genome.wustl.edu) ORF1 UTP-glucose-1-phosphate uridylyltransferase UTP-glucose-1-phosphate uridylyltransferase UTP-glucose-1-phosphate uridylyltransferase
ORF2 ORF2 ORF2 ORF2
ORF3 OrfX OrfX OrfX
ORF4 Wza Wza Wza
ORF5 Yor5 Yor5 Yor5
ORF6 Yco6 Yco6 Yco6
ORF7 glycosyltransferase undecaprenolphosphate gal-1-P transferase flippase, wzx ORF8 glycosyltransferase rhamnosyl transferase hypothetical protein ORF9 mannosyl transferase glycosyl transferase mucoviscosity-associated protein A ORF10 CapD polysaccharide biosynthesis protein putative glycosyl transferase hypothetical protein ORF11 flippase, Wzx putative glycosyl transferase GDP-mannose 4,6-dehydratase ORF12 hypothetical 65.4 kDa protein in CPS region glycosyltransferase GDP-mannose-4-keto-6-D epimerase ORF13 acetyltransferase Wzy GDP-mannose mannosyl hydrolase ORF14 sugar transferases predicated ORF putative colanic biosynthesis glycosyl transferase ORF15 6-phosphogluconate
dehydrogenase predicted acyltransferases
putative colanic acid UDP-glucose lipid carrier ORF16 mannose-1-phosphate guanylyltransferase flippase,Wzx 6-phosphogluconate
dehydrogenase ORF17 phosphormannonutase dehydrogenase 6-phosphogluconate
dehydrogenase
GDP-mannose pyrophosphorylase( manC) ORF18 putative transport protein dTDP-glucose 4,6-dehydratase phosphomannomutase(manB ) ORF19 putative transport protein glucose-1-phosphate
thymidylyltransferase
UDP-glucose 6-dehydrogenase( ugd)
dTDP-6-deoxy-L-mannose-dehydrogenase
putative nucleotide sugar epim9erase( wbnF) dTDP-6-deoxy-D-glucose
-3,5-epimerase
rhamnosyl transferase
Table 5. Features of the ORFs encoded in K2 cps region from k. pneumoniae.
ORF Signal peptide predicated
Putative transmembrane
regions
Putative function Organism (name of protein) % identity/ % similarity
Accession no Orf1 N 1 UTP-glucose-1-phosphate uridylyltransferase E. coli (GalF) 99/98 AAN52283 Orf2 Y 6 unknown E. coli (COG0671) 85/87 ZP_00272700
Orf3 Y 1 unknown E. coli (OrfX) 97/99 AAD21561
Orf4 Y 1 putative outer membrane lipoprotein E. coli (Wza) 91/96 AAD21562
Orf5 N 0 protein-tyrosine-phosphatase E. coli (Wzb) 67/85 AAD29991
Orf6 N 2 tyrosine-protein kinase E. coli (Wzc) 64/79 AAD21564
Orf7 N 1 glycosyltransferase P. fluorescens (COG0438) 36/54 ZP_00263750
Orf8 N 0 glycosyltransferase B. japonicum (bll2376) 31/51 ZP_00127611
Orf9 N 0 mannosyltransferase A. actinomycetemcomitans (orf6) 35/53 BAA94398
Orf10 N 10 unknown A. actinomycetemcomitans (orf5) 24/42 BAA94397
Orf11 N 12 flippase S. typhimurium (Wzx) 47/67 AAG24819
Orf12 Y 3 unknown K. pneumoniae (orf12) 97/97 Q48458
Orf13 N 0 acetyltransferase S. pneumoniae (cps9vM) 30/48 AAM62297
Orf14 N 4 sugar transferases K. pneumoniae (RfbP) 95/95 C56146
Orf15 N 1 6-phosphogluconate dehydrogenase E. coli (Gnd) 98/98 I84555
Orf16 N 0 mannose-1-phosphate guanylyltransferase E. coli 97/97 I57096
Orf17 N 0 phosphormannonutase dehydrogenase E. coli (RfbK) 98/99 BAA07746
Wzy
Fig. 1. Schematic illustration of the functional roles of the CPS core components.
(1) Yor5 regulates the phosphorylation level of Yco6. (2) Yco6 phosphorylates the
CPS synthesis enzymes to affect CPS production. (3)Yor5 affects CPS polymerization by regulates the interaction of Yco6 and Wzx.
