Date 2015/August/26
Type of manuscript: Original article
New title: Association of Meloxicam use with the risk of acute pancreatitis: A Case-control study Running head: meloxicam and acute pancreatitis
Authors' full names:
Shih-Wei Lai MD 1,2, Cheng-Li Lin MS 1,3, Kuan-Fu Liao MD and MS 4,5,6 1College of Medicine, China Medical University and 2Department of Family
Medicine, China Medical University Hospital, Taichung, Taiwan
3Management Office for Health Data, China Medical University Hospital, Taichung,
Taiwan
4College of Medicine, Tzu Chi University, Hualien, Taiwan
5Department of Internal Medicine, Taichung Tzu Chi General Hospital, Taichung,
Taiwan
6Graduate Institute of Integrated Medicine, China Medical University
Corresponding author: Kuan-Fu Liao, Department of Internal Medicine, Taichung Tzu Chi General Hospital, No.66, Sec. 1, Fongsing Road, Tanzi District, Taichung City, 427, Taiwan
Phone: 886-4-2205-2121; Fax: 886-4-2203-3986 E-mail: kuanfuliaog@gmail.com
ABSTRACT
Background and Objective. No sufficient research focuses on the relationship between meloxicam use and acute pancreatitis. The study aimed to explore this issue in Taiwan. Methods. This case-control study was conducted using the database of the Taiwan National Health Insurance Program. In all, there were 6780 cases aged 20-84 years who were newly diagnosed with acute pancreatitis during the period of 1998-2011 and 21393 controls without acute pancreatitis. Both cases and controls were matched with sex, age, and comorbidities. The odds ratio and 95% confidence interval were measured to explore the association between acute pancreatitis and meloxicam use and comorbidities using the multivariable unconditional logistic regression model. Results. After controlling for potential confounding factors, the adjusted odds ratio of acute pancreatitis was 1.76 for subjects with current use of meloxicam (95% confidence interval 1.30, 2.40), as compared with subjects who never used meloxicam. The adjusted odds ratio decreased to 1.29 for subjects with late use of meloxicam (95% confidence interval 0.82, 2.03), but without statistical significance. Conclusions. Current use of meloxicam is associated with increased odds of acute pancreatitis. Clinicians should consider the potential risk of acute pancreatitis when prescribing meloxicam.
Keywords: acute pancreatitis; meloxicam; Taiwan National Health Insurance Program
Bulleted points
1. Current use of meloxicam is associated with increased odds of acute pancreatitis. 2. Meloxicam might have a unique effect on risk of acute pancreatitis, even in
INTRODUCTION
Meloxicam is one of nonsteroidal anti-inflammatory drugs and it is now classified as a new preferential cyclooxygenase-2 (COX-2) inhibitor. It is commonly used to treat osteoarthritis and rheumatoid arthritis with markedly clinical effects. Meloxicam is well tolerated with less gastrointestinal side effects including bleeding, ulceration and perforation, as compared with traditional nonsteroidal anti-inflammatory drugs. Other main adverse events of meloxicam include peripheral edema and hypertension. Although no case of acute pancreatitis was reported to be possibly related to
meloxicam use, but the U.S. Food and Drug Administration has published that among 9080 people taking meloxicam having side effects since 2000 to 2012, 55 people (0.61%) had pancreatitis. In a case-control study in Denmark, the authors found that non-steroidal anti-inflammatory drugs were significantly associated with increased odds of acute pancreatitis, but meloxicam was not studied.
To date, no sufficient background information to hypothesize that there might be a link between meloxicam use and acute pancreatitis. However, on the basis of the U.S. Food and Drug Administration report, we think there could be a relationship between meloxicam use and acute pancreatitis. We therefore conducted a population-based case-control study using the database of the Taiwan National Health Insurance Program to explore whether there is a relationship between meloxicam use and acute pancreatitis.
METHODS
Design and study population
A population-based case-control study was conducted analyzing the database of the Taiwan National Health Insurance Program. This program began in March 1, 1995. It has covered about 99% of the whole 23 million citizens living in Taiwan.
The details of the program were well written in previous papers. This study was approved by the Ethics Review Board of China Medical University and Hospital in Taiwan (CMUH-104-REC2-115).
