Study on Curing Kinetics and Curing Mechanism of Epoxy
Resin Based on Diglycidyl Ether of Bisphenol A and
Melamine Phosphate
Wen-Yi Chen,1 Yez-Zen Wang,2Feng-Chih Chang1
1Institute of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan
2Department of Chemical Engineering, National Yun-Lin University of Science and Technology, Yun-Lin, Taiwan
Received 22 February 2003; accepted 11 November 2003
ABSTRACT: The curing kinetics of the diglycidyl ether of bisphenol A/melamine phosphate (DGEBA/MP) was ana-lyzed by the DSC technique. The Kissinger and Flynn–Wall– Ozawa methods were applied to determine the dynamic kinetics of the DGEBA/MP system. The activation energies obtained by these two methods were 83.9 and 85.6 kJ/mol, respectively. An autocatalytic equation was applied to de-termine the isothermal curing kinetics of the DGEBA/MP system. The DGEBA/MP system exhibits autocatalytic be-havior in the isothermal curing procedure, whose kinetics fits well with the autocatalytic mechanism. The obtained
isothermal curing activation energy of the DGEBA/MP sys-tem was 110.0 kJ/mol. The curing mechanism of DGEBA with melamine phosphate was investigated using FTIR,13
C solid-state NMR, and 31
P solid-state NMR. It involved an epoxide–amine reaction, etherification of phosphoric acid and epoxy, dehydration, and thermal oxidation of the hy-droxyl group of the DGEBA/MP system.© 2004 Wiley Peri-odicals, Inc. J Appl Polym Sci 92: 892–900, 2004
Key words: epoxy resin; melamine phosphate; kinetics (polym.); FTIR; activation energy
INTRODUCTION
Epoxy resins have been widely used in coatings, ad-hesives, insulating materials, and composites because of their great versatility, low shrinkage, good chemical resistance, adhesion, and high-grade electrical insula-tion. Like most organic polymeric materials, the flam-mability of epoxy resins is rather high, thus limiting their applications in certain areas. Therefore, mel-amine phosphate is introduced herein as a curing agent and a flame retardant to improve the flame retardation of epoxy resins.
An optimal curing process depends on understand-ing the curunderstand-ing kinetics, the curunderstand-ing mechanism, and accurate modeling of the curing process. This model-ing includes determination of the mechanism, or ap-propriate kinetic equation for the analyzed system, measurement of the reaction orders, and activation energies of the reaction. An accurate model not only helps to predict curing behavior for process design and control, but also can be used to predict aging and degradation of thermosetting polymer systems. It can further be used to compare the curing behavior of different systems or formulations using different ma-trices, catalysts, fillers, and additives.
The current understanding of the mechanism and kinetics of curing lags the rapid increase in
formula-tions and the industrial applicaformula-tions of epoxy-based resins and composite systems. The mechanism and kinetics of curing are not yet comprehensively under-stood, and an accurate model of an optimal curing process has yet to be clearly established.1To date, no
study has been reported on modeling the curing of the diglycidyl ether of bisphenol A/melamine phosphate (DGEBA/MP) system.
An appropriate method must be used to measure, accurately, the cure kinetics parameters: the degree of conversion (␣), conversion rate (d␣/dt), and the acti-vation energy (E).
The rate of conversion can therefore be defined as follows: d␣ dt ⫽ dH/dt ⌬HRxn (1)
where⌬HRxnis the heat of reaction. DSC techniques
use two basic approaches: isothermal curing is carried out at a single cure temperature and at a given cure cycle, and dynamic curing is carried out at a constant heating rate.
Dynamic kinetics
Kinetic analyses were performed using two kinetic models, the Kissinger and the Flynn–Wall–Ozawa4 – 6 models. These two methods were used in this study, instead of other nonisothermal methods, because they Correspondence to: F.-C. Chang (Changfc@mail.nctu.edu.tw).
Journal of Applied Polymer Science, Vol. 92, 892–900 (2004) © 2004 Wiley Periodicals, Inc.
do not require prior knowledge of the reaction mech-anism to quantify kinetic parameters.
