Oxygen Consumption, Ammonia-N Excretion, and
Growth Rate in Juvenile Green-Neon Shrimp
(Neocaridina denticulata) Exposed to Chlordane
and Lindane
Da-Ji Huang1 and Hon-Cheng Chen2*
1
Institute of Zoology, National Taiwan University, Taipei, Taiwan 106, ROC 2
Institute of Fisheries Sciences, National Taiwan University, Taipei, Taiwan 106, ROC
ABSTRACT
The purpose of this study was to investigate the chronic toxicity of chlordane and lindane as well as their sublethal concentration effects on green-neon shrimp (Neocaridina denticulata), a common habitant of freshwater systems of eastern Asia and Hawaii. In this study, the effects of experimental concentrations of 1 and 10 ng/L of chlordane and 0.1 and 1 µg/L of lindane on the growth rate, the
duration of the molt cycle, oxygen consumption (QO2), ammonia-N excretion
(ENH4+), and the O:N ratio were examined. Results indicated that growth rates of
the were lower than those of the control group, and the molt cycle duration times had also been altered. In all treatments, we also observed that QO2 increased in
the first few days, then it gradually returned to normal during the latter part of the
experiment from days 14 to 28. Furthermore, a decrease in ENH4+ and an
increase in the O:N ratio were observed in chlordane-treated groups, while
lindane showed significantly increased ENH4+ and a decreased O:N ratio
compared to the control. Accordingly, the results of this study demonstrate that lower concentrations of both chlordane and lindane toxicity to N. denticulate, even though their effects varied.
Key words: chlordane, chronic toxicity, lindane, Neocaridina denticulata INTRODUCTION
Chlordane and lindane, both organochlorine pesticides (OCPs), have been extensively used over the last 2 decades. OCPs are some of the most dangerous pesticides because of their toxicity, stability, high liposolubility, and long biological half-life. OCPs exhibit high degres of bioaccumulation and biomagnification through the food chain, and these chemicals are known to have carcinogenic, teratogenic, and endocrine-disrupting effects on humans and wildlife (Klaassen, 2001). Therefore, many developing and
developed countries, due to its serious toxicity have prohibited the use of these compounds for many years, even though OCPs are still being detected in ecosystems (Chen et al., 1999). Therefore, the influence of OCP residues on the development of wildlife has become a major concern.
Toxicity is the capacity of a chemical agent to adversely affect the activity of a living organism: its growth, health, life span, and/or reproductive capacity. Adverse effects also include behavioral changes in individual organisms and ecological changes that affect collective
1
________________________________________________________________________________________________________________________
*Corresponding author: Room 605, Institute of Fisheries Science, National Taiwan University,
No. 1 Roosevelt Road, Sec. 4, Taipei 106, Taiwan, R.O.C.
populations. More commonly, animals are subjected to low-dose toxic chemical stresses arising from exposure to sublethal concentrations, so there is a need to assess the effects of toxic compounds on aquatic organisms. Several studies have used growth rate, oxygen consumption, ammonia-N excretion, etc. as indicators to reflect alterations after exposure to a chemical (Schweer, 2002).
The growth rate is an index associated with stress and common signal in chronic-toxicity studies. The growth of an organism is generally used as a sensitive and reliable endpoint in chronically-toxicological investigations. It has clear potential for providing predictions of the harmful properties of a chemical present in a watercourse (Rosas et al., 2001; Benimeli et al., 2003). Molting is either directly or indirectly involved in the expression of growth, and it may be examined through toxicological testing. Because noticeable growth can only occur as a result of molting, any disruption of molting can result in alterations in growth (Skinner, 1985; Schweer, 2002).
Oxygen consumption and ammonia-N excretion are widely considered to be critical factors for evaluating the physiological responses of crustaceans (Claybrook, 1983; McMahon and Wilkens, 1983; McMahon, 2001). These changes in metabolic substrate usage can be measured by monitoring the oxygen: nitrogen (O:N) ratio of test organisms. The O:N ratio indicates the relationship between the amount of oxygen consumed by an organism and the amount of nitrogen excreted, and shows the relative role protein catabolism plays in the organism’s energy budget. Theoretical values of
O:N of between 3 and 16 have been suggested for the catabolism of protein, whereas catabolism of equal quantities of proteins and lipids yields O:N values of between 50 and 60. Greater values of O:N correspond to increases in lipid and carbohydrate catabolism (Mayzaud and Conover, 1988). Studies have concluded that changes in the O:N ratio measured among test organisms can serve as a sensitive indicator which provides for the relatively early detection of reproductive impacts by contaminants (Schweer, 2002).
