INTRODUCTION
In 40-50% of people with diabetes mellitus type 1 or type 2, detectable peripheral neuropathy develops within 10 years of the onset of disease, and the neuropathic pain associated with symptomatic disease is frequently
bothersome(1,2)
. Foot ulceration, which depends on the degree of foot insensitivity(3), and amputation are impor-tant and costly sequelae of diabetic neuropathy(4)
. Autonomic dysfunction has also been reported as a common complication in patients with diabetes(5,6)
and can lead to sexual dysfunction and postural hypotension.
From the 1
Department of Neurology, En Chu Kong Hospital,
2
Institute of Preventive Medicine College of Public Health, National Taiwan University, and 3
Department of Neurology, National Taiwan University Hospital.
Received December 8, 2004. Revised December 29, 2004. Accepted February 14, 2005.
Reprint requests and correspondence to: Yu Sun MD, Department of Neurology, En Chu Kong Hospital, No. 399, Fuhsin Road, San-shia, Taipei, Taiwan.
E-mail: sunyu@ms4.hinet.net.
Effectiveness of Vitamin B12 on Diabetic Neuropathy:
Systematic Review of Clinical Controlled Trials
Yu Sun
1,2, Mei-Shu Lai
2, and Chien-Jung Lu
3Abstract- The clinical effectiveness of vitamin B12 and its active coenzyme form on diabetic neuropathy is
uncertain. Therefore, we searched the English- and non-English-language literature on this topic by using MEDLINE (Ovid, PubMed), the Cochrane Controlled Trials Register, and related papers. We identified seven randomized controlled trials from June 1954 to July 2004 and reviewed them for the clinical effective-ness of vitamin B12 according to the following parameters: Measurement scales of somatic and autonomic symptoms or signs; vibrometer-detected thresholds of vibration perception; and, electrophysiologic mea-sures such as nerve conduction velocities and evoked potentials. Three studies involved the use of vitamin B complex (including B12) as the active drug, and four used methylcobalamin. Two studies were of fairly good quality (Jadad score = 3/5), and five were of poor quality (Jadad score ≤ 2/5). Both the vitamin B12 combination and pure methylcobalamin had beneficial effects on somatic symptoms, such as pain and paresthesia. In three studies, methylcobalamin therapy improved autonomic symptoms. Effects on vibration perception and electrophysiological measures were not consistent. With both the vitamin B12 combination and pure methylcobalamin, symptomatic relief was greater than changes in electrophysiological results. However, more high-quality, double-blind randomized controlled trials are needed to confirm the effects of vitamin B12 on diabetic neuropathy.
Key Words: Systematic review, Randomized controlled trial, Vitamin B12, Methylcobalamin, Diabetic
neuropathy
This autonomic dysfunction is difficult to treat and only partially responsive to current therapy.
Vitamin B12 plays a vital role in the metabolism of fatty acids essential for the maintenance of nerve myelin. Prolonged B12 deficiency can lead to nerve degeneration and irreversible neurological damage(7)
. Diabetic neu-ropathy, with or without B12 deficiency, had been treat-ed with neurotropic vitamins for decades. In routine clin-ical practice, formulations that can be taken orally are usually used. Vitamin B12 is available in three forms: cyanocobalamin, hydrocobalamin, and methylcobal-amin. The first is the most widely available and least expensive, but some experts prefer the other two forms.
The treatment of diabetic neuropathy can be a frus-trating experience for both physicians and patients, and the clinical effectiveness of vitamin B12 therapy on dia-betic peripheral neuropathy is still unclear. The purpose of this review was to investigate and evaluate the report-ed effectiveness of vitamin B12 supplements to provide evidence-based recommendations for clinical practice.
METHODS
Search strategy
We conducted a systematic review of English- and non-English-language articles using MEDLINE (Ovid, PubMed), the Cochrane Controlled Trials Register, and related papers from June 1954 to July 2004. Additional references were identified by searching bibliographies or related publications. Studies reported in abstracts or con-ference presentations were excluded from the review because they inadequately reported their methodologies and results and because they had not undergone peer review. We used three main Medical Subject Headings: (1) Trials: randomized controlled trial or controlled-clin-ical trial or double-blind method or clincontrolled-clin-ical trial; (2) Vitamin B12: methylcobalamin, cyanocobalamin or hydroxycobalamin; (3) Neuropathy: diabetic polyneu-ropathy, diabetic peripheral neuropathy.