According to the method of Kissinger, the activation energy can be obtained from the maximum reaction rate, where d(d␣/dt)/dt is zero at a constant-heating rate condition. The resulting relation can be expressed as
d关ln共q/Tm2兲兴
d共1/Tm兲 ⫽ ⫺
E
R (2)
where Tmis the temperature at which the rate is
max-imum and q is a constant heating rate. Therefore, a plot of ln(q/Tm2) versus 1/Tmgives the activation
en-ergy without the need to make any assumption about the conversion-dependent function.
Based on Doyle’s approximation,7 Flynn–Wall– Ozawa developed an alternative method to calculate the activation energy:
log共q兲⫽log
冋
AEg共␣兲R
册
⫺2.315⫺0.457E
RT (3)
Based on this equation, a more accurate value of the activation energy can be obtained by iteration or a least-squares technique to improve the linear approx-imation of the temperature integration term.
Isothermal kinetics
The conversion and the rate of conversion vary con-tinuously throughout the process of reaction as the
uncured resin is isothermally cured. Using DSC the conversion is measured as
␣t⫽
⌬Hh
⌬HRxn
(4) where the subscript t indicates the value at time t and ⌬Hh is the heat of reaction at time t. An alternative
method is to measure the heat evolved during the crosslinking of the partially cured sample. This latter method becomes necessary when the exothermic energy is too small to be detected by the former method. A distinct advantage of the latter method is that it allows Tg and the conversion to be measured simultaneously.8,9 Using this method, the
percent-age of conversion is calculated as ␣t⫽
⌬HRxn⫺ ⌬Hr
⌬HRxn
(5) where⌬Hris residual heat of reaction. The analysis of
autocatalyzed curing reactions assumes, in contrast, that at least one of the reaction products is also in-volved in the propagation reaction characterized by an accelerating isothermal conversion rate. The kinetics of autocatalyzed reactions is described as follows8:
d␣ dt ⫽ k⬘␣
m(1⫺␣)n (6)
where m and n are the reaction orders and k⬘(T) is the specific rate constant.
The curing of the epoxy resin, with amino curing agents, involves three principal reactions: epoxide–pri-mary amine addition; epoxide–secondary amine reaction; and etherification of epoxide–hydroxyl, branching, and crosslinking, respectively.10 –15 These chemical reactions cause a complicated change in the physical state, from a viscous liquid to a gel, and even-tually vitrify the material. FTIR and NMR were used to characterize chemical reactions of the epoxy resin.
The kinetics and mechanism of curing DGEBA with melamine phosphate have not yet been reported. This
study reports that the melamine phosphate is able to function as a hardener and a flame retardant of the epoxy resin.
EXPERIMENTAL Materials
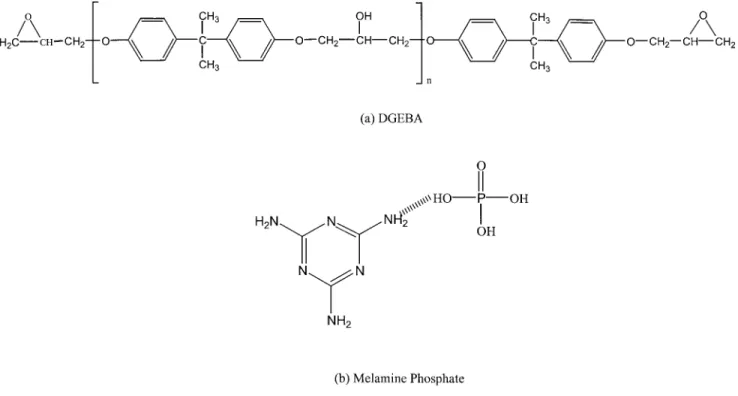
The liquid epoxy resin used in this study was a dig-lycidyl ether of bisphenol A (BE-188) from Chang Chun Chemical Plastics of Taiwan with an equivalent
Figure 2 Typical dynamic DSC thermogram of DGEBA/MP.
Figure 3 Activation energy of DGEBA/MP obtained by the Kissinger method and the Flynn–Wall–Ozawa method.
epoxy molecular weight of 188 g/eq. The hardener used was melamine phosphate (MP, C3N6H3䡠H3PO4),
also from Chung-Shan Institute of Science and Tech-nology, Taiwan. Figure 1 presents the chemical struc-tures of these compounds.
