General Cardiology
Cardiology 2000;93:137–141
A Randomized Crossover Evaluation of
Antianginal Efficacy and Safety of
Nitrolingual-Spray and Nitroglycerin Tablet
Form in Coronary Artery Disease Patients
Kuo-Liong Chien
aFung-Chang Sung
bChia-Lun Chao
aTa-Chen Su
aMing-Fong Chen
aYuan-Teh Lee
aaDepartment of Internal Medicine, National Taiwan University Hospital, and bSchool of Public Health,
National Taiwan University, Taipei, Taiwan, ROC
Dr. Kuo-Liong Chien, Department of Internal Medicine
ABC
© 2000 S. Karger AG, BaselKey Words
Coronary artery disease
WTreadmill exercise test
WNitroglycerin
WClinical trial
Abstract
Twenty-eight coronary artery disease patients with more
than 50% stenosis in at least one major coronary artery
completed this randomized crossover clinical trial for the
comparison of efficacy and safety of Nitrolingual-Spray
and nitroglycerin (NTG) tablets. Exercise time was
lengthened to 399.1 s (spray) or 408.5 s (tablets),
com-pared to a baseline of 387.3 s. Ischemic burden
de-creased to about –4.0 mm with both forms, compared to
–7.5 mm at baseline (ANOVA: p = 0.003). The ischemic
time improved to 137.2 s (spray) or 152.9 s (tablets),
com-pared to 253.4 s at baseline (ANOVA: p = 0.005). Patients
taking tablets experienced more episodes of
hypoten-sion and/or headache compared to patients taking the
spray. Nitrolingual-Spray is as effective and safe as NTG
tablets for the treatment of symptomatic coronary heart
disease.
Copyright © 2000 S. Karger AG, Basel
Introduction
Sublingual nitroglycerin (NTG) is an established
thera-py for the treatment of effort angina [1]. Nonetheless, the
pharmacological activity of NTG tablets diminishes
grad-ually, particularly if it is not properly stored [2–6]. Tallett
[7] suggested that patients have NTG tablets dispensed at
least every 12 weeks, even when following instructions for
optimal storage. Lack of knowledge about the tablet form
limits the usefulness of the drug [8, 9]. New formulations
of NTG with greater stability are now available.
Several clinical trials have demonstrated that NTG
spray has a more rapid action of angina relief than NTG
tablets [10–13] due to its rapid absorption [14]. We
con-ducted a randomized crossover clinical trial in Taiwan to
compare the antianginal efficacy and safety of the most
commonly used NTG tablets (0.6 mg) with a new spray
formulation (Nitrolingual-Spray, 0.4 mg per puff; Pohl
Boskamp, Hohenlockstedt, Germany) for treatment of
coronary artery disease (CAD) patients with myocardial
ischemia, documented by a treadmill exercise test (TxT).
Patients and Methods
Thirty CAD patients with x50% stenosis in at least one of the three major coronary arteries were recruited into this randomized crossover clinical trial. All patients gave informed consent. The hos-pital ethics review committee had approved the study. There were 25 men and 5 women (average age, 59.4 B 8.5 years; table 1). All patients had symptomatic angina, documented CAD by coronary angiography, and a positive TxT. Patients with any of the following criteria were excluded: myocardial infarction within the previous 6 months, congestive heart failure (NYHA x3), symptomatic valvular heart disease, or cardiomyopathy.
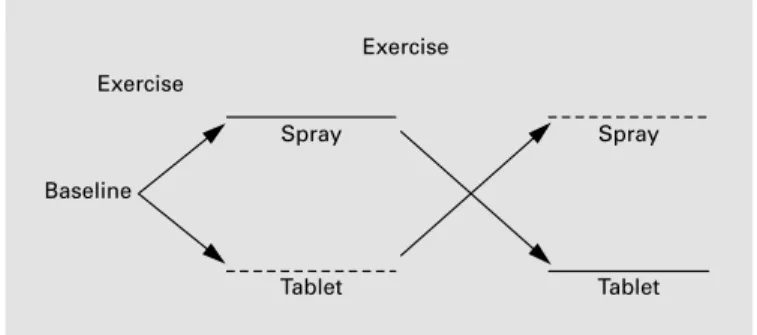
All patients performed a TxT without NTG tablets or spray to establish baseline performance. They were then randomly assigned to either the spray-to-tablet or tablet-to-spray groups (fig. 1). In the spray-to-tablet group, patients underwent a TxT immediately after
Fig. 1. Study protocol. –––– ST group: 1 puff (0.4 mg) of NTG oral
spray → 1 tablet (0.6 mg) of sublingual NTG. - - - TS group: 1 tablet (0.6 mg) of sublingual NTG → 1 puff (0.4 mg) of NTG oral spray.
taking one 0.4-mg NTG spray puff. After resting for at least 30 min, the patients took an NGT 0.6-mg tablet sublingually and then per-formed another TxT. In the tablet-to-spray group, the drug form application was reversed. All TxT were performed using the standard Bruce protocol. ST-segment depression of at least 1.0 mm was con-sidered significant for exercise-induced ischemia. The stress test was considered negative if at least 85% of the predicted maximal heart rate (defined as 220 – age in years) was achieved without significant ST-segment changes. Those patients with maximal heart rates ! 85% of the predicted rate were excluded from the study. The end point of the TxT was extreme fatigue and exhaustion.