Invertebrates constitute the vast majority of animal species on earth, and crustaceans represent an important and diverse group. Therefore many invertebrate toxicity test protocols are routinely used in regulatory toxicity testing (Schweer, 2002). The green-neon shrimp (Neocaridina
denticulata) is distributed in rivers
throughout eastern Asia and the Hawaiian islands (Shy and Yu, 1998, Englund and Cai, 1999). The major characteristic of N. denticulata is the appearance of the oval-like endopod on the first pleopod. In males, the endopod of the second pleopods have an appendix masculine that is oval-shore and has cilia around it. In the female, the endopods of the second pair of pleopods bear an appendix only (Shy and Yu, 1998).
Even though N. denticulata is a common shrimp in fresh waters of Taiwan, only a few attempts have so far been made to determine the effects of chlordane and lindane in freshwater systems on N. denticulata (Chen et al., 1999). Therefore, the purpose of this study was to investigate the effects of exposure to sublethal concentrations of these two OCPs on oxygen consumption, ammonia-N excretion, duration of the molt cycle, and growth
rate in juveniles of N. denticulata. The results may provide objective information which can be applied to minimize the impacts of chlordane and lindane on aquatic ecosystems.
MATERIALS AND METHODS
Animal maintenance and chemicals Green-neon shrimp (Neocaridina
denticulata) were taken from rivers in
Taipei County, northern Taiwan for laboratory testing. They were transferred to a 50-L glass aquarium after being identified. This aquarium was equipped with a water-cycling device; the pH was maintained at 7.4~7.8; the dissolved oxygen concentration was greater than 7.3 mg/L; and the hardness was 38~45 mg
CaCO3/L. The temperature was
maintained at 25 ± 1 °C, and a 12-h light-dark photoperiod was used. Under these conditions, shrimp were fed twice a day and acclimated for 2 weeks before testing. Newly hatched shrimp (7 d old, 1.5 mm in body length, and 0.5 mg in body weight) were used for the growth tests; juveniles (6 ± 0.2 mm in body length, and 3 ± 0.4 mg in body weight) were used for the molting, oxygen consumption, and ammonia-N excretion tests.
Chlordane and lindane were purchased from Sigma (St. Louis, MO, USA). Stock solutions of chlordane (10 mg/L) and lindane (100 mg/L) were prepared in acetone. It was reported that the values of the 96-h
LC50 for chlordane and lindane are
127.03 ng/L and 9.36 µg/L for N.
denticulata, respectively (Huang and
Chen, 2004). Hence, experimental concentrations of chlordane and lindane were 1 and 10 ng/L, and 0.1 and 1 µg/L, respectively. In the control group, only acetone was added.
Toxicity tests with water renewal every 48 h were based on the Standard
Guide for Conducting Acute Tests with Shrimps (EPA/ROC, 1998).
Growth rate
Groups of 50 newly hatched shrimp were randomly sampled and placed in 10-L glass beakers, with exposure times of 120 d. Body length and body weight were measured using an electronic ruler and electronic balance at 7-d intervals. Body length was measured as the distance from the base of the eyestalk to the tip of the telson (Hung et al., 1993).
Molt (duration of the molt cycle) Juvenile shrimp were individually placed in 100-ml glass beakers containing 50 ml of test medium for each concentration of each chemical and the control. The incidence of molting among all specimens was checked every day, and the shed exoskeletons were carefully removed. The experiments ended after the shrimp had completed their 6th molt. Oxygen consumption, ammonia-N excretion, and the O:N ratio
Groups of 15 juvenile shrimp were randomly sampled and placed in 10-L glass beakers. Control and exposed samples were taken at intervals of 30 min (acute), and 3, 7, 14, and 28 d for estimation of oxygen consumption and ammonia-N excretion.