Eligible studies
We included reports if they described randomized controlled trials (RCTs) of any type of vitamin B12
ther-apy in patients with diabetes peripheral neuropathy. We also included studies of the coenzyme forms of B12, such as methylcobalamin, cyanocobalamin, or hydroxy-cobalamin in either the oral or injection form. Trials involving combination therapy were eligible only if vita-min B12 or its coenzyme form was one of the treatment agents. Diabetic neuropathy was defined as peripheral large- or small-fiber neuropathy resulting in autonomic or somatic sensory symptoms.
Our primary outcome measure was the clinical effec-tiveness, as assessed by using three main parameters: 1) Clinical scores of somatic and autonomic symptoms or signs; 2) Vibrometer-detected thresholds of vibration perception; and, 3) Electrophysiological measures such as nerve conduction velocities (NCVs) and somatosenso-ry evoked potentials. We excluded uncontrolled trials, observational studies, animal experiments, and studies focusing on only a specific population such as patients with uremia. Studies of vitamins used for other purposes (eg, encephalopathy, dementia, anemia) were also excluded.
Quality assessment
We assessed the following methodological features that were most relevant to the control of bias by follow-ing the guidelines of Jadad scorfollow-ing system: Randomization, random allocation concealment, mask-ing of treatment allocation, blindmask-ing, and withdrawals(8,9)
. Two reviewers independently applied the inclusion crite-ria. One of them extracted the data and assessed its qual-ity; and the other reviewer checked these results, and any noted differences were resolved by consensus.
Information gathered
To compare clinical effectiveness across studies, consistent information was selected whenever possible. We gathered the following information: Number of patients examined, intervention with monotherapy or a combination regimen, study design, duration of study and follow-up, and outcome measures and results. Clinical effectiveness was assessed by means of a narra-tive comparison of the different outcomes, which includ-ed the mean change or proportion of patients whose
results changed from baseline, mean clinical scores, and mean differences between control and active agents.
RESULTS
Quantity and quality of studies
We identified seven clinical controlled trials that met our inclusion criteria (Fig.)(10-16)
. All studies were pub-lished in English, except 1, which was pubpub-lished in Chinese. Three studies used vitamin B complex (B1, B6, and B12) as a combination agent(10-12)
, whereas the other four used pure methylcobalamin as the main treatment (13-16)
. In the studies of vitamin B complex, a synthetic derivative of thiamin, ie, Benfotiamine (B1), and cyanocobalamin (B12) 250 µ g were given. One study did not mentioned randomization in its clinical con-trolled trials. Because the investigators described the study design in detail, with no differences between the study groups, we included this study in our review. Although six studies mentioned randomization, all lacked an adequate description, notably how the random-ization was generated. None of the studies included an intent-to-treat analysis. Table 1 shows the quality assess-ments of the studies. Only two studies involved a dou-ble-blind process and were judged of fairly good quality (Jadad score = 3/5)(12,15)
. The other five studies were of poor quality (Jadad score ≤ 2/5)(10,11,13,14,16)
.
Table 2 shows the characteristics of the studies. The mean ages of the patients enrolled were all around 50-60
Figure.Flow diagram shows how we identified RCTs that met our criteria.
