Migratory pattern and habitat use of tropical eels Anguilla spp. (Teleostei : Anguilliformes : Anguillidae) in the Philippines, as revealed by otolith microchemistry
全文
(2) Briones et al.: Migratory pattern of Anguilla eels in the Philippines annuli in 1899 (Campana, 1999). Because their accretion is on a daily basis, critical information about the age and growth rate can be determined for individual fish. Additionally, trace elements that are absorbed into the otolith structure can provide information on the environments that the fish has lived in and the physiological history. The trace element composition of the increments can be used to reconstruct the environmental histories of many fishes. Deposition of the strontium (Sr) in the otoliths is positively correlated with the salinity of the ambient water (Tzeng, 1996). Thus, Sr:Ca ratios in otoliths can explain the migratory environmental histories of diadromous fishes (Tzeng & Tsai, 1994; Jessop et al., 2002; Arai et al., 2004). Differences in the ratios Sr:Ca concentration of the otoliths represent the different environmental migratory patterns of eels. As the eels migrate from their spawning grounds in the open ocean into the rivers and estuaries of the Philippines, they experience different water temperatures as well as different salinity gradients (i.e., marine, brackish and freshwater). They also experience a series of ontogenetic changes, going through the yolk-sac, leptocephalus, glass eel and elver stages. Environmental and physiological changes may all have the potential to affect the incorporation of strontium into the otoliths of eels (Kalish, 1989; Sadovy & Severin, 1992). The Sr:Ca ratio in the otolith is positively related to salinity (Secor et al., 1995; Farrell & Campana, 1996; Chang et al., 2004) and negatively correlated to ambient temperature (Townsend et al., 1992). However, the influence of temperature on Sr:Ca ratios in the otolith is minimal (Campana, 1999). The elusive life cycle of the anguillid eels has attracted many scientists to study the life history and migratory patterns of this fish. Studies have found diverse migratory behaviour and habitat uses in temperate eels (Daverat et al., 2006). The estuarine/marine residency of anguillid eels was believed to occur more frequently in higher latitudes where productivity is usually lower in freshwater than in the coastal areas (Tsukamoto et al., 2002). However, this hypothesis was based on the studies of temperate eels and may not be valid for tropical eels. Furthermore, it is still unknown if the tropical eels show high plasticity in habitat use and movements between fresh and sea water. This study will extend our understanding of the life history and movement of the tropical eel species.. MATERIALS AND METHODS Fish collection and sampling areas. – All eel samples were collected from different sampling sites in the Cagayan and Kalinga Provinces in the Northern part of Luzon Island, the Philippines (Fig. 1). A total of 9 individuals were collected in the Chico River (Pinukpuk, Kalinga Province), approximately 60 km from its draining point at the Cagayan River and about 100 km from the river mouth or estuary. Two eel samples were collected from the Buguey River, 8 individuals from the Casili Creek and 3 individuals from the Abulog River, all of which are located in the Cagayan. 142. Province. The eels from the Chico River were caught by electrofishing, while the eels from the Buguey River, Casili Creek and Abulog River were obtained with the use of fish pots (Fig. 2) and fish hooks with earthworm bait. The eels were preserved immediately in an ice box after capture. The total length (TL) and body weight of the eels were measured to the nearest 0.1 cm and 0.1 g, respectively. Otolith preparation and microchemistry analysis. – The largest pair of the eel’s otoliths, known as the sagittae, were dissected from the vestibular apparatus and prepared for microchemistry analysis and age determination. The dissected otoliths were washed with distilled water, dried at room temperature and placed in sealed Eppendorf tubes. The preserved otoliths were brought to the Fisheries Biology Laboratory, College of Life Science, National Taiwan University, Taipei, Taiwan for further processing and analysis following the methods of Tzeng et al. (2002, 2003a, b) and Shiao et al. (2003). Briefly, the otoliths (with the distal side facing upwards) were embedded in a silicon rubber mould with an Epofix kit (Streurs A/S Denmark) and hardened in an oven for 40 minutes at a 60oC. The embedded otoliths were then sectioned with the use of a low-speed circular saw (Beuhler Metaserv, Buehler, UK Ltd.) to remove excess Epofix resin to facilitate polishing. The otoliths were then ground and polished on a Buehler Metaserv grinder-polisher (Buehler, UK Ltd.) at a speed of 300 rpm with wet-polishing paper of 2,000-grit for first the polishing and 2,400-grit for the second polishing. The polished otoliths were periodically rinsed in running water and checked under a compound light microscope. When the core was revealed, final polishing was done with a Metaserv 2000 grinder-polisher with wet-polishing cloth and a Gamma Micropolisher II Deagglumerated Alumina 0.05 μm (Buehler, USA) at 300 rpm to smoothen the surface of the otolith for the electron probe microanalyzer (EPMA) analysis. The polished Epofix resin block with otoliths were coated with a thin layer of conductive carbon paint (SPI Supplies, Division of Structure Probe Inc., USA) to increase the electron conductance and to reduce X-ray diffraction. The microchemistry analysis for Sr:Ca ratios of the otolith was done at the EPMA Laboratory, Institute of Earth Sciences, Academia Sinica, Nankang, Taipei. The concentrations of the strontium and calcium in the otoliths were measured along a transect from the primordium to the edge with an electron probe microanalyzer EPMA (JEOL JXA-8900R) equipped with wavelength dispersive X-ray spectrometers. Quantitative analysis was conducted with 15 kV accelerating voltage, 3 nA for the beam with a 5 × 4 μm (× 20,000) rectangular scanning beam size. The wavelength dispersive spectrum at the strontium Lα peak position was measured for 80 seconds for the peak and 20 seconds for each of the upper and lower baselines. The peak concentration of calcium at Kα was measured for 20 seconds and 10 seconds for each of the upper and lower baselines. Synthesized strontianite (SrCO3) and calcite (CaCO3) from the Department of Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington, DC, were used as.