The oxygen consumption examination was based on the method described by Chinni et al. (2000). After being weighed, animals were placed into an oxygen-consumption detection bottle (WTW KF12, Weilheim, Germany) with a microprocessor oximeter (WTW
OXI196). Oxygen consumption (QO2,
µg O2/g/h) was calculated as follows:
QO2 = ∆ppm × 1/BW × V × 1/t;
where QO2 is the amount of oxygen
(∆ppm) consumed in the interval t (h),
BW is the wet body weight (g) of the
individual, and V is the volume (ml) of the oxygen-consumption detection
bottle.Oxygen consumption tests were
estimated for a period of 6 h. Additionally, the water within the bottle at the start as well as at the end of oxygen consumption analysis was immediately sampled to examine the level of ammonium-N before its transformation to nitrite or nitrate because of oxidation and activities of some microorganisms. Examination of ammonium-N within the water was detected using an ammonia electrode (Mettler-Toledo, type-15 230 3000, Urdorf, Switzerland). Ammonium-N
excreted (ENH4+, µg NH4+/g/h) by
animals was calculated as follows:
ENH4+ = ∆ppb × 1/BW × V × 1/T;
where ENH4+ is the amount of
ammonium (∆ppb) generated in the total experimental time T (h). In addition, the oxygen: nitrogen (O:N) ratios were also calculated as the ratio of atoms of oxygen consumed to atoms of nitrogen excreted in the above intervals.
Statistical analysis
All values of growth rate, molting rate, oxygen consumption, and ammonia-N excretion tests were analyzed by analysis of variance using Microcal™ vers. 6.0. (Northampton, MA, USA). Experimental and control values were compared using Student’s
t-test (paired assay, p < 0.05).
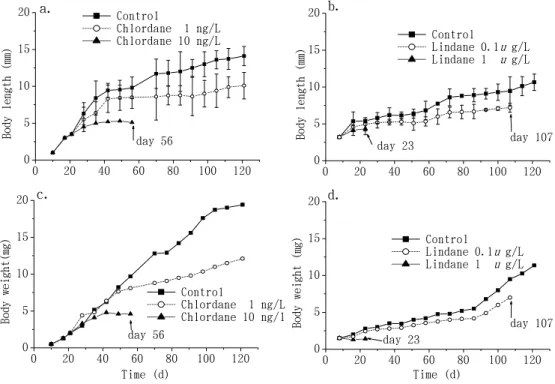
0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 0 5 1 0 1 5 2 0 a . d a y 5 6 B o d y l e n g t h ( m m ) C o n t r o l C h l o r d a n e 1 n g / L C h l o r d a n e 1 0 n g / L 0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 0 5 1 0 1 5 2 0 b . d a y 1 0 7 d a y 2 3 B o d y l e n g t h ( m m ) C o n t r o l L i n d a n e 0 . 1 u g / L L i n d a n e 1 u g / L 0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 0 5 1 0 1 5 2 0 c . d a y 5 6 B o d y w e i g h t ( m g ) T i m e ( d ) C o n t r o l C h l o r d a n e 1 n g / L C h l o r d a n e 1 0 n g / l 0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 0 5 1 0 1 5 2 0 d . d a y 1 0 7 d a y 2 3 B o d y w e i g h t ( m g ) T i m e ( d ) C o n t r o l L i n d a n e 0 . 1 u g / L L i n d a n e 1 u g / L
Figure 1. Growth rate of body length (mean ± S.D., n = 10) and body weight measured
after exposure to chlordane (a, c) and lindane (b, d). Mortality rates of 100% were observed on days 56, 107, and 23 for the groups exposed to 10 ng/L chlordane, 0.1 µg/L lindane, and 1 µg/L lindane, respectively.
RESULTS
Growth rate
Growth rates were measured as the decrease in body weight and body length (compared to the control group) (Fig. 1) as affected by different treatments. Mortality rates of 100% were observed at 56, 107, and 23 d for the groups subjected to 10 ng/L chlordane, and 0.1 and 1 µg/L lindane, respectively. The higher the concentration of pesticides the shrimp were exposed to, the lower the growth rates we observed.