Table 1. Quality assessments of included RCTs of vitamin B12 therapy for diabetic neuropathy
Intervention and study Randomization* Double Effective Intent-to-treat Dropout Jadad score
blinding blinding‡ analysis rate (%)
Vitamin B12complex
Winkler et al10
Yes No No No 0 1
Simeonov et al11 Yes No No No 0 1
Stracke et al12
Yes Yes Yes No 0 3
Methylcobalamin
Li13 Yes No No No 0 1
Shindo et al14
No† No No No 0 0
Yaqub et al15 Yes Yes Yes No 14 3
Devathasan et al16 Yes No No No 12 1
*None of the studies described how the randomization was generated; †Patient characteristics of the study and control groups did not differ
significantly; ‡Masking of treatment and matching placebo; Main items of Jadad scores are as follows: randomization, random allocation
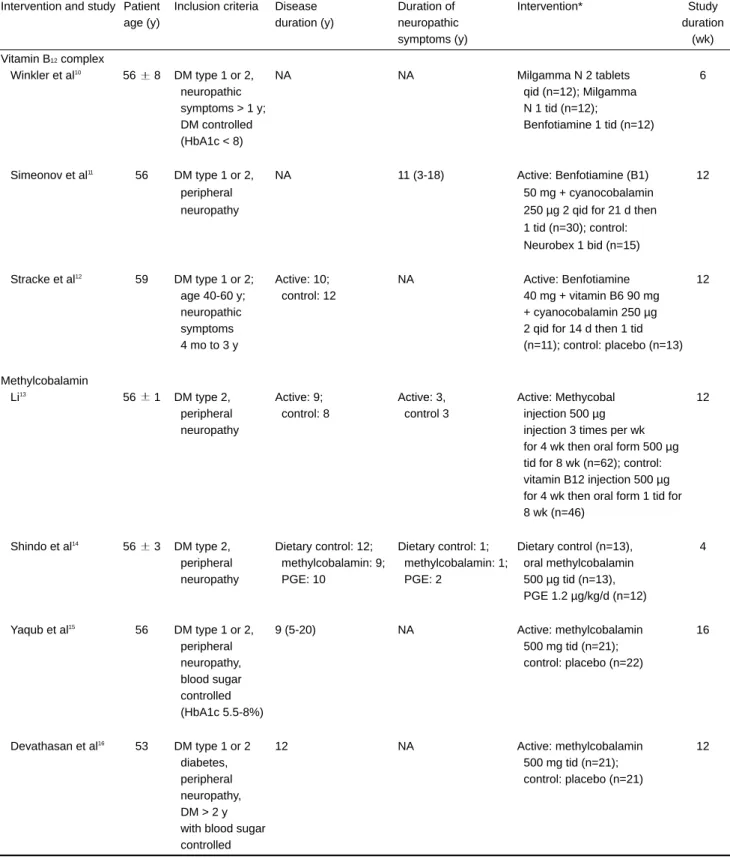
Table 2. Characteristics of included studies of vitamin B12 therapy for diabetic neuropathy
Intervention and study Patient Inclusion criteria Disease Duration of Intervention* Study
age (y) duration (y) neuropathic duration
symptoms (y) (wk)
Vitamin B12complex
Winkler et al10
56 8 DM type 1 or 2, NA NA Milgamma N 2 tablets 6
neuropathic qid (n=12); Milgamma
symptoms > 1 y; N 1 tid (n=12);
DM controlled Benfotiamine 1 tid (n=12)
(HbA1c < 8)
Simeonov et al11 56 DM type 1 or 2, NA 11 (3-18) Active: Benfotiamine (B1) 12
peripheral 50 mg + cyanocobalamin
neuropathy 250 µg 2 qid for 21 d then
1 tid (n=30); control: Neurobex 1 bid (n=15) Stracke et al12
59 DM type 1 or 2; Active: 10; NA Active: Benfotiamine 12
age 40-60 y; control: 12 40 mg + vitamin B6 90 mg
neuropathic + cyanocobalamin 250 µg
symptoms 2 qid for 14 d then 1 tid
4 mo to 3 y (n=11); control: placebo (n=13)
Methylcobalamin Li13
56 1 DM type 2, Active: 9; Active: 3, Active: Methycobal 12
peripheral control: 8 control 3 injection 500 µg
neuropathy injection 3 times per wk
for 4 wk then oral form 500 µg tid for 8 wk (n=62); control: vitamin B12 injection 500 µg for 4 wk then oral form 1 tid for 8 wk (n=46)
Shindo et al14
56 3 DM type 2, Dietary control: 12; Dietary control: 1; Dietary control (n=13), 4
peripheral methylcobalamin: 9; methylcobalamin: 1; oral methylcobalamin
neuropathy PGE: 10 PGE: 2 500 µg tid (n=13),
PGE 1.2 µg/kg/d (n=12)
Yaqub et al15 56 DM type 1 or 2, 9 (5-20) NA Active: methylcobalamin 16
peripheral 500 mg tid (n=21);
neuropathy, control: placebo (n=22)
blood sugar controlled (HbA1c 5.5-8%) Devathasan et al16
53 DM type 1 or 2 12 NA Active: methylcobalamin 12
diabetes, 500 mg tid (n=21);
peripheral control: placebo (n=21)
neuropathy, DM > 2 y with blood sugar controlled
DM indicates diabetes mellitus; NA: not available; PGE: prostaglandin E1. Data are the mean standard deviation, mean, or mean (range),
unless otherwise noted. *Milgamma N is benfotiamine 40 mg + vitamin B6 90 mg + cyanocobalamin 250 µg; Neurobex, 100 mg thiamin + vita-min B6 20 mg + vitavita-min B12 100 µg (form of B12 not mentioned).