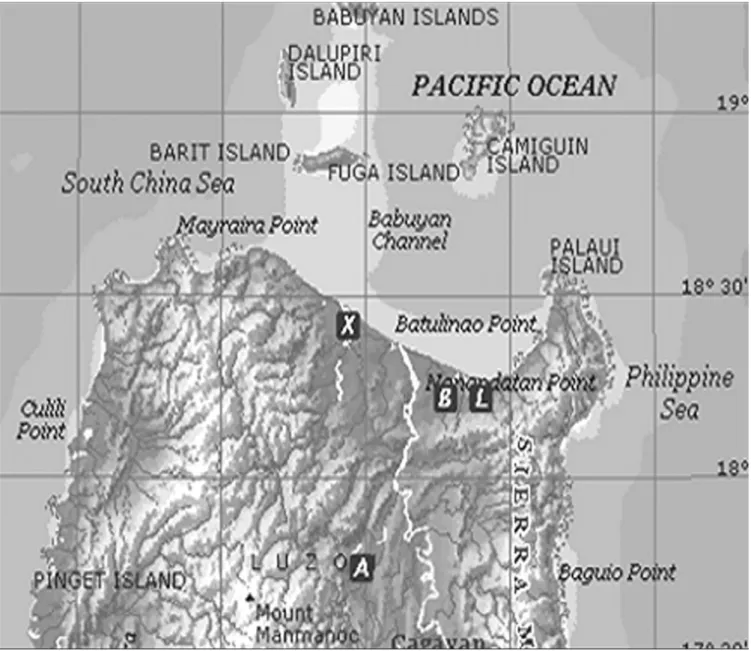
(3) THE RAFFLES BULLETIN OF ZOOLOGY 2007 standards to calibrate the concentration of strontium and calcium in the eel otoliths. The weight ratio of the Sr:Ca ratio was calculated after correction with the PRZ (phi-rho-z) method.. ages of the eels were still estimated and data is provided in Table 1, although the ages and growth rates of the tropical eels were not the main concern of this study.. Age determination. – After microchemistry analysis, the otoliths were polished to remove the carbon coating and etched for 1 to 2 minutes with 5% EDTA to reveal the annuli for age determination. Yearly growth increments in the otoliths were examined using a compound light microscope. The age of the eel was determined through the growth increments or annuli in their otoliths deposited on a yearly basis (Fig. 3). The criteria which were used to interpret the otolith annuli in temperate eels were also adapted in this study. False annuli were distinguished from the supernumerary check based on their width, optical density, relative positions and degree of continuity around the otolith circumference (Oliveira, 1996; Greynoth, 1999). However, the interpretation of otolith annuli in tropical eels is sometimes very difficult and doubtful due to the existence of some false annuli. The. RESULTS Species composition and sizes. – A summary of species composition, sample size, mean total length, mean weight and mean age of the eels is presented in Table 1. A total of 22 eels were collected from the different sampling sites from different rivers in Northern Luzon, the Philippines. These species were Anguilla marmorata, A. bicolor bicolor and A. bicolor pacifica. The species composition of the eels differed among rivers. In the Chico River (17o37'N 121o25'E) which is approximately 100 km away from the estuary, all eels collected were identified as A. marmorata. In the Buguey River (18 o16'N 121 o50'E) and Abulog River (18 o26'N 121o26'E), respectively 9 and 3 km away from their estuaries, all the eels consisted of A. bicolor pacifica. In the Casili Creek. Fig. 1. The location of the sampling sites. A = Chico River; B = Casili Creek; X = Abulog River; L = Buguey River.. 143.