Study subjects
Cases were subjects aged 20-84 years who were newly diagnosed with acute
pancreatitis according to International Classification of Diseases 9th Revision Clinical
Modification (ICD-9 code 577.0) during the period of 1998-2011. The index date for each case was the date of diagnosing acute pancreatitis. Control subjects without acute pancreatitis were randomly selected from the same database. Both cases and controls were matched with sex, age (per 5 years), and comorbidities. The index date for controls was randomly appointed a month and day with the same index year of the matched acute pancreatitis cases. Subjects who had chronic pancreatitis (ICD-9 code 577.1) or pancreatic cancer (ICD-9 code 157) before the date of diagnosing acute pancreatitis were excluded from the study. The underlying comorbidities before the date of diagnosing acute pancreatitis were included as follows: alcohol-related disease, biliary stone, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, hypercalcemia, hypertriglyceridemia, as well as cardiovascular disease including coronary artery disease, heart failure, cerebrovascular disease and peripheral atherosclerosis. Based on ICD-9 codes, the diagnosis accuracy of comorbidities included has been written in previous studies. History of prescriptions for other cyclooxygenase-2 inhibitors available in Taiwan was also included in the study.
Definition of meloxicam exposure
According to the prescription history, we can estimate the last remaining one tablet of meloxicam. To reduce biased results, subjects whose last remaining one tablet of
meloxicam was noted > 1 month before the date of diagnosing acute pancreatitis were excluded from the study. Current use of meloxicam was defined as subjects whose last remaining one tablet of meloxicam was noted ≤ 14 days before the date of diagnosing acute pancreatitis or those still having meloxicam tablets at the date of diagnosing acute pancreatitis. Late use of meloxicam was defined as subjects whose last remaining one tablet of meloxicam was noted between 15-30 days before the date of diagnosing acute pancreatitis. Subjects who never had meloxicam prescription were classified as never use of meloxicam.
Statistical analysis
The distributions of sex, age, medication use and comorbidities were compared between the cases and the controls using the Chi-square test for categorized variables and t-test for continuous variables. Variables found to be significant in the univariable unconditional logistic regression model were then included in the multivariable unconditional logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were measured to explore the association between acute pancreatitis and meloxicam use and comorbidities. All data processing and statistical analyses were performed with the SAS software version 9.2 (SAS Institute, Inc., Cary, North Carolina, USA). A two-tailed P value < 0.05 was considered statistically significant. RESULTS
Characteristics of the study population
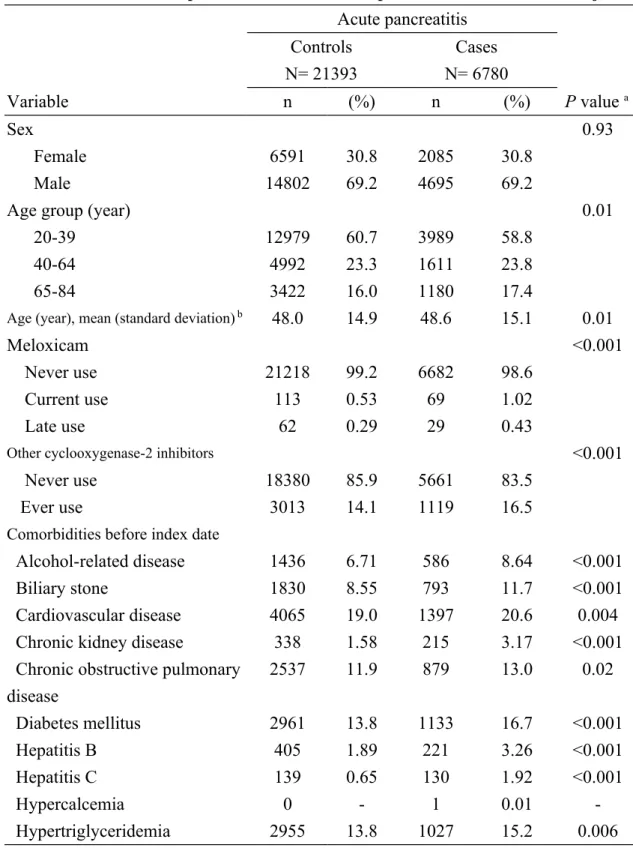
Table 1 demonstrates the characteristic profiles between the cases and the controls. There were 6780 cases of acute pancreatitis and 21393 controls with similar distributions of sex and age. The cases were likely to have higher proportions of current use of meloxicam, late use of meloxicam, ever use of other cyclooxygenase-2 inhibitors, alcohol-related disease, biliary stone, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B,
hepatitis C, and hypertriglyceridemia than the controls, with statistical significance (Chi-square test, P < 0.05 for all). The mean ages (standard deviation) were 48.6 (15.1) years in the cases and 48.0 (14.9) years in the controls, without statistical significance (t-test, P = 0.01).
Association between acute pancreatitis and meloxicam use
After controlling for age, other cyclooxygenase-2 inhibitors, alcohol-related disease, biliary stone, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, and
hypertriglyceridemia, the multivariable unconditional logistic regression model demonstrated that the adjusted odds ratio of acute pancreatitis was 1.76 for subjects with current use of meloxicam (95% confidence interval 1.30, 2.40), as compared with subjects with never use of meloxicam. The adjusted odds ratio decreased to 1.29 for subjects with late use of meloxicam (95% confidence interval 0.82, 2.03), but without statistical significance (Table 2).