Dynamic-curing kinetics
A stoichiometric ratio of epoxy to amine was used in this DGEBA/MP system. The dynamic curing kinetics of the DGEBA/MP system was determined using a DuPont 2910 differential scanning calorimeter (Du-Pont, Boston, MA) operated in a nitrogen atmosphere. The sample weight of 6 –7 mg was placed in a sealed aluminum sample pan. A dynamic curing reaction
scan was conducted from 30 to 350°C at a heating rate of 5, 10, 15, or 20°C/min and a nitrogen flow rate of 40 mL/min.
Isothermal-curing kinetics
Isothermal curing was conducted at four different temperatures: 200, 210, 220 and 230°C.
FTIR
The sample was placed on a KBr pellet and FTIR spectrum was obtained at 220°C using a Nicolet Ava-tar 320 FTIR (Nicolet Analytical Instruments,
Madi-Figure 4 Typical isothermal DSC thermograms of DGEBA/MP at various curing temperatures.
son, WI), to analyze the epoxide content of the system at a resolution of 2 cm⫺1.
Solid-state NMR
High-resolution solid-state 13C-NMR and 31P-NMR
experiments were carried out at room temperature using a Bruker DSX-400 spectrometer (Bruker Instru-ments, Billerica, MA) operated at resonance frequen-cies of 399.53–100.47 and 399.53–161.72 MHz for 13C
and31P, respectively. The13C and31P CP/MAS
spec-tra were measured with a 3.9-s 90° pulse, with 3-s pulse delay time, acquisition time of 30 ms, and 2048 scans. All NMR spectra were taken at 300 K using broadband proton decoupling and normal cross-po-larization pulse sequencing. A magic angle sample spinning (MAS) rate of 5.4 kHz was used to eliminate resonance broadening attributed to the anisotropy of the chemical shift tensors.
RESULTS AND DISCUSSION Dynamic kinetics
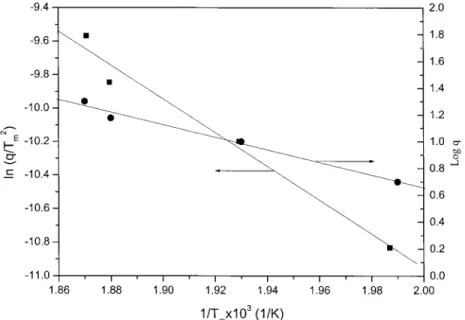
Both Kissinger and Flynn–Wall–Ozawa methods as-sume that the DSC peak exotherm is isoconversion and its value is independent of the heating rate. These two methods were applied to the data obtained in the dynamic heating experiments at different heating rates between 5 and 20°C/min used in this work. Figure 2 shows the heat flow measured by DSC cured under various heating rates. The temperature of max-imum rate increases from 229.9 to 260.8°C with the increase of the heating rate.
By applying the Flynn–Wall–Ozawa and Kissinger methods of the maximum reaction rates (peaks of the DSC thermogram), activation energies can be deter-mined by slopes of the lines shown in Figure 3. The obtained activation energies, based on the Kissinger and Flynn–Wall–Ozawa methods, are 83.9 and 85.6 kJ/mol, respectively. The activation energy, obtained by the Flynn–Wall–Ozawa method, is only slightly higher than that obtained by the Kissinger method, and the difference is insignificant.16
Isothermal kinetics
The kinetics of epoxy–amine reactions has been well established in the literature.17–21In the DSC curve of
dynamic curing for the DGEBA/MP system, at a heat-ing rate of 10°C/min as shown in Figure 2, Ti(199.2°C) is the initial temperature of the curing reaction, Tp (244.8°C) is the peak maximum temperature, and Tf (276.4°C) is the final temperature, respectively. Based on this result, this study used isothermal curing tem-peratures of 200, 210, 220, and 230°C.
Figure 6 13
C solid-state NMR of (A) melamine phosphate and (B) DGEBA cured with melamine phosphate.