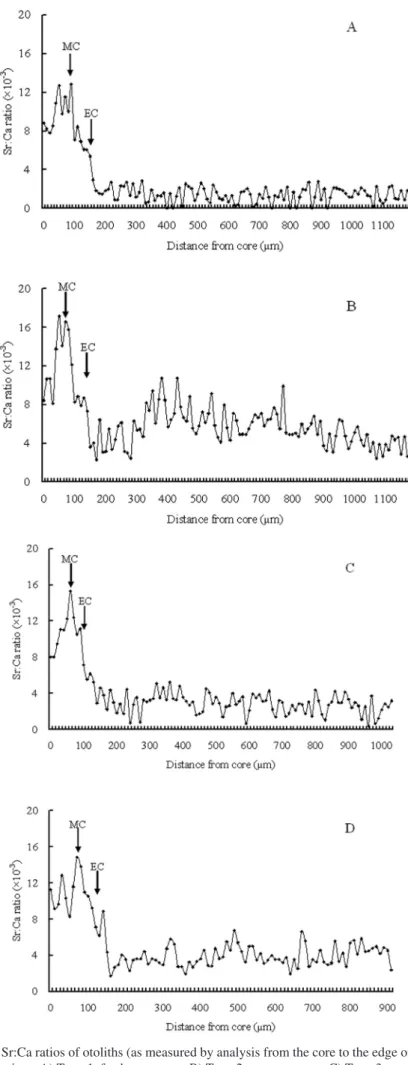
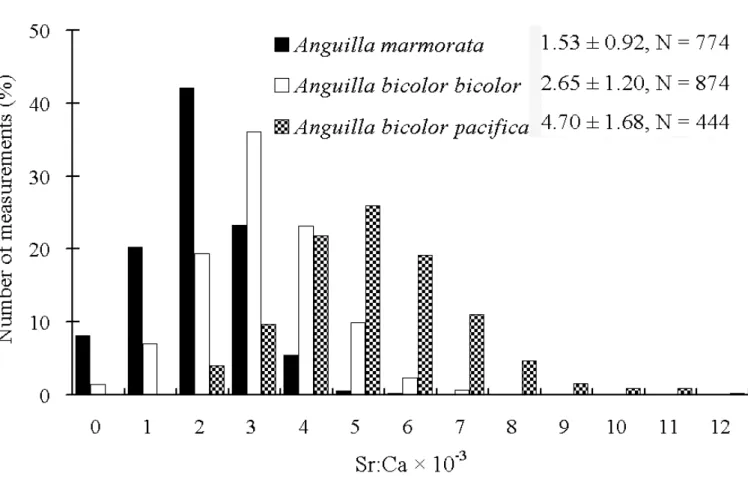
(4) Briones et al.: Migratory pattern of Anguilla eels in the Philippines Table 1. Species composition, sample size, mean total length, mean weight and mean age of Anguilla marmorata, A. bicolor bicolor and A. bicolor pacfica collected from different sampling sites. Size parameters are in the form of mean ± SD. Site. Species. A. A. marmorata. B. Sample Size. Total Length, cm (Range). Weight, g (Range). Age, years (Range). 9. 32.7 ± 5.9 (24.4 - 39.3). 113.5 ± 21.9 (84.7 - 139.2). 6.8 ± 1.6 (5 - 9). A. bicolor bicolor. 8. 42.4 ± 9.9 (32.7 - 58.8). 132.6 ± 98.5 (52 - 335). 9.0 ± 1.3 (7 - 11). X. A. bicolor pacifica. 3. 35.6 ± 5.4 (29.5 - 40.0). 108.6 ± 10.4 (100.2 - 120.3). 7.6 ± 3.1 (5 - 11). L. A. bicolor pacifica. 2. 39.8 ± 2.6 (38.0 - 41.6). 105.2 ± 21.0 (90.4 - 120.1). 4.8 ± 2.1 (7 - 10). A = Chico River, Kalinga Province; B = Casili Creek, Cagayan Province; X = Abulog River, Cagayan Province; L = Buguey River, Cagayan Province.. (18o16'N 121o41'E) which is approximately 3.5 km from the Cagayan River and 13 km away from the estuary, the eels were identified as A. bicolor bicolor. Classification of migratory pattern and habitat use. – Anguilla bicolor bicolor and A. bicolor pacifica specimens collected in the lower reaches of the river close to the estuary, had a wide range of otolith Sr:Ca ratios. The otolith Sr:Ca ratios ranged from 0 - 7 × 10-3 for A. bicolor bicolor and 2 - 12 × 10-3 for A. bicolor pacifica, respectively (Fig. 4). The wide range of otolith Sr:Ca ratios indicated that the habitat use of A. bicolor bicolor and A. bicolor pacifica comprised of fresh, brackish and marine waters. On the other hand, specimens of A. marmorata were all collected in the upper reaches, hence these eels represent the range of otolith Sr:Ca ratios of freshwater eels. Most otolith Sr:Ca ratios of A. marmorata were < 4 × 10-3, with only 6% of values > 4 × 10-3. The value of 4 × 10-3 was used to separate freshwater and brackish/sea water residency in a previous study of the same species (Shiao et al., 2003). Therefore, this same value was used to discriminate freshwater residency from brackish/ marine residency in the present study. The migratory pattern and habitat use of each eel was interpreted by examining the temporal pattern of the Sr:Ca ratio of the otoliths, which revealed the ambient salinity in marine, estuarine and freshwater environments. The migratory patterns which revealed the life histories of the eels in the Philippines were thus classified into three types: 1) Type 1, freshwater; 2) Type 2, marine; 3) Type 3a,. Fig. 2. A fish pot used to collect eel samples (fish pot is provided courtesy of Dr. Tereso A. Abella).. 