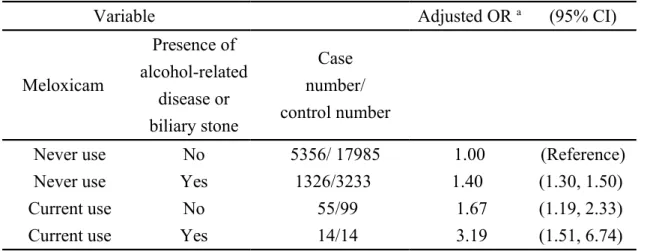
Association of acute pancreatitis stratified by current use of meloxicam and alcohol-related disease and biliary stone
After controlling for age, other cyclooxygenase-2 inhibitors, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, and hypertriglyceridemia, as a reference of subjects with never use of meloxicam and without alcohol-related disease and biliary stone, the adjusted odds ratio of acute pancreatitis was 1.67 in those with current use of
meloxicam and without alcohol-related disease and biliary stone (95% CI 1.19, 2.33). The adjusted odds ratio of acute pancreatitis was 1.40 in those with never use of meloxicam and with alcohol-related disease or biliary stone (95% CI 1.30, 1.50). The adjusted odds ratio of acute pancreatitis increased to 3.19 in those with current
use of meloxicam and with alcohol-related disease or biliary stone (95% CI 1.51, 6.74) (Table 3).
DISCUSSION
In this retrospective analysis based on a healthcare database, we found that current use of meloxicam was associated with increased odds of acute pancreatitis (adjusted OR 1.76), but late use of meloxicam was not associated with acute pancreatitis. These results indicate that only people currently taking meloxicam could have the risk of acute pancreatitis. Those who once took meloxicam but are not taking it now might not have the risk. As we know, alcoholism and biliary stone are two most common causes of acute pancreatitis. In order to reduce biased results, we tested the
association of acute pancreatitis stratified by current use of meloxicam and alcohol-related disease and biliary stone. Table 3 demonstrates that even in absence of alcohol-related disease and biliary stone, current use of meloxicam alone still was associated with increased odds of acute pancreatitis (adjusted OR 1.67). These results indicate that meloxicam might have a unique effect on risk of acute pancreatitis.
To date, no case of acute pancreatitis was reported to be possibly related to
meloxicam use. No systematic study focuses on the relationship between meloxicam use and acute pancreatitis. We cannot compare our results with others. Only The U.S. Food and Drug Administration has reported this kind of adverse event, but without mentioning the causal-effect relationship. Similarly, the underlying mechanism between meloxicam use and acute pancreatitis cannot be completely determined in this observational study. A further research is needed to clarify this issue.
Although this is the first population-based case-control study to explore the relationship between meloxicam use and acute pancreatitis in Taiwan, there are several critical limitations, particularly those concerning data analysis and
date of diagnosing acute pancreatitis may not be the day of this disease onset, the possibility of meloxicam use to treat acute pancreatitis-related pain cannot be completely excluded. There could be a chance for protopathic bias. A prospective study or case report is necessary to clarify the causal-effect relationship. Second, because the indications for meloxicam prescription were not addressed in this study, we were not sure whether the underlying indications were associated with acute pancreatitis. Therefore, meloxicam use can only be regarded as increased relative risk, not absolute risk of acute pancreatitis. Third, according to the reported data from the U.S. Food and Drug Administration, the likelihood of meloxicam-related acute pancreatitis was quite low, accounting for only 0.61% among patients having meloxicam-related side effects. Meloxicam-related acute pancreatitis may be not an important and interesting clinical issue. It is hard to convince the readers of the applicability of our data because no case was reported for meloxicam-related acute pancreatitis. Furthermore, we really detect the association between meloxicam use and acute pancreatitis. These results can provide the undated evidence for this issue. Forth, the cases had higher proportions of alcohol-related disease, biliary stone, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, and hypertriglyceridemia than the controls, but these underlying comorbidities seem to be not the indications for
meloxicam prescription. Therefore, the association between meloxicam use and acute pancreatitis cannot be confounded by these underlying comorbidities. Fifth, the exact causes of acute pancreatitis were not documented in this database due to the natural limitation. Therefore, we were not sure how many patients of acute pancreatitis were really caused by meloxicam use. Sixth, to date, more than 500 drugs are listed in the World Health Organization (WHO) database to be potentially associated with acute
pancreatitis. Therefore, it appears to be very difficult to include all offending drugs for analysis