Molting
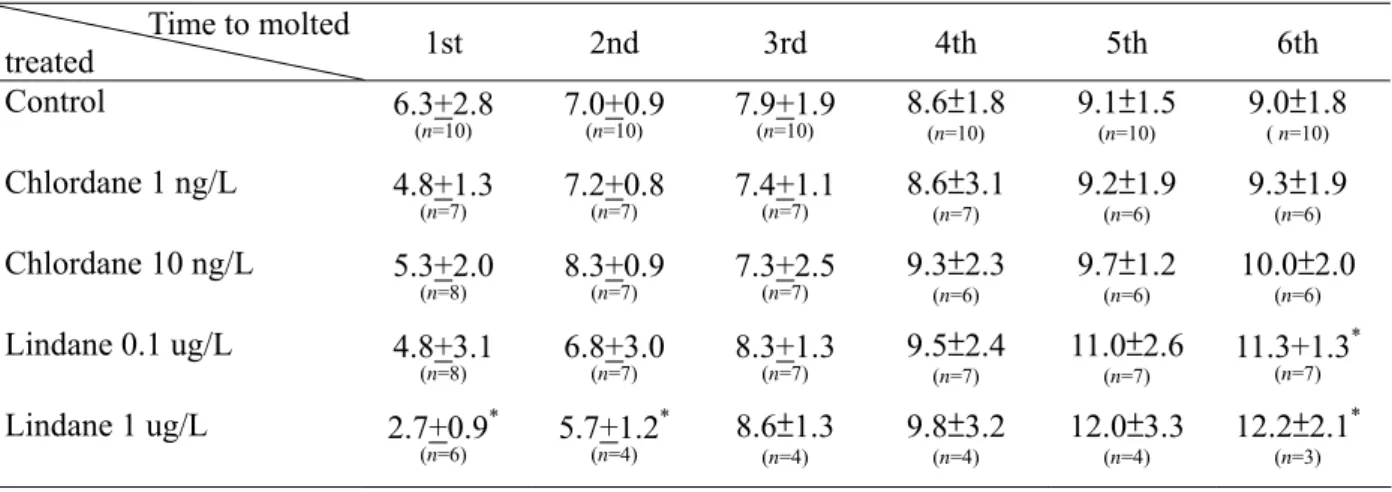
Results from the experiment on molting rates are shown in Table 1. From the data sets of control shrimp, we observed that the molt cycle duration continued for the entire experimental period. After being treated with chlordane and lindane, the exposed shrimp especially the group exposed to 1 ug/L lindane (p < 0.05) showed a decline in the duration before their first molt compared to the controls. However, molt cycle durations from the 3rd to the 6th molts of the treated shrimp tended to be longer than those of the control, and the results for the 6th molt of shrimp
exposed to 0.1 and 1 ug/L lindane treatments showed statistically significant differences from the control (p < 0.05).
Oxygen consumption, ammonia-N excretion, and the O:N ratio
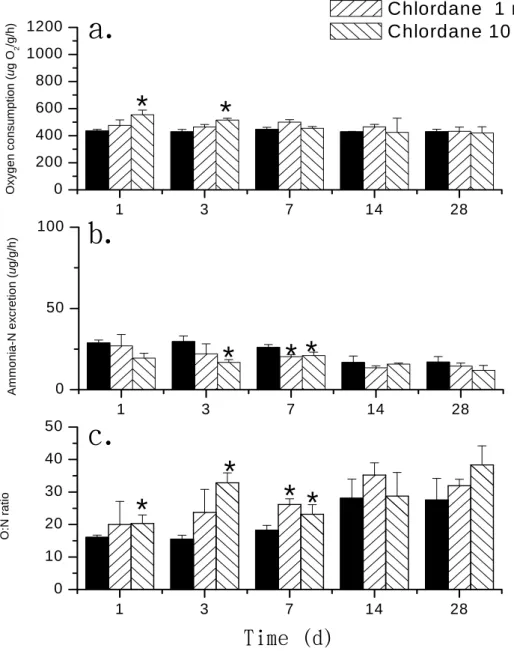
Results in Figs. 2 and 3 show the
oxygen consumption (QO2),
ammonia-N excretion (ENH4+), and
O:N ratio. The QO2 values of shrimp
treated with 10 ng/L chlordane were significantly higher than those of control shrimp after 1 and 3 d of exposure (p < 0.05). We also observed
that QO2 values of shrimp treated with
0.1 and 1 µg/L lindane were higher than those of the control after exposure for 7 d and for 1, 3, and 7 d, respectively (p < 0.05). After exposure
for 14 d, the QO2 from the chlordane-
and lindane-treated shrimp had gradually recovered. Results for
ENH4+ showed that the level in the 1
ng/L chlordane-treated shrimp was significantly lower than that of the control group after 7 da of exposure (p < 0.05), and the level in 10 ng/L chlordane-treated shrimp was significantly lower than that of the control group after both 3 and 7 d of exposure (p < 0.05). After exposure to Table 1. Duration of the molt cycle of juvenile Neocaridina denticulata in the control and treated groups shown
with units of day (mean ± S.D.). The number of shrimp (n) is shown in parentheses. Mean values of the treated groups with an asterisk (*) significantly differ from those of the control groups (p < 0.05).