144. estuarine but freshwater-favouring eels (where the Sr:Ca ratio was < 4 × 10-3 over more than half of their life span from elver stage) and Type 3b, estuarine but marine-favouring eels (where the Sr:Ca ratio was > 4 × 10-3 over more than half of their life span from elver stage). The mean Sr:Ca ratio of the otoliths was calculated from the elver check to the edge. All eel species were treated together using the same criteria due to the inadequate sample size of each individual species. We could not compare the otolith Sr:Ca ratios between upper and lower reaches within each species as A. marmorata was only found in the upper reaches while A. bicolor bicolor and A. bicolor pacifica were only found in the lower reaches near the estuaries. The differences in absorption of strontium in the otoliths among these two species were presumably very minor and are assumed not to affect the interpretation of their migratory life histories. Life history patterns as indicated by Sr:Ca ratios of the otoliths. – Changes in the Sr:Ca ratios of the otoliths of the Philippine eels are presented in Figure 5. The temporal changes in the Sr:Ca ratios of the otoliths were similar among individuals from the primordium to the elver check. This implies that the environmental histories during their marine leptocephali stage were similar. The increase in Sr:Ca ratios from approximately 8 - 12 × 10-3 at the primordium to a peak of approximately 14 - 18 × 10-3 at a distance of 50 - 100 μm from the primordium which represents the metamorphosis stage from leptocephali to glass eels (Arai et al., 1997). The Sr:Ca profiles beyond the otolith elver check to the edge revealed the migratory patterns that were diverse among species which classified the life history of eels into three types: Type 1: freshwater eel. – The mean Sr:Ca ratio of the otoliths from the elver check to the edge was 2.01 ± 0.15 × 10-3 and ranged from 1.17 to 2.89 × 10-3. Eleven eels (50 % of the total 22 samples) were classified under Type 1, with eight A. marmorata from the Chico River and three A. bicolor bicolor from Casili Creek. This indicates that the eels migrated into the freshwater immediately after their elver stage. The Sr:Ca ratios fluctuated between 0 and 3 × 10-3 along the transect line which the electron beam ran along during the processing of the otoliths. The high strontium concentrations in the core represent the marine leptocephali stage. The Sr:Ca ratios.