years. Two studies of methylcobalamin included only patients with diabetes type 2(13,14)
. Patients with vitamin B12 deficiency were excluded in only one study(12). In
most of the studies, the mean duration of diabetes melli-tus was 9-12 years. The patients in one study had
long-term neuropathic symptoms(11)
. Vitamin B complex, including vitamin B1, B6, and B12, was the interven-tional agent in three studies(10-12)
. In one study, the inject-ed form of methylcobalamin was given in the active group, followed by the oral form; however, the study
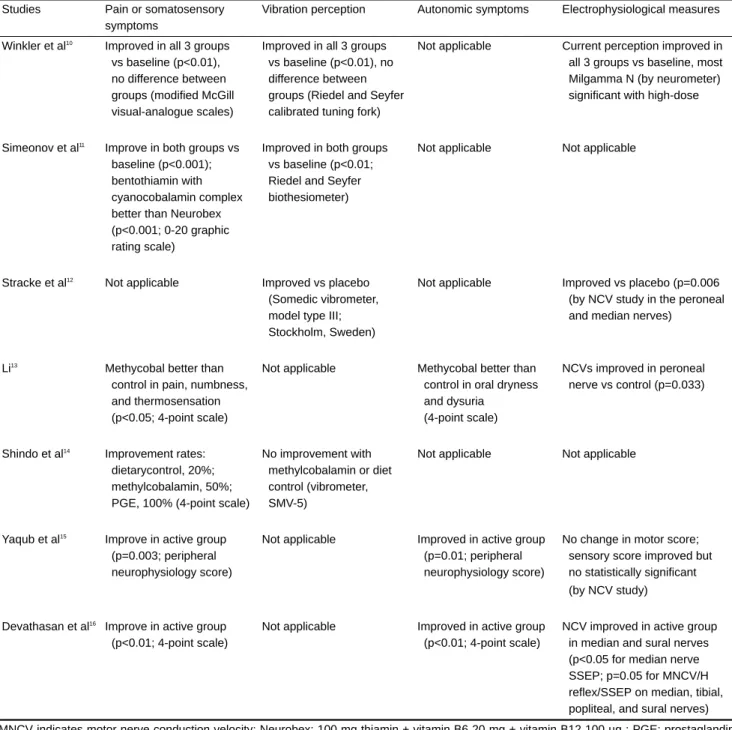
Table 3. Clinical effectiveness of vitamin B6 therapy for diabetic neuropathy
Studies Pain or somatosensory Vibration perception Autonomic symptoms Electrophysiological measures
symptoms
Winkler et al10 Improved in all 3 groups Improved in all 3 groups Not applicable Current perception improved in
vs baseline (p<0.01), vs baseline (p<0.01), no all 3 groups vs baseline, most
no difference between difference between Milgamma N (by neurometer)
groups (modified McGill groups (Riedel and Seyfer significant with high-dose
visual-analogue scales) calibrated tuning fork)
Simeonov et al11 Improve in both groups vs Improved in both groups Not applicable Not applicable
baseline (p<0.001); vs baseline (p<0.01;
bentothiamin with Riedel and Seyfer
cyanocobalamin complex biothesiometer)
better than Neurobex (p<0.001; 0-20 graphic rating scale)
Stracke et al12 Not applicable Improved vs placebo Not applicable Improved vs placebo (p=0.006
(Somedic vibrometer, (by NCV study in the peroneal
model type III; and median nerves)
Stockholm, Sweden) Li13
Methycobal better than Not applicable Methycobal better than NCVs improved in peroneal
control in pain, numbness, control in oral dryness nerve vs control (p=0.033)
and thermosensation and dysuria
(p<0.05; 4-point scale) (4-point scale)
Shindo et al14 Improvement rates: No improvement with Not applicable Not applicable
dietarycontrol, 20%; methylcobalamin or diet
methylcobalamin, 50%; control (vibrometer,
PGE, 100% (4-point scale) SMV-5)
Yaqub et al15
Improve in active group Not applicable Improved in active group No change in motor score;
(p=0.003; peripheral (p=0.01; peripheral sensory score improved but
neurophysiology score) neurophysiology score) no statistically significant
(by NCV study)
Devathasan et al16 Improve in active group Not applicable Improved in active group NCV improved in active group
(p<0.01; 4-point scale) (p<0.01; 4-point scale) in median and sural nerves
(p<0.05 for median nerve SSEP; p=0.05 for MNCV/H reflex/SSEP on median, tibial, popliteal, and sural nerves) MNCV indicates motor nerve conduction velocity; Neurobex: 100 mg thiamin + vitamin B6 20 mg + vitamin B12 100 µg ; PGE: prostaglandin E1; SMV-5: Suzuki-Matsuoka vibrometer; SSEP: somatosensory evoked potential.