TABLE I
Data of Isothermal Curing of DGEBA/MP for Various Curing Temperatures Curing temperature (°C) Reaction rate constant (K, min⫺1) m n m⫹ n 200 0.13 0.97 0.87 1.84 210 0.31 1.05 1.48 2.53 220 0.84 1.19 1.99 3.18 230 3.66 1.48 3.20 4.68 Average reaction order 1.17 1.88 3.05 Activation energy (Ea, kJ mol⫺1) 110.0
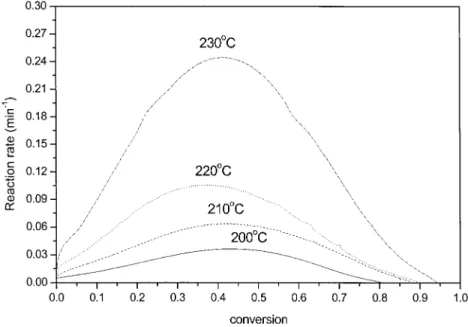
Figure 4 shows the typical isothermal DSC thermo-grams of the DGEBA/MP system. The maximum times, for the reaction peaks at curing temperatures of 200, 210, 220, and 230°C, are 29.8, 16.9, 9.9, and 5.7 min, respectively. The reaction rate at any temperature increases with time and passes through a maximum. The maximum reaction peak becomes higher, and less time is needed, as the isothermal temperature is in-creased. Figure 5 plots the reaction rate versus conver-sion of the DGEBA/MP system. This result indicates that the curing reaction of the DGEBA/MP system is strongly autocatalytic in nature and thus eq. (6) is applicable. Table I shows kinetic parameters obtained for DGEBA cured with MP. The reaction orders (n ⫹
m) of isothermal curing at 200, 210, 220, and 230°C
obtained from eq. (6) are 1.84, 2.53, 3.18, and 4.68, respectively. The R2coefficient of fitted data is 0.998. Many previous studies have assumed that the overall reaction order is 2 for the epoxy–amine reaction of the epoxy/amine system, at a relatively lower curing tem-perature, and that etherification and homopolymer-ization reactions could be neglected.22–25Other studies
have also reported that the reaction order (n⫹ m) of the epoxy–amine reaction was near 2 under isother-mal curing.26,27In this study, the overall order of the curing reaction of the DGEBA/MP system at 200°C is 1.84. Therefore, the DGEBA/MP system undergoes an epoxy–amine reaction at low curing temperature.
Figure 2 shows that the initial curing temperature (Ti) of the DGEBA/MP system was 199.2°C. Therefore,
it is similar to that of isothermal curing at 200°C for the DGEBA/MP system. At 210 and 220°C, the overall curing reaction orders of the DGEBA/MP system are 2.53 and 3.18, respectively. The etherification of an epoxide ring and a hydroxyl group forms an ether linkage, which becomes significant at high curing tem-perature in certain epoxy resin/amine systems. The importance of this reaction, in terms of its effect on the network, was previously reported.28,29 Many studies have assumed that the overall reaction order was 3 for etherification of the epoxy/amine system at later stages of curing and at a high curing temperature.30 –32 The literature indeed demonstrated that the reaction order of etherification is near 3.31The obtained overall
Figure 7 31
P solid-state NMR of (A) melamine phosphate and (B) DGEBA cured with melamine phosphate.
curing reaction order of the DGEBA/MP system was 3.18, close to 3, at 220°C. Therefore, at a high curing temperature of 220°C, the DGEBA/MP system exhib-ited etherification between the epoxide ring and the hydroxyl group. At 230°C, the overall curing reaction order of the DGEBA/MP system was 4.68.
The reaction rate constants at isothermal-cure tem-peratures of 200, 210, 220, and 230°C were 0.13, 0.31, 0.84, and 3.66 min⫺1, respectively. The reaction rate constant increased with increasing isothermal
temper-ature and the activation energy of isothermal curing was 110.0 kJ/mol.