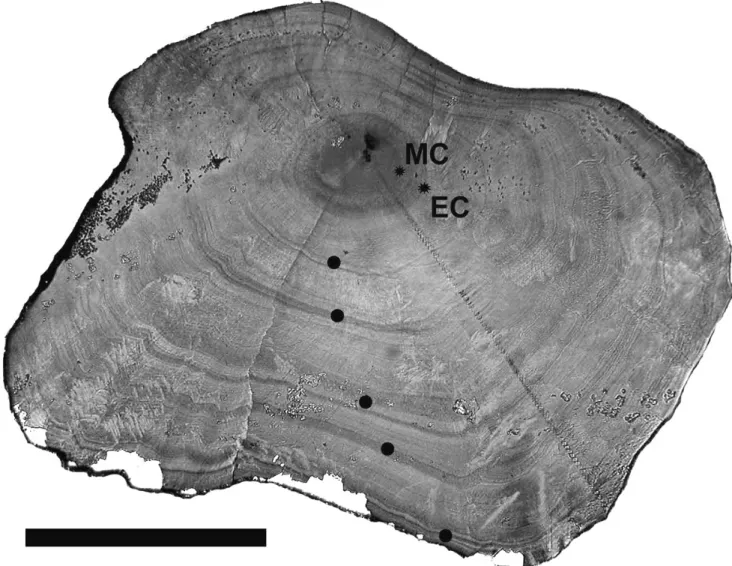
(5) THE RAFFLES BULLETIN OF ZOOLOGY 2007 suddenly decreased, indicating that the eels migrated to the estuaries (brackish water) during their elver stage. The Sr:Ca ratios continued to decrease, indicating the movement and residency of the eels in the upstream freshwater environment. Type 2: marine eel. – Two individuals (9.09% of the total 22 samples) of A. bicolor pacifica from the Abulog River were classified under Type 2 where the mean Sr:Ca concentration of the otoliths from the elver check to the otolith edge was 4.85 ± 0.95 × 10-3. After the elver stage, the Sr:Ca ratio of the otoliths decreased to 2 - 3 × 10-3, approximately 150 μm from the core, then increased to an average of 5.52 × 103 . This suggests that the eels entered the freshwater after the elver stage, then migrated back to the saline water and stayed there until their silvering stage. Type 3: estuarine contingent. – The mean Sr:Ca ratio of the eels in this group varied considerably among individuals. Type 3 eels exhibited a variety of temporal patterns in their otoliths. The eels did not have a consistent low or high Sr:Ca ratio as those of the Type 1 and 2 eels. Type 3 eels were subdivided according to the duration of their stay in the estuaries and the temporal patterns of the Sr:Ca ratios of the otoliths. Type 3a (estuarine but freshwater-favouring eels). had a Sr:Ca ratio of < 4 × 10-3 over more than half of their life span from elver stage. Type 3b (estuarine but marinefavouring eels) had Sr:Ca ratios > 4 × 10-3 over more than half of their life span from elver stage. Five individuals (22.73% of the total 22 samples) were classified under Type 3a, with one individual of A. marmorata from the Chico River and four individuals of A. bicolor bicolor from the Casili Creek. Four individuals (18.18% of the total 22 samples) were classified under Type 3b, with three A. bicolor pacifica (two from the Abulog River and one from the Buguey River) and one A. bicolor bicolor from the Casili Creek. Difference in habitat preference among species. – There was a difference in habitat preference between the species as shown in Table 2. Anguilla marmorata preferred freshwater while A. bicolor pacifica preferred a marine environment. On the other hand, A. bicolor bicolor were distributed between the freshwater and the marine environments.. DISCUSSION Reconstructing the migratory patterns and determining the habitat preference in relation to the salinity of the ambient. Fig. 3. An otolith sample from a Type 1, freshwater eel, Anguilla marmorata (31 cm TL), collected in the Chico River. *EC = elver check; *MC = metamorphosis check; dots = annuli. Scale bar = 500 μm.. 145.
(6) Briones et al.: Migratory pattern of Anguilla eels in the Philippines Table 2. Percentages of Anguilla marmorata, A. bicolor bicolor and A. bicolor pacifica having different life history patterns as inferred from otolith Sr:Ca ratios. Species. Sample Size. Life History Patterns (%) Type 1. Type 2. Type 3a. Type3b. A. marmorata. 9. 88.9. 0.0. 11.1. 0.0. A. bicolor bicolor. 8. 37.5. 0.0. 50.0. 12.5. A. bicolor pacifica. 5. 0.0. 40.0. 0.0. 60.0. Total. 22. 50.0. 9.1. 22.7. 18.2. Type 1 = freshwater contingent; Type 2 = seawater contingent; Type 3a = estuarine but freshwater-favouring contingent; Type 3b = estuarine but seawater-favouring contingent.. environment of the freshwater eels in the Philippines were made possible through the Sr:Ca ratios revealed in the otoliths. Generally, the concentration of strontium is about 100-fold greater in seawater (8.7 × 10-5 M) than in freshwater (9 × 10-7 M) (Campana, 1999). Accordingly, marine-resident fish take up and deposit more strontium in their otoliths compared to freshwater fish. Only in exceptional cases do fish living in strontium-rich freshwater absorb strontium into their otoliths to the same levels as seawater fish (Kraus & Secor 2004), but such strontium-rich freshwater fish did not occur in this study. Clearly, the migratory patterns and habitat preferences of the eels were different among species (Table. 2). For example, A. marmorata in the Philippines preferred freshwater, which is the similar case in Taiwan (Shiao et al., 2003). Elvers are freshwater-oriented stages and they move up the river during onshore migration (Tzeng et al., 2002, 2003a, 2003b). As the eels start their migration after the elver stage, the Sr:Ca ratio of the otoliths becomes < 4 × 10-3, indicating that the eels inhabit the freshwater environment (Shiao et al., 2003; Jessop et al., 2004). This is also true for the Philippine A. marmorata, where samples were collected from the upper reaches of the Chico River which corresponded to the low. Fig. 4. Frequency distribution of otolith Sr:Ca ratios measured after the elver check mark to the edge of the otolith of Anguilla marmorata, A. bicolor bicolor and A. bicolor pacifica. The mean otolith Sr:Ca ratios (± SD) and the total measurements were given in the legend.. 146.