Time to molted treated 1st 2nd 3rd 4th 5th 6th Control 6.3+2.8 (n=10) 7.0+0.9(n=10) 7.9+1.9(n=10) 8.6±1.8(n=10) 9.1±1.5 (n=10) 9.0±1.8 ( n=10) Chlordane 1 ng/L 4.8+1.3 (n=7) 7.2+0.8(n=7) 7.4+1.1(n=7) 8.6±3.1(n=7) 9.2±1.9(n=6) 9.3±1.9(n=6) Chlordane 10 ng/L 5.3+2.0 (n=8) 8.3+0.9(n=7) 7.3+2.5(n=7) 9.3±2.3(n=6) 9.7±1.2 (n=6) 10.0±2.0 (n=6) Lindane 0.1 ug/L 4.8+3.1 (n=8) 6.8+3.0(n=7) 8.3+1.3(n=7) 9.5±2.4(n=7) 11.0±2.6 (n=7) 11.3+1.3 * (n=7) Lindane 1 ug/L 2.7+0.9* (n=6) 5.7+1.2 * (n=4) 8.6±1.3 (n=4) 9.8±3.2 (n=4) 12.0±3.3 (n=4) 12.2±2.1* (n=3) 69
0.1 and 1 µg/L lindane, levels of
ENH4+ in the treated shrimp were
significantly higher than those of the control on days 1, 7, 14, and 28, and on days 7, 14, and 28, respectively (p < 0.05). Values of the O:N ratio in the 1 and 10 ng/L chlordane-treated groups were higher then those of the
control group on day 7 and on days 1, 3, and 7, respectively (p < 0.05). Treatment with lindane produced opposite results as those seen in the chlordane groups, as we observed obvious drops in the O:N ratios on days 7, 14, and 28 (p < 0.05). 1 3 7 14 28 0 10 20 30 40 50
*
*
*
*
c .
O: N ra ti oT i m e ( d )
1 3 7 14 28 0 50 100*
*
*
b .
Am mon ia-N e x cretio n (u g/g/h ) 1 3 7 14 28 0 200 400 600 800 1000 1200*
*
a .
Oxyge n co nsum ption ( u g O 2 /g/h) Control Chlordane 1 ng/L Chlordane 10 ng/LFigure 2. Oxygen consumption (a), ammonia-N excretion (b), and the O:N ratio (c) of the
control, as well as individuals exposed to 1 and 10 ng/L chlordane in juvenile
Neocaridina denticulata (mean ± S.D., n = 15). Mean values of the treated groups
1 3 7 14 28 0 10 20 30 40 50
*
*
*
*
*
*
*
*
a
c .
O:N r a tioT i m e ( d )
1 3 7 14 28 0 50 100 150 200 250 300 350*
*
*
*
*
*
*
*
*
b .
A m monia-N ex cretio n ( u g/g/h ) 1 3 7 14 28 0 200 400 600 800 1000 1200* *
*
*
a
a .
Ox y gen cons umption ( u g O 2 /g/h)Control
Lindane 0.1 ug/L
Lindane 1 ug/L
Figure 3. Oxygen consumption (a), ammonia-N excretion (b), and the O:N ratio (c) of the
control as well as individuals exposed to 0.1 and 1 µg/L lindane in juvenile
Neocaridina denticulata (mean ± S.D., n = 15). Mean values of treated groups with
an asterisk (*) significantly differ from those of the control groups (p < 0.05).