Curing mechanism
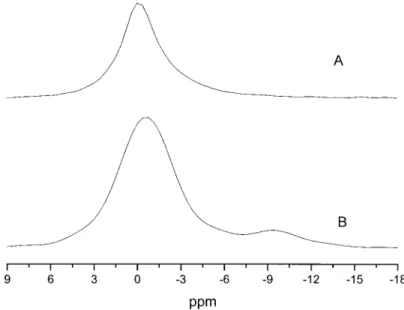
The curing mechanism of the epoxy/amine system was characterized by NMR and FTIR. Figure 6 shows the13C solid-state NMR spectrum of the mel-amine phosphate. The absorption peaks of the –NACON– group, and its hydrogen bonding with
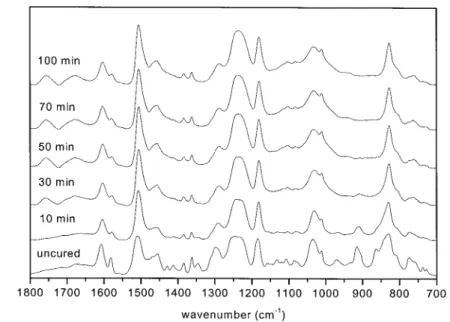
Figure 8 FTIR spectra, at various times, of DGEBA/MP at 220°C.
Scheme 2 Epoxide–amine reaction of DGEBA/MP and DGEBA/melamine.
hydroxyl, are at 157 and 164 ppm, respectively. Figure 6 shows the13C solid-state NMR spectrum of DGEBA cured with melamine phosphate, at 220°C for 100 min, where the peak at 164 ppm almost disappears, implying that most melamine phos-phate is decomposed into melamine and phosphoric acid as shown in Scheme 1. Figure 7(A) shows the
31P solid-state NMR spectrum of the melamine
phosphate and the DGEBA cured with melamine phosphate where the absorption peak of phosphoric acid is at 0 ppm.
For the amine– epoxide reaction, Figure 8 shows the IR absorption spectra of the initial (uncured) and after heat treatments at 220°C for various time periods. A comparison of these spectra, for curing at 220°C, re-veals that the stretching vibration band of the epoxy ring at 915 cm⫺1decreases and shifts downward. At the same time, the absorption peak of the NH2group
at 774 cm⫺1also decreases. These results are related to the epoxide–amine reaction for the epoxy/amine sys-tem. Scheme 2 presents the mechanism of the ep-oxide–amine reaction. Figure 9 shows the 13C solid-state NMR spectrum of DGEBA cured with melamine at 220°C for 100 min, which is essentially the same as the DGEBA cured with melamine phosphate. There-fore, the epoxide–amine reaction of the DGEBA/MP
system indeed occurs between melamine and the ep-oxy.
Figure 8 shows that the absorption peak of the –POOOC– group at 1016 cm⫺1increases with curing time, implying that the DGEBA/MP system under-goes etherification during curing. Figure 7(B) shows the 31P solid-state NMR spectrum of DGBEA cured with melamine phosphate at 220°C, with major and minor peaks at 0 and⫺9 ppm, respectively. The major peak corresponds to the phosphoric content, whereas the minor peak is attributed to P(AO)(OC) groups. Scheme 3 shows the mechanism of the etherification of the DGEBA/MP system.
For dehydration and thermal oxidation, Figure 8 shows that the absorption peak, of the –CAC– group at 1670 cm⫺1, increases with curing time because of dehydration of the DGEBA/MP system occurring during later stages of curing,33as shown in Scheme 4. The absorption peak, at 1757 cm⫺1as shown in Figure 8, is the ketone group resulting from the thermal oxi-dation of the hydroxyl group in the DGEBA/MP sys-tem occurring in the later stages of curing, as shown in Scheme 5. The area of this ketone peak increases with curing time, as shown in Figure 8. The IR spectra are able to demonstrate that dehydration and thermal ox-idation of the hydroxyl group of the DGEBA/MP
Scheme 3 Etherification of DGEBA/H3PO4.
Figure 9 13
system occur simultaneously in the later stage of cur-ing.
CONCLUSIONS
The Kissinger and Flynn–Wall–Ozawa methods were
applied in defining dynamic kinetics of the
DGEBA/MP system. The activation energies, obtained by these two methods, are 83.9 and 85.6 kJ/mol, respectively. For isothermal-curing kinetics, the DGEBA/MP system has an autocatalytic behavior and the isothermal-cure activation energy is 110.0 kJ/mol. The curing mechanism of the DGEBA/MP system involves the epoxide–amine reaction, etherification of phosphoric between phosphoric acid and epoxy, de-hydration, and thermal oxidation of the hydroxyl group of the DGEBA/MP system.