(7) THE RAFFLES BULLETIN OF ZOOLOGY 2007 Sr:Ca concentration of the otoliths. This implies that the migratory pattern of A. marmorata involves movement from the highly-saline seawater to brackish water to a purely freshwater environment. As the Chico River is approximately 100 km away from the estuary, it is unlikely to be influenced by tidal movements. However, for A. bicolor bicolor and A. bicolor pacifica specimens, the Sr:Ca ratios of their otoliths were not consistently at the higher or lower levels, which means that the elvers did not migrate upstream but rather inhabited the estuary most of the time during their growth phase. The migratory behaviour of the Philippine eels during the yellow stage or growth phase diverged into the estuarine contingent (consisting of the freshwater-favouring and marine-favouring eels) and the seawater contingent. Both contingents were dominated by A. bicolor bicolor and A. bicolor pacifica. The habitat preference of A. bicolor bicolor and A. bicolor pacifica during their growth phases may be opportunistic and not obligatory. This has been observed in the European eels (Tzeng et al., 1997), Japanese eels (Tzeng et al., 2003a, b) and American eels (Jessop et al., 2002). This is the first study to clearly demonstrate that the tropical eels can inhabit brackish/sea water completely or move frequently between fresh- and seawater during the yellow eel growth stage. This result suggests that the ability of Anguilla spp. to reside in environments of various salinities may have existed in the ancestral species before the speciation event separating the tropical and temperate eels. Tsukamoto et al. (2002) hypothesized that the higher proportions of brackish/marine eels occur at the higher latitudes than in the subtropical to tropical latitudes. However, little is known about the proportion of the different contingents at the lower latitudes. Based on this study, A. marmorata seems to be freshwateroriented, A. bicolor bicolor brackish water-oriented and A. bicolor pacifica marine water-oriented. It is difficult to identify a simple rule that can satisfactorily explain the proportion of sympatric species and their contingents in tropical areas. The tropical rivers are usually inhabited by more than one eel species. Interspecific competition for food and space may have the strongest influence on habitat use and may mask the habitat preference of the disadvantaged species. The Philippine eels were widely distributed in freshwater, estuarine or seawater environments during their growth phase (yellow stage). Selection of the growth habitat may depend on food availability and carrying capacity of the environment. Another possible reason for the estuarine habitat preference of A. bicolor bicolor and A. bicolor pacifica is their body’s osmoregulation mechanisms. Tzeng et al. (2003a) argued that if the eels stayed in the estuaries, their osmoregulatory cost would be lower than for those in either freshwater or marine environments. The authors also determined that the salinity of the eel’s body fluid is approximately 10.5 - 14 ppt, which is close to that of brackish water. Hence, brackish water residency results in savings in the energy that would be used to maintain a homeostatic osmotic potential of the body fluid. When the elvers migrate from the offshore seawater areas to. upstream freshwater areas, they must overcome the osmotic pressure imposed by the salinity gradient in the environments. If they stayed in the estuary, their osmoregulatory cost would be lower than in the freshwater or marine environments. Also, the migration from the seawater areas to the freshwater upper reaches carries greater risks and energy costs than simply just residing in the estuaries (Shiao et al., 2003). The costs of migration include mortality resulting from the actual migration as well as the changes in environmental conditions that the fish may not be able to tolerate (specifically osmoregulatory stress in diadromous fishes) (McDowall, 1988). This may be one of the reasons why some eels (e.g., A. bicolor bicolor and A. bicolor pacifica) prefer to stay in the estuaries rather than migrate into the rivers or other freshwater environments. Anguilla marmorata is an ancient tropical species of freshwater eel that has a preference for freshwater in the growth phase which causes them to migrate upstream (Shiao et al., 2003). Interspecific competition, environmental factors, productivity of the environment and adaptation may play important roles in the freshwater habitat preference