report did not describe which form of B12 was injected in the control group(13). Three other studies used the oral
form of pure methylcobalamin as the main treatment(14-16)
. The duration of intervention in all included studies ranged from 4 to 16 weeks.
Clinical effectiveness
Overall, the measuring parameters or scoring sys-tems differed between studies; therefore, a meta-analysis was hard to do. Of the six trials(10,11,13-16)
in which pain or somatosensory symptoms were measured, all involved different scoring scales and all showed a statistically sig-nificant beneficial outcome with vitamin B complex or methylcobalamin treatment compared with baseline or placebo results (Table 3). Of the four trials in which the vibration perception threshold was tested, 3(10-12)
showed a beneficial outcome and 1(14)
did not show a significant improvement with methylcobalamin treatment. Methylcobalamin improved autonomic symptoms in three studies(13,15,16)
. As for assessment of peripheral nerve function from electrophysiological studies, one study used a neuromotor assessment process to measure the current perception threshold and demonstrated a benefi-cial outcome with vitamin B complex. Of the trials that included testing of NCVs, one study of vitamin B com-bination therapy and one study of methylcobalamin revealed beneficial outcomes(12,16)
compared with place-bo. In one study, outcomes were better with methyl-cobalamin than with conventional vitamin B12 (though the form of B12 was not described clearly) in terms of autonomic symptoms, somatosensory symptoms, and electrophysiological results(13)
. However, one double-blinded placebo-controlled trial showed no change or significant improvement on NCV with methylcobalamin compared with placebo(15)
.
DISCUSSION
We conducted this systematic review to explore the efficacy of vitamin B12 therapy on diabetic neuropathy in limited RCTs and also to assess the quality of the included studies. A clinical trial of this treatment regi-men was published as early as 1954(17)
. It was an
obser-vational study without randomization or a matched placebo or blinding process. Although diabetic neuropa-thy has been treated with neurotropic vitamins for decades, high-quality RCTs of this intervention are still lacking. Most RCTs have not used a double-blind design; this is a problem because selection bias cannot be reduced without adequate randomization and use of blind controls(8)
. Furthermore, the small number of par-ticipants in most RCTs may reduce the validity of the findings.
Other problems in this review were the variability of the intervention (which included multicomponent vita-min B, the injected form of methylcobaloavita-min, or the oral form of methylcobalamin) and the diverse scoring systems used in measuring outcomes. Therefore, quanti-tative pooling of the data was not feasible for our analy-sis of the results. Instead, we individually summarized the efficacies of viamin B12 acording to four main items: Pain and somatosensory symptoms, autonomic symptoms, vibration perception, and electrophysiologic measures (mainly NCV). Our results showed that treat-ment with either vitamin B complex or pure methyl-cobalamin had beneficial effects on somatosensory symptoms, such as pain and paresthesia, though two RCTs of the vitamin complex with cyanocobalamin did not involve a double-blind design. Those latter RCT’s may therefore have resulted in overestimation of the treatment effects(8)
. As for methylcobalamin therapy, its benefits on autonomic symptoms (improved in three studies) were as consistent as its effects on pain and somatosensory symptoms (improved in all four studies), and they were relatively reliable, as shown in one place-bo-controlled trial with a double-blind design. Vitamin B complex treatment slightly improved the vibration per-ception threshold, whereas methylcobalamin provided no benefit. Improvement in neurophysiological parame-ters was not as evident as changes in clinical signs and symptoms. A possible explanation could be that the duration of treatment was not long enough for the long fibers to regenerate.