We compared the following variables of the two groups to base-line exercise performance: (1) exercise duration: the time from start to maximal exercise; (2) ischemic burden: total ST-segment depres-sion during exercise, and (3) ischemic time: time duration from ap-perance to disappearance of ST-segment depression during exercise and resting periods. Secondary end points compared to baseline included rate-pressure product (RPP), blood pressure patterns, and adverse effects such as hypotension, palpitation, headache, and dizzi-ness.
Efficacy was assessed in terms of exercise capacity by symptom-limited exercise time, total work performed, time to onset of angina, and ST-segment change.
Statistical Analysis
All values are presented as means B SD. The results were ana-lyzed for exercise period 1 and then for period 2, as a crossover design. Data were grouped by drug form and then analyzed. Analysis of variance was used to test the differences of measurements among baseline, and NTG spray and tablet treatments. The differences in test variables between NTG spray and tablets were analyzed using linear regression, with random effect incorporated in the model. Sig-nifcance of differences in adverse events was tested using the ¯2 or
Fisher’s exact test. p values ! 0.05 were considered significant for all parameters tested.
Table 1. Demographic characteristics of
the study subjects ST group (n = 15) TS group (n = 15)
Men 12 13
Women 3 2
Age, years (mean B SD) 59.2B19.8 59.6B5.6
Range 40–75 47–70
Body weight, kg (mean B SD) 69.07B12.56 69.06B6.42
Body height, cm (mean B SD) 165.6B8.1 168.8B5.8
History of angina 10 10 Hypertension 7 8 Diabetes mellitus 4 1 Hyperlipidemia 13 9 Smoking Yes 3 3 No 6 7 Quit 6 5
ST = 1 puff (0.4 mg) of NTG oral spray → 1 tablet (0.6 mg) of sublingual NTG; TS = 1 tablet (0.6 mg) of sublingual NTG → 1 puff (0.4 mg) of NTG oral spray.
Initial
Results
Of the 30 patients enrolled, 28 completed the protocol,
and 2 patients withdrew because of severe headache while
taking NTG tablets. The two treatment groups were
simi-lar in basic demographic characteristics (table 1). The
mean heart rate, systolic blood pressure, diastolic blood
pressure and RPP were similar among baseline, NTG
spray, and NTG tablet treatment (table 2).
The duration of exercise increased from 387.3 s at
baseline to 399.1 s with NTG spray treatment and 408.5 s
with NTG tablet treatment (p = 0.26; table 2). The
aver-age ischemic burden was reduced from –7.50 mm at
base-line to –3.97 mm with NTG tablets and –4.04 mm with
NTG spray (ANOVA: p = 0.003). While the two
treat-ment groups showed similar reductions in ST-segtreat-ment
depression, ischemic burden in both treatment groups
was significantly less at baseline (fig. 2a). The average
ischemic time was also shortened with both forms of
NTG compared with baseline (ANOVA: p = 0.005,
table 2, fig. 2b).
Two patients in the NTG spray group did not develop
ST depression of 11 mm, even at the end of exercise.
Thus, we used the heart rate and blood pressure values at
the peak of exercise as indicators of ischemia
develop-ment. The time required before ischemia developed
dur-ing exercise was prolonged in both treatment groups
(355.4 s with NTG spray and 336.9 s with NTG tablets)
compared to baseline (287.6 s, p = 0.30, table 2). Average
heart rate, systolic blood pressure and RPP during
isch-emia were significantly higher with NTG spray than with
NTG tablets. Figure 2c shows that greater stresses were
required to develop ischemia in the spray group than in
the tablet group.