DISCUSSION
Chlordane and lindane were toxic to green-neon shrimp, N. denticulata, even with exposures to low toxicity
values of 1 and 10 ng/L chlordane and 0.1 and 1 µg/L lindane. In this study, we describe their effects on the growth rate, duration of the molt cycle,
oxygen consumption, ammonia-N excretion, and the O:N ratio. Growth rates of shrimp treated with both of these OPCs were lower than that of the control. Exposure of newly hatched N.
denticulata juveniles to 10 ng/L
chlordane, and 0.1 and 1 µg/L lindane resulted in 100% mortality within 56, 23, and 107 d, respectively (Fig. 1). Similar results were found in a mysid (Mysidopsis bahia) on studies contaminants retard growth rates (McKenney and Celestial, 1996). The growth rate of arthropods is closely related to the molt cycle duration; increases or decreases in the time duration of molts may affect growth rates (Schweer, 2002). The results from this study showed that circumstances of the treated groups differed compared with that of control group. After treatment with chlordane and lindane, a trend appeared in which molt cycle durations from the initial molt decreased, while they increased from the 4th to the 6th molts. And this circumstance was obvious in both lindane-treated groups which showed significant differences from the controls (p < 0.05). Molting, one of the key physiological processes of arthropods, is under hormonal control, and it is susceptible to negative effects of contaminants especially endocrine- disrupting chemicals. Molting is primarily regulated by interactions of
molt-stimulating hormones (ecdysteroids) and nervous system
secretions produced in the cephalo- thorax with molt-inhibiting hormones produced in the eyestalks (Skinner, 1985; Subramoniam, 2000). Touart (1982) found that the pesticide,
diflubenzuron (Dimilin®), increased
the duration of the molt cycle in the mysid shrimp, M. bahia, and probably acted on the mysid endocrine system as a molt inhibitor. Even so, direct
results on the effects of chlordane and lindane on the action mechanism of ecdysteroids of N. denticulata were not available in the present study. Furthermore, another consideration concerning the effects of chlordane and lindane on the molt cycle and growth is that the reduced growth rate in N. denticulata possibly resulted from a transfer of energy from growth mechanisms as the organism attempted to counteract the stress (McKenney et al., 1991, Khan et al., 1992).
In the present study, we observed that
QO2 increased on the first day after
being exposed to 10 ng/L chlordane and 0.1 or 1 µg/L lindane which implies that chlordane and lindane might alter the respiratory rate after acute exposure (Figs. 2, 3). Changes in
QO2 caused by pesticides indicate
metabolic alterations, which might affect larval growth. These results agreed with those reported by other authors on the respiratory metabolism
of white shrimp, Penaeus vannamei,
challenged with sublethal doses of pesticides (Mckenney et al., 1991; Galindo et al., 1996). However, we
found that QO2 gradually returned to a
normal condition from day 7 or 14. Thus, we considered that gills of treated N. denticulata were not seriously damaged or destroyed by chlordane (1 and 10 ng/L) and lindane (0.1 and 1 µg/L), and they still could recover their functions in oxygen
absorption. ENH4+ is the waste product
of protein metabolism, and it may be another metabolic waste which indicates that N. denticulata was affected by chemical toxicity after
considering QO2 and calculating the
O:N ratio. We observed that the
situations with ENH4+ and the O:N
ratio after exposure to chlordane and lindane entirely differed in our
experiment. Decreases in ENH4+ and
increases in the O:N ratio were observed in the chlordane-treated groups (Fig. 2b, c). However, lindane showed a totally different effect, in
that ENH4+ increased and the O:N
ratio significantly decreased compared
to the controls (Fig. 3b, c). The main
energy source of crustaceans is from protein (free amino acid) degradation,
and ENH4+ is produced from proteins.
Nevertheless, when a crustacean is in a situation where it requires energy for the long term or the free amino acids in the body are limited, it can transfer its energy source from proteins to lipids in order to enhance its energy (Mayzaud and Conover, 1988). McKenney et al. (1991) indicated that exposure of M. bahia to the defoliant, DEF (S, S, S,-tri-n-butyl phosphoro-
trithioate), caused a decrease in ENH4+,
but an increase in the O:N ratio probably because M. bahia changes the substance used for its metabolic energy from proteins to lipids in order to enhance its energy for resisting the effect of the toxicity, as occurred in shrimp exposed to chlordane. Mayzaud and Conover (1988) showed that an increasing metabolism rate of crustaceans affected by stress can cause the O:N ratio to drop to below 4. In our lindane-treated groups, we deduced that N. denticulate greatly increased its metabolism rate in order to remove the lindane and resist toxicity after the organism was affected by the toxicity of lindane; this
possibly caused the rise in ENH4+ and
the fall in the O:N ratio. But, even with the effects of lindane, it failed to transfer its energy source to lipids. Thus N. denticulata degraded a great deal of protein due to its consumption
of energy in an effort to resist the toxicity of lindane, and this produced a large amount of nitrogen-containing degradation products. After having been affected by lindane for 28 d, the nonstop consumption of protein caused physiological recession, and the quantity of ammonia being discharged was much less compared to that on the 14th day. At present, we are unable to determine which physiological mechanism which was affected by lindane prevented the transfer to lipid metabolism.