of A. marmorata, indicating that they are physically and physiologically adapted to this habitat. Declining eel stocks in the other parts of the world may be advantageous for the Philippines to become an exporter of eels. The Philippines is fortunate not to have many of the problems experienced by other countries such as waterway pollution, diseases and in particular, excessive over-harvesting of the glass eel growth stage. China, Japan and Taiwan have already begun to import eels at this growth stage from Europe, Australia and the Philippines to supplement their domestic requirement of eel seed stock. As natural stocks in Europe are declining, there have been strong calls from environmentalists and farmers to limit the export of glass eels. In addition, losses during transit are unacceptably high, thus this supply of eels is anticipated to decrease in the near future. Instead of exporting glass eels, the Philippines could add value to this commodity by growing them locally. The Philippines has an advantage over other countries with its good shipping facilities and location close to the main eel-consuming countries. Hence, a better understanding of the migratory patterns and habitat use by wild eels would provide useful information for aquaculture. In summary, the migratory pattern and habitat preference of the freshwater eels in the Philippines differed among the species, as inferred by the Sr:Ca ratios of their otoliths. They were distributed in the freshwater, brackish water and marine environments during their growth phase (yellow stage). Environmental conditions may have influenced the diverse habitat preference of A. bicolor bicolor, whereas osmoregulatory costs may have influenced the residency of A. bicolor pacifica in the estuaries. However, A. marmorata migrated to its preferred habitat in the upper reaches of freshwater rivers because of several possible reasons. Through the otolith Sr:Ca ratios and microstructure analysis, we can clearly reconstruct the life histories and the migratory patterns of the freshwater eels of the Philippines from the high-salinity. 147.
(8) Briones et al.: Migratory pattern of Anguilla eels in the Philippines. Fig. 5. Temporal changes in the Sr:Ca ratios of otoliths (as measured by analysis from the core to the edge of the otolith) from eels collected from different rivers in the Philippines. A) Type 1, freshwater type, B) Type 2, seawater type, C) Type 3a, estuarine but freshwater-favouring type, D) Type 3b, estuarine but seawater-favouring type.. 148.
(9) THE RAFFLES BULLETIN OF ZOOLOGY 2007 marine environment to the low-salinity estuarine and freshwater environments in rivers. The present study is the first to determine the migratory patterns and life histories of fish in the Philippines through the Sr:Ca ratios in their otoliths.. Kraus, R. T. & D. H. Secor, 2004. Incorporation of strontium into otoliths of an estuarine fish. Journal of Experimental Marine Biology and Ecology, 302: 85-106.. ACKNOWLEDGMENTS. Oliveira, K., 1996. Field validation of annular growth rings in the American eel Anguilla rostrata, using tetracycline-marked otoliths. Fishery Bulletin, 94: 186-189.. We thank the Office of the Student Affairs and the College of Fisheries-Freshwater Aquaculture Center, Central Luzon State University, the Philippines for providing financial assistance for travel to Taiwan. We also thank Dr. Arsenia G. Cagauan for support and assistance and Engr. Perfecto B. Garcia for assistance in the collection of eels.. LITERATURE CITED Arai, T., A. Kotake, P. M. Lokman, M. J. Miller & K. Tsukamoto, 2004. Evidence of different habitat use by New Zealand freshwater eels Anguilla australis and A. dieffenbachii, as revealed by otholith microchemistry. Marine Ecology Progress Series, 266: 213-225. Arai, T., T. Otake & K. Tsukamoto, 1997. Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Marine Ecology Progress Series, 161: 17-22. Campana, S. E., 1999. Chemistry and composition of fish otoliths: pathways, mechanism and applications. Marine Ecology Progress Series, 188: 263-297. Chang, C. W., S. H. Lin, Y. Iizuka & W.-N. Tzeng, 2004. Relationship between Sr:Ca ratios in the otolith of grey mullet Mugil cephalus and ambient salinity: validation mechanism and applications. Zoological Studies, 43: 74-85. Daverat, F., K. E. Limburg, I. Thibault, J.-C. Shiao, J. J. Dodson, F. Caron, W. -N. Tzeng, Y. Iizuka & H. Wickström, 2006. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Marine Ecology Progress Series, 308: 231-241. Ege, V., 1939. A revision of the genus Anguilla Shaw. A systematic, phylogenetic and geographical study. Dana-Report, 16(3): 1256. Farrell, J. & S. E. Campana, 1996. Regulation of calcium and strontium deposition on the otoliths of juvenile tilapia, Oreochromis niloticus. Comparative Biochemistry and Physiology, 115(A): 103-109. Graynoth, E., 1999. Improved otolith preparation, ageing and backcalculation techniques for New Zealand freshwater eels. Fishery Research, 42: 137-146. Jessop, B. M., J.-C. Shiao, Y. Iizuka & W.-N. Tzeng, 2002. Migratory behaviour and habitat use by American eel Anguilla rostrata as revealed by otolith microchemistry. Marine Ecology Progress Series, 233: 217-299. Jessop, B. M., J.-C. Shiao, Y. Iizuka & W.-N. Tzeng, 2004. Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Marine Ecology Progress Series, 272: 231-244.. McDowall, R. M., 1988. Diadromy in Fishes: Migrations Between Freshwater and Marine Environments. Timber Press, Oregon. 308 pp.. Sadovy, Y. & K. P. Severin, 1992. Trace elements in biogenic aragonite: Correlation of body growth rate and strontium levels in the otoliths of the white grunt, Haemulon plumieri (Pisces: Haemulidae). Bulletin of Marine Science, 50: 237-257. Secor, D. H., A. Henderson-Arzapalo & P. M. Piccoli, 1995. Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes? Journal of Experimental Marine Biology and Ecology, 192: 15-33. Shiao, J.-C., Y. Iizuka, C. W. Chang & W.-N. Tzeng, 2003. Disparities in habitat use and migratory behavior between tropical eel Anguilla marmorata and temperate eel A. japonica in four Taiwanese rivers. Marine Ecology Progress Series, 261: 233-242. Tesch, F. W., 2003. The Eel. Blackwell Publishing, Oxford. 408 pp. Townsend, D. W., R. L. Radtke, S. Corwin & D. A. Libby, 1992. Strontium:calcium ratios in juvenile Atlantic herring Clupea harengus otoliths as a function of water temperature. Journal of Experimental Marine Biology and Ecology, 160: 131-140. Tsukamoto, K., J. Aoyama & M. J. Miller, 2002. Migration, speciation and the evolution of diadromy in anguillid eels. Canadian Journal of Fisheries and Aquatic Science, 59: 19891998. Tzeng, W.-N., 1996. Effects of salinity and ontogenetic movements on strontium:calcium ratios in the otolith of Japanese eel Anguilla japonica Temminck and Schlegel. Journal of Experimental Marine Biology and Ecology, 199: 111-112. Tzeng, W.-N. & Y. C. Tsai, 1994. Changes in otolith microchemistry of the Japanese eel Anguilla japonica, during its migration from the ocean to the rivers of Taiwan. Journal of Fish Biology, 45: 671-683. Tzeng, W.-N., K. P. Severin & H. Wickstrom, 1997. Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Marine Ecology Progress Series, 149: 73-81. Tzeng, W.-N., J.-C. Shiao & Y. Iizuka, 2002. Use of otolith Sr:Ca ratios to study the riverine migratory behaviors of Japanese eel Anguilla japonica. Marine Ecology Progress Series, 245: 213221. Tzeng, W.-N., Y. Iizuka, J.-C. Shiao, Y. Yamada & H. P. Oka, 2003a. Identification and growth rates comparison of divergent migratory contingents of Japanese eel (Anguilla japonica). Aquaculture, 216: 77-86. Tzeng, W.-N., J.-C. Shiao, Y. Yamada & H. P. Oka, 2003b. Life history pattern of Japanese eel Anguilla japonica in Mikawa Bay, Japan. American Fisheries Society Symposium, 33: 285293.. Kalish, J. M., 1989. Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. Journal of Experimental Marine Biology and Ecology, 132: 151178.. 149.
(10)
數據
相關文件
Consistent with the negative price of systematic volatility risk found by the option pricing studies, we see lower average raw returns, CAPM alphas, and FF-3 alphas with higher
6 《中論·觀因緣品》,《佛藏要籍選刊》第 9 冊,上海古籍出版社 1994 年版,第 1
The first row shows the eyespot with white inner ring, black middle ring, and yellow outer ring in Bicyclus anynana.. The second row provides the eyespot with black inner ring
You are given the wavelength and total energy of a light pulse and asked to find the number of photons it
Understanding and inferring information, ideas, feelings and opinions in a range of texts with some degree of complexity, using and integrating a small range of reading
Wang, Solving pseudomonotone variational inequalities and pseudocon- vex optimization problems using the projection neural network, IEEE Transactions on Neural Networks 17
Hope theory: A member of the positive psychology family. Lopez (Eds.), Handbook of positive
volume suppressed mass: (TeV) 2 /M P ∼ 10 −4 eV → mm range can be experimentally tested for any number of extra dimensions - Light U(1) gauge bosons: no derivative couplings. =>