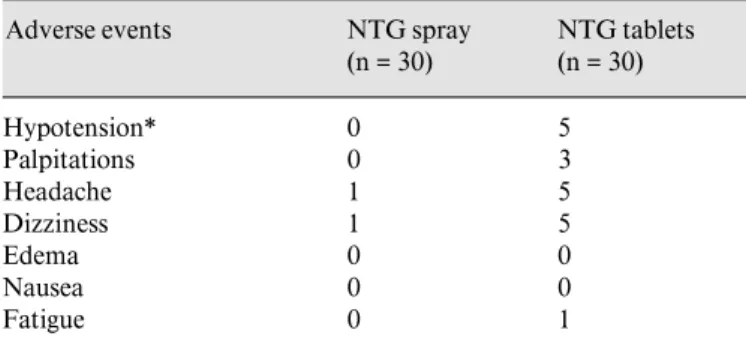
There were no significant differences in the frequency
and types of reported adverse events between the two
treatment groups; nonetheless, more patients experienced
hypotension, headache, and dizziness with NTG tablets
(5 patients) than with NTG spray (0 patients; table 3).
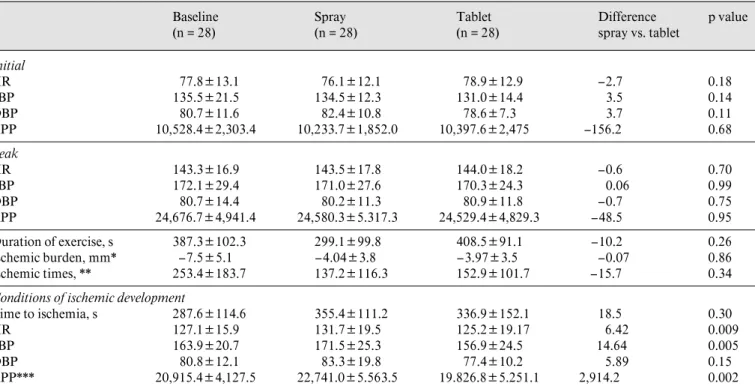
Table 2. Comparison of hemodynamic parameters in patients at baseline, with NTG spray, and NTG tablet groups
Baseline (n = 28) Spray (n = 28) Tablet (n = 28) Difference spray vs. tablet p value HR 77.8B13.1 76.1B12.1 78.9B12.9 –2.7 0.18 SBP 135.5B21.5 134.5B12.3 131.0B14.4 3.5 0.14 DBP 80.7B11.6 82.4B10.8 78.6B7.3 3.7 0.11 RPP 10,528.4B2,303.4 10,233.7B1,852.0 10,397.6B2,475 –156.2 0.68 Peak HR 143.3B16.9 143.5B17.8 144.0B18.2 –0.6 0.70 SBP 172.1B29.4 171.0B27.6 170.3B24.3 0.06 0.99 DBP 80.7B14.4 80.2B11.3 80.9B11.8 –0.7 0.75 RPP 24,676.7B4,941.4 24,580.3B5.317.3 24,529.4B4,829.3 –48.5 0.95 Duration of exercise, s 387.3B102.3 299.1B99.8 408.5B91.1 –10.2 0.26 Ischemic burden, mm* –7.5B5.1 –4.04B3.8 –3.97B3.5 –0.07 0.86 Ischemic times, ** 253.4B183.7 137.2B116.3 152.9B101.7 –15.7 0.34
Conditions of ischemic development
Time to ischemia, s 287.6B114.6 355.4B111.2 336.9B152.1 18.5 0.30
HR 127.1B15.9 131.7B19.5 125.2B19.17 6.42 0.009
SBP 163.9B20.7 171.5B25.3 156.9B24.5 14.64 0.005
DBP 80.8B12.1 83.3B19.8 77.4B10.2 5.89 0.15
RPP*** 20,915.4B4,127.5 22,741.0B5.563.5 19.826.8B5.251.1 2,914.2 0.002
Crossover analyses were also performed to compare the means of various variables between spray and tablet groups, with p values. HR = Heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; ischemic burden = total ST-segment depression; ischemic time = time from ST depression appearance to disappearance. ANOVA * p = 0.0028, indicating total ST depression among the three treatments were significantly different. ** p = 0.0054, *** p = 0.046.
Fig. 2. Comparisons of ischemic burden (a), ischemic time (b), and RPP (c) at the onset of ischemia.
Discussion
This crossover clinical trial compared the effectiveness
of NTG spray and tablets for treatment of
exercise-induced ischemia in CAD patients. The results
demon-strated that exercise duration, total ST-segment
depres-sion, and ischemic time were improved with both spray
and tablet forms compared with baseline. There were
sig-nificant differences in heart rate, blood pressure, and RPP
during exercise-induced ischemia between the two
treat-Table 3. Adverse events with NTG spray and tablet treatments
Adverse events NTG spray
(n = 30) NTG tablets (n = 30) Hypotension* 0 5 Palpitations 0 3 Headache 1 5 Dizziness 1 5 Edema 0 0 Nausea 0 0 Fatigue 0 1
Two patients withdrew due to severe headache with the tablet form. * p = 0.051 (Fisher’s exact test).
ments. Patients taking the spray form seemed to tolerate
higher stress before ischemia developed than patients
tak-ing the tablet form.