The stable development of a population of an organism depends on the health conditions of individuals (Colborn et al., 1993). We found that
N. denticulata was affected by
exposure to low concentrations of chlordane (1 and 10 ng/L) and lindane (0.1 and 1 µg/L). However, low concentrations of chlordane (< 14.2 µg/L) and lindane (< 20.6 ng/L) can still be detected in freshwater systems (Abou-Arab et al., 1995; Chen et al.,
1999; Galindo et al., 1999). When
chemicals such as chlordane and lindane reach wildlife through the food chain, they may cause physiological problems (Colborn et al., 1993). As a result, decreases in wildlife populations may well be expected. This is an urgent situation which requires our attention in order to determine biological safe concentrations, if the reproductive systems and behaviors of N.
denticulata in our environment are not
to be disrupted. We are currently working on related studies to further understand the effects of chlordane and lindane on N. denticulata, especially on the structure of the gills, renal gland, and other tissues caused by these two pesticides.
REFERENCES
Abou-Arab, A. A. K., M. N. E. Gomaa, A. Badawy and K. Naguib (1995) Distribution of organochlorine pesticides in the Egyptian aquatic ecosystem. Food Chem. 54: 141-146.
Benimeli, C. S., M. J. Amoroso, A. P. Chaile and G. R. Castro (2003) Isolation of four aquatic streptomycetes strain capable of growth on organochlorine pesticides. Bioresource Technol. 89: 113-138.
Chen, H.-C., Y.-S. Wang and J.-H. Yaun (1999) Standard methods to study and detect the bioaccumulation in fish and shellfish (EPA-88-1502-03-01),
Environmental Protection Administration of Taiwan, Taipei.
(in Chinese)
Chinni, S., R. N. Khan and P. R. Yallapragada (2000) Oxygen consumption, ammonia-N excretion, and metal accumulation
in Penaeus indicus postlarvae exposed to lead. Bull. Environ. Contam. Toxicol. 64: 144-151. Claybrook, D. L. (1983) Nitrogen
metabolism. In The biology of crustacea. Vol. 5—Internal anatomy and physiological regulation. L. H. Mantel, ed. Academic Press, New York, pp. 163-214.
Colborn, T., F. S. vom Saal and A. M. Soto (1993) Developmental effects of endocrine disrupting chemicals in wildlife and humans. Environ. Health Perspect. 101: 378-384. Englund, R. A. and Y. Cai (1999) The
occurrence and description of
Neocaridina denticulate sinensis
(Kemp, 1918) (Crustacea: Decapoda: Atyidae), a new introduction to the Hawaiian Islands. Bishop Mus. Occasional
Papers 58: 58-65.
EPA/ROC (1998) Standard guide for conducting acute tests with shrimp: static renewal test for
Neocaridina denticulata (NIEA
B905.10B). Environmental Protection Administration of Taiwan, Taipei. (in Chinese)
Gaindo, R. J. G., J. A. Medina and C. Villagrana (1996) Physiological and biochemical changes in shrimp larvae (Penaeus vannamei) intoxicated with organochlorine pesticides. Mar. Pollut. Bull. 32: 872-875.
Galindo, R. J. G., V. U. Fossato, C. V. Lizarraga and F. Dolci (1999) Pesticides in water, sediments, and shrimp from a coastal lagoon off the Gulf of California. Mar. Pollut. Bull. 38: 837-841.
Huang, D.-J. and H.-C. Cheng (2004) Effects of chlordane and lindane on testosterone and vitellogenin levels in green neon shrimp (Neocaridina denticulata). Int. J. Toxicol. 23(2): 91-96.
Hung, M. S., T. Y. Chan and H. P. Yu (1993) Atyid shrimps (Decapoda: Caridea) of Taiwan, with descriptions of three new species. J. Crust. Biol. 13: 481-503.