References
1. Boey, F. Y. C.; Qiang, W. Polymer 2000, 41 2081. 2. Richardson, M. J. Pure Appl Chem 1992, 64, 1789.
3. Richardson, M. J. In: Clorimetry and Thermal Analysis of Poly-mers; Matho, V. B. F., Ed.; Hanser: New York, 1993; p. 91. 4. Kissinger, H. E. Anal Chem 1957, 29, 1702.
5. Flynn, J. H.; Wall, L. A. J Res Natl Bur Stand Part A Phys Chem 1996, 70A, 487.
6. Ozawa, T. Bull Chem Soc Jpn 1965, 38, 1881. 7. Nam, J.; Seferis, J. C. J Appl Polym Sci 1993, 50, 1555. 8. Farris, R. J.; Lee, C. Polym Eng Sci 1983, 23, 586. 9. Fava, R. A. Polymer 1968, 9, 137.
10. Kamal, M. R. Polym Eng Sci 1974, 14, 23.
11. Dusek, L. Adv Polym Sci 1986, 1, 78.
12. Wang, X.; Gillham, J. K. J Appl Polym Sci 1991, 43, 2267. 13. Kreibich, U. T.; Schmid, R. J Polym Sci Part C 1975, 53, 177. 14. Dusek, K.; Plestil, J.; Lednicky, F. Polymer 1978, 19, 393. 15. Keenan, J. D.; Seferis, J. C. J Appl Polym Sci 1979, 24, 2375. 16. Munns, T. E.; Seferis, J. C. J Appl Polym Sci 1983, 28, 2227. 17. Serier, A.; Pascault, J. P.; L. T. My, J Polym Sci Part A: Polym
Chem 1991, 29, 209.
18. Li, Y. S.; Li, M. S.; Chang, F. C. J Polym Sci Part A: Polym Chem 1999, 37, 3614.
19. Barral, L.; Cano, J.; Lopez, J.; Lopez-Bueno, I.; Nogueira, P.; Abad, M. J.; Ramirez, C. J Polym Sci Part B: Polym Phys 2000, 38, 351.
20. Lin, Y. G.; Sautereau, H.; Pascault, J. P. J Polym Sci Part A: Polym Chem 1986, 24, 2171.
21. Barral, L.; Cano, J.; Lopez, J.; Lopez-Bueno, I.; Nogueira, P.; Abad, M. J.; Ramirez, C. Polymer 2000, 41, 2657.
22. Mijovic, J.; Kim, J. H.; Slaby, J. J Appl Polym Sci 1984, 29, 1449.
23. Moroni, A.; Mijovic, J.; Pearce, E.; Foun, C. C. J Appl Polym Sci 1986, 32, 3761.
24. Mijovic, J.; Wang, H. T. J Appl Polym Sci 1989, 37, 2661. 25. Ryan, M. E.; Dutta, A. Polymer 1979, 20, 203.
26. Khanna, U.; Chanda, M. J Appl Polym Sci 1993, 49, 319. 27. Stefani, P. M.; Moschiar, S. M.; Aranguren, M. I. J Appl Polym
Sci 2001, 79, 1771.
28. Bokare, U. M.; Ghandi, S. K. J Polym Sci Part A: Polym Chem 1980, 18, 857.
29. Duesk, K. Polym Bull 1985, 13, 321.
30. Xu, L. S.; Schlup, J. R. J Appl Polym Sci 1998, 67, 895. 31. Liu, Y. F.; Zhao, M.; Shen, S. G.; Gao, J. G. J Appl Polym Sci 1998,
70, 1991.
32. Bonnaus, L.; Pascault, J. P.; Sautereau, H. Eur Polym Mater 2000, 36, 1313.
33. Kim, B. S.; Inoue, T. Polymer 1995, 36, 1985.
Scheme 5 Thermal oxidation of DGEBA/MP.
Scheme 4 Dehydration of DGEBA/MP.