Previous clinical trials have reported several
differ-ences in efficacy between the two NTG spray and tablet
formulations [10, 12]. NTG spray has a longer shelf life
with no stability problems, but NTG tablets carried by
patients are often out of date and have decreased efficacy
[5, 9]. While both tablet and spray NTG are convenient to
use, the spray form may have a greater advantage, as it
causes fewer headache and hypotension episodes than the
tablet form. Although this was an actively controlled
study, it was not double-blinded and, therefore, there may
have been some information bias on the part of the
observers or patients. However, the similar distributions
of demographic and risk factors in both groups implies
that the comparison is acceptable. In comparing NTG
tablets (0.6 mg) with the metered-dose NTG spray
(0.4 mg), the difference in formulation might account for
the improved results in the NTG spray group in our study.
Lee et al. [14] reported an earlier onset of action with
NTG spray compared to tablets. We did not find
signifi-cant differences between spray and tablet forms, except
when ischemia developed.
Crossover designs reduce between-individual variance
and improve the efficiency of the sample size [15]. On the
other hand, crossover studies may suffer from carry-over
effect and/or period effect. Because the elimination
half-life following both sublingual administration of NTG
spray and tablets ranges from 2.5 to 4.4 min [14], a
carry-over effect was avoided by taking a long enough rest
before undergoing the next crossover treatment. The
peri-od effect was adjusted by a mperi-odel fitting technique,
incor-porating random effect into the fitted linear regression
model.
Based on the efficacy, adverse effects, storage
conve-nience, and hemodynamic parameters,
Nitrolingual-Spray should be considered as a therapy for CAD patients
with angina pectoris. It may be a superior preparation
compared to the tablet form and may have clinical
advan-tages.
Acknowledgment
This study was supported by the Chang-Long Company, Taiwan, and the authors thank Miss Shu-Hui Chu and Miao-Chen Wang for the preparation of the manuscript.
References
1 Opie LH: Drugs and the heart: Nitrates. Lancet 1980;i:750–753.
2 Page DP, Carson NA, Buhr CA, Flinn PE, Wells CE, Randall MT: Stability study of nitro-glycerin sublingual tablets. J Pharm Sci 1975; 64:140–147.
3 Fusari SA: Nitroglycerin sublingual tablets. 1. Stability of conventional tablets. J Pharm Sci 1973;62:122–129.
4 Russell VA, Lynch M: Storage of glyceryl trini-trate tablets in dispensing containers. Pharm J 1973;211:466–468.
5 O’Hanrahan M, McGarry K, Kelly JG, Horgan J, O’Malley K: Diminished activity of glyceryl trinitrate. Br Med J 1982;284:1183–1184. 6 Marty J, Shaw J, Hunt D: The stability of
glyce-ryl trinitrate tablets during patient use. Aust NZ J Med 1983;13:147–150.
7 Tallett ER: Stability of glyceryl trinitrate tab-lets. Br Med J 1982;284:1403.
8 Maclean FK, Hunt D, Marty JJ, Sloman JG, Shaw J: An assessment of the clinical use of gly-ceryl trinitrate in a hospital out-patient popula-tion. Aust NZ J Med 1980;10:12–14. 9 Van den Burg MJ, Cooper WD, Kimber GR:
Does the lack of patient knowledge about glyce-ryl trinitrate limit the usefulness of the drug? Br J Clin Pharmacol 1985;20:246P–247P. 10 Chevigne M, Renier J, Rigo P, Denjoulin JC,
Collignon P, Kulbertus HE: Efficacy of nitro-glycerin in spray form. Rev Med Intern 1980;1: 265–272.
11 Kattus AA, Alvaro AB, Zohman LR, Coulson AH: Comparison of placebo, nitroglycerin and isosorbide dinitrate for effectiveness of relief of angina and duration of action. Chest 1979;1: 17–23.
12 Kimichi A, Lee G, Amsterdam E, Fujii K, Krieg P, Mason DT: Increased exercise toler-ance after nitroglycerin oral spray: A new and effective therapeutic modality in angina pecto-ris. Circulation 1983;67:124–127.
13 Parker JO, Van Koughnett KA, Farrell B: Ni-troglycerin lingual spray: Clinical efficacy and dose response relationship. Am J Cardiol 1986; 57:1–5.
14 Lee G, Low R, Price J, Nguyen T, Mason DT: Efficacy of Nitrolingual®-Spray. Clin Res 1980;
28:191.
15 Jones B, Kenward MG: Design and Analysis of Cross-Over Trials. London, Chapman & Hall, 1989.