Khan, A. J. Barbieri, S. Khan and F. Sweeney (1992) A new short-term mysid toxicity test using sexual maturity as an endpoint. Aquat. Toxicol. 23: 97-105.
Klaassen, C. D. (2001) Casarett and Doull’s toxicology. McGraw-Hill, New York.
Mayzaud, P., and R. L. Conover (1988) O:N atomic ratio as a tool to describe zooplankton metabolism. Mar. Ecol. Prog. Ser. 45: 289-302. McKenney, C. L. and D. M. Celestial
(1996) Modified survival, growth and reproduction in an estuarine
mysid (Mysidopsis bahia) exposed to hormone analogue through a complete life cycle. Aquat. Toxicol. 35: 11-20.
McKenney, C. L., T. L. Hamaker and E. Matthews (1991) Changes in the physiological performance and energy metabolism of an estuarine mysid (Mysidopsis bahia) exposed in laboratory through a complete life cycle to defoliant DEF. Aquat. Toxicol. 19: 123-135.
McMahon, B. R. and J. L. Wilkens (1983) Ventilation, perfusion, and oxygen uptake. In The biology of crustacea. Vol. 5—Internal anatomy and physiological regulation. L. H. Mantel, ed. Academic Press, New York, pp. 290-372.
McMahon, B. R. (2001) Respiratory and circulatory compensation to hypoxia in crustaceans. Respir. Physiol. 128: 349-364.
Roasa, C., G. Cuzon, G. Gaxiola, Y. L. Priol, C. Pascual, J. Rossignyol, F. Contreras, A. Sanchex and A. V. Wormhoudt (2001) Metabolism and growth of juveniles of
Litopenaeus vannamei: effect of
salinity and dietary carbohydrate levels. J. Exp. Mar. Biol. Ecol 259: 1-22.
Schweer L. G. (2002) Draft detailed review paper on mysid life cycle toxicity test. (68-W-01-023), U.S. Environmental Protection Agency, Washington, DC.
Shy, J. Y. and Yu, H. P. (1998) Freshwater shrimps of Taiwan. National Museum of Marine Biology and Aquarium, Taipei. (in Chinese).
Skinner, D. M. (1985) Molting and regeneration. In The biology of crustacea Vol. 9. D. E. Bliss and L. H. Mantl, eds. Academic Press, New York, pp. 44-146.
Subramoniam, T. (2000) Crustacean ecdysteroids in reproduction and embryogenesis. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 125: 135-156.
Touart L. W. (1982) The effects of diflubenzuron on molting and regeneration of two estuarine crustaceans, the mysid shrimp
Mysidopsis bahia and the grass
shrimp Palaemonetes pugio. M.S. thesis. Univ. of West Florida, Pensacola, FL.
可氯丹( Chlordane )及林丹( Lindane )對多齒新米蝦
( Neocaridina denticulata )所造成之慢性毒性影響
黃大駿1、陳弘成2 1國立臺灣大學動物學研究所 2國立臺灣大學漁業科學研究所 摘 要 可氯丹(chlordane)及林丹(lindane)為長效性有機氯殺蟲劑。本研究目的在瞭 解環境中殘留的可氯丹及林丹對多齒新米蝦( Neocaridina denticulata )所造成 之慢性毒性影響。將多齒新米蝦曝露於亞致死濃度之可氯丹(1 及 10ng/L)及林丹 (0.1 及 1µg/L)下來進行試驗,於曝露後測定多齒新米蝦成長速率、脫殼時間、耗氧(QO2)、排氨量(ENH4+)及氧氮比(O:N ratio)改變的情形。實驗結果顯示可氯丹
及林丹均會抑制成長及改變脫殼時間,並使曝露初期各組的耗氧量上升;而在實 驗後期的第14 至 28 天,各試驗組的耗氧量則會逐漸回復正常值。此外,可氯丹 的試驗組中排氨量降低,氧氮比增加;而林丹的試驗組則呈現排氨量上升,氧氮 比減少的情形。由此可知,多齒新米蝦的成長的確受到亞致死濃度之可氯丹(1 及10ng/L)及林丹(0.1 及 1µg/L)的毒性影響。 關鍵詞:可氯丹、林丹、多齒新米蝦、慢性毒