K2 strain Chedid, Japan
NTUH-K2044, Taiwan
MCG 78578, Washington
Fig. 2. Comparative analysis of the K. pneumoniae cps gene clusters. The solid
A
M 1 2 M 1 2 763 bp 600 bpB
M 3 4 M 3 4 2200 bp 412 bpFig. 3. Identification of yor5 (A) and yco6 (B) mutation by Southern blot analysis. The arrows represent the PCR products. Left panel, photographs of an ethidium bromide-stained agarose gel. Lanes M: DNA maker; 1: PCR product using primer pair yor3 and yor4 in K. pneumoniae CG43-S3, 2: PCR product using primer pair yor3 and yor4 in K. pneumoniae CG43-S3-yor5-, 3: PCR product using primer pair yco6E1 and yco6E3 in K. pneumoniae CG43-S3, 4: PCR product using primer pair yco6E1and yco6E3 in K. pneumoniae CG43-S3-yco6-. Right panels, Southern blot analysis using probes from coding regions of yor5 and yco6, respectively.
A
B
CG43 CG43-yor5- CG43-yco6-B
0 0.2 0.4 0.6 0.8 1 1.2 1.4 0 2 4 6 8 10 12 time(h) OD 60 0 CG 43 Yor5 mutant Yco6 mutant U9451Fig. 4. Phenotype analysis of CG43, CG43-yor5- and CG43-yco6-. U9451, a galU mutant, is used as a negative control because galU encodes the enzyme UDP-glucose pyrophosphorylase that regulates the supply of UDP-galactose and UDP-glucose, two major precursors for the biosynthesis of CPS (Chang et al., 1996) (A) Overnight culture of wild type strain CG43S3, CG43-yor5- and CG43-yco6- were subjected to 5000 g centrifugation for 5 min. (B) Growth curves of the bcteria CG43, CG43-yor5- , CG43-yco6- and U9451 at 37 ℃ in LB broth.
2 folds serial dilution
CG43
yor5
-yco6
-U9451
Fig. 5. Comparison of CPS production of CG43-S3, CG43-S3-yor5-, CG43-S3-yco6-, and U9451. The CPS were extracted with hot-phenol and treated with protease K, appeared to be measured and probed with K2 antiserum. The frames represent equal amount of CPS analyzed with ScanAlyze (Michael Eisen , Stanford University).
0 2 4 6 8 10 12 14
S3 yor5- yco6-
galU-Bacterial Strains
Gl
ucuronic acid con
tent (μg
/10
9 CFU)
Fig. 6. Glucuronic acid contents of CG43, CG43-yor5- , CG43-yco6- and U9451. CPS of the bacteria from 500 µl of overnight culture was extracted and then hydrolyzed by H2SO4, and then 3-hydroxydiphenol was added to measure the
absorbance at 520 nm. Glucuronic acid was used as standard for the quantification of uronic acid.
A
Fig. 7. Both Yor5 and Yco6 affected the polymeric CPS production. The CPS was extracted with hot-phenol, treated with protease K and analyzed with 5% DOC PAGE. Panel A shows immune blot analysis probed with polyclonal K2 antiserum. Panel B shows the alcian blue-silver stained DOC PAGE. CPS-P represents high molecular weight polymeric CPS. CPS-O represents low molecular weight
CG43 yor5- yco6- U9451
CPS-P
CPS-O
B
CG43 yor5- yco6- U9415CPS-P
CG43 yco5- yor6- U9415
Fig. 8. Neither yor5 nor yco6 mutation affected the oligomeric CPS synthesis. The CPS were extracted with hot-phenol and treated with protease K and the CPS analyzed with alcian blue-silver stained 10% DOC PAGE. Alcian blue is a cationic dye and capable of binding to negative CPS to increase the sensitivity in CPS detection.
kDa M 1 2 3 4 5 6 7 8 200.0 116.3 66.3 55.4 36.5 31.0
Fig. 9 Purification of the His6-Yor5 recombinant protein. The His6-Yor5 was overexpressed in E. coli NovaBlue (DE3) and purified through Ni+2 affinity column.
The purified proteins were resolved by 12.5% SDS PAGE and then stained with Coomassie blue. Lane 1, protein molecular weight marker; lane 2, the whole cell lysate; lane 3, soluble proteins; lane 4, binding buffer elution; lane 5:washing buffer elution; lane 6 to lane 8, the purified fractions.