Some investigators have reported that antidiabetes drugs, such as metformin, may induce B12 deficiency(18)
. Therefore, vitamin B12 or methylcobalamin treatment
may correct this deficiency state and possibly convert its related neuropathy. This mechanism might explain its inconsistent effects in the treatment of diabetic neuropa-thy. In our review, only one RCT excluded subjects with vitamin B12 deficiency. Future subgroup analysis of dia-betic participants with or without B12 deficiency in clin-ical trials of vitamin therapy is important.
In conclusion, treatment with both combination agents (vitamin B complex with cyanocobalamin) and pure methylcobalamin appeared to improve symptomatic relief more than electrophysiologic results among patients with diabetic neuropathy. However, more high-quality, double-blind RCTs are needed to confirm the clinical effectiveness of vitamin B12 and its active coen-zyme.
REFERENCES
1. Partanen J, Niskanen L, Lehtinen J, et al. Natural history of peripheral neuropathy in patients with non-insulin-depen-dent diabetes mellitus. N Engl J Med 1995;333:89-94. 2. Anonymons. The effect of intensive treatment of diabetes
on the development and progression of long-term compli-cations in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977-86.
3. Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration percep-tion thresholds. A prospective study. Diabetes Care 1994; 17:557-60.
4. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. Diabetes in America. 2nd ed. Bethesda, MD: National Diabetes Data Group, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1995:409-28.
5. Ziegler D, Gries FA, Fies FA, et al. The epidemiology of diabetic neuropathy: Diabetic Cardiovascular Autonomic Neuropathy Multicenter Study Group. J Diabetes Complications 1992;6:49-57.
6. Pfeifer MA, Weinberg CR, Cook DL, et al. Autonomic
neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care 1984;7:447-45.
7. Scalabrino G, Buccellato FR, Veber D, et al. New basis of the neurotrophic action of vitamin B12. Clin Chem Lab Med 2003;41:1435-7.
8. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12.
9. Jadad AR, Moore RA, Carroll D, et al. Assessing the quali-ty of reports of randomized clinical trials: is blinding neces-sary? Control Clin Trials 1996;17:1-12.
10. Winkler G, Pal B, Nagybeganyi E, et al. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung 1999; 49:220-4.
11. Simeonov S, Pavlova M, Mitkov M, et al. Therapeutic effi-cacy of “Milgamma” in patients with painful diabetic neu-ropathy. Folia Med (Plovdiv) 1997;39:5-10.
12. Stracke H, Lindemann A, Federlin K. A benfotiamine-vita-min B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes 1996;104:311-6.
13. Li G. Effect of mecobalamin on diabetic neuropathies. Beijing Methycobal Clinical Trial Collaborative Group. Zhonghua Nei Ke Za Zhi 1999;38:14-7.
14. Shindo H, Tawata M, Inoue M, et al. The effect of prostaglandin E1.alpha CD on vibratory threshold deter-mined with the SMV-5 vibrometer in patients with diabetic neuropathy. Diabetes Res Clin Pract 1994;24:173-80. 15. Yaqub BA, Siddique A, Sulimani R. Effects of
methyl-cobalamin on diabetic neuropathy. Clin Neurol Neurosurg 1992;94:105-11.
16. Devathasan G, Teo WL, Mylvaganam A. Methylcobalamin in chronic diabetic neuropathy. A double-blind clinical and electrophysiological study. Clin Trials J 1986;23:130-40. 17. Shuman CR, Gilpin SF. Diabetic neuropathy: controlled
therapeutic trials. Am J Med Sci 1954;227:612-7.
18. Gilligan MA. Metformin and vitamin B12 deficiency. Arch Intern Med 2002;162:484-5.