21.5
14.4 6.0
1 2 3 4 5 6
A
(min)30 0 1 5 15 30
B
1.6 1.4 1.2 Yor5 1 0.8 0.6 Control 0.4 0.2 0 2.5 10 0 5 15 30 45 60 (min)Fig. 10. Assessment of the phosphatase activity of the recombinant Yor5. The phosphatase activity of Yor5 was determinated by measurement of the decomposition of Para-nitrophenyl phosphate (pNPP) at 37 ℃ (A). Tube 1 to 5 each contained 4 µg/ml Yor5 in PNPP reaction buffer. Tube 6 is the blank containing only the PNPP reaction buffer. The phosphatase activity of Yor5 with PNPP as the substrate is 3.2×10-7 moles/min/mg (B).
ig. 11. Purification of the His6-Yco6E23 recombinant protein. Panel A: Yco6E23 TM helix kDa M 1 36.5 55.4 21.5 14.4 kDa M 1 2 3 4 5 6 7 66.3 36.5 21.5 14.4 97.4 6.0 55.4 31.0
Transmemebrane domain kinase domain
kinase domain Yco6E23 Yco6E23
A
C
TyrosineB
TM helix cluster 31.0 Fis a truncated form of Yco6 Panel B His6-Yco6E23 was overexpressed in E. coli and
purified by Ni+2 affinity column and resolved on 12.5% SDS PAGE using Coomassie Blue-staining. M, protein marker; lane 1, total protein; lane 2, soluble proteins; lane 3, binding buffer elution; lane 4: washing buffer elution; lanes 5 to 7, the eluted fractions. Panel C: Purification of the His6-Yco6E23 recombinant protein by raising the
imidazole concentration of wash buffer to 125 mM, M: protein maker; Lane 1: is the purified recombinant protein Yco6E23.
Yco6E23 + + + + 0 5 15 30 (min) Yco6E23 + + + + Yor5 + + + + Yor5 - - - - High MW Yco6E23 Yco6E23 complex
Fig. 12. In vitro dephosphorylation assay. The reaction mixes were separated with 12.5 % SDS PAGE, and the radioactive proteins were visualiz by InstantImagerTM (Packard Instrument Company).
A
B
114.0 88.0 50.7 50.7 35.5 35.5 28.2 28.2 21.0 kDa M 1 2 3 4 5 Yco6E23 Yco6E23 complex 88.0ig. 13. Identification of Yco6E23 complex. The protein samples were separated Yco6E23
Yco6E23
Yor5 complex
F
with 12.5% SDS PAGE. The Western blot probed with anti-phosphotyrosine monoclonal antibody (Sigma P3300) was shown in panel A. Panel B represents the blot probed with anti-His.tag monoclonal antibody (Novagen). M, protein maker; lane 1, Yco6E23: lane 2, Yco6E23 (100 ℃); lane 3, Yor5; lane 4, Yco6E23+Yor5; lane 5 (100 ℃)
ig. 14. Comparsion of the phosphotyrosine proteins between wild type and 114.0 kDa 50.7 28.2 35.5 M F
CG43-S3-yco6-. Panel A shows Coomassie Blue-stained SDS-PAGE; Panel B shows the corresponding Western blot probed with monoclonal anti-phosphotyrosine antibody (Sigma P3300). M, protein maker; lane 1, CG43-S3 whole cell lysate; lane 2, CG43-S3-yco6- whole cell lysate; The arrows ( ) represent probable targets of Yco6 and the arrow ( ) represents Yco6.
88.0 M 21.0 114.0 50.7 28.2 35.5 1 2 1 2 88.0 kDa Yco6 21.0
A
B
ig. 15. The Yco6-interacting proteins identified using immunoprecipitation.
M 1 2 M 1 2 114.0 50.7 28.2 35.5 21.0 114.0 50.7 28.2 35.5 21.0 kDa a 88.0 88.0 F
Immunoprecipitation was used with monoclonal anti-phosphotyrosine antibody (Sigma PT66) from the whole cell protein. Then the protein-antibody complex was cached by proteinA/G. Panel A shows Coomassie Blue-stained SDS PAGE; Panel B shows corresponding Western blot probed with monoclonal anti-phosphotyrosine antibody (Sigma P3300). M, protein maker; lane 1, CG43-S3; lane 2, CG43-S3-yco6-. The arrows ( ) represent probable targets of Yco6 and the arrow ( ) represents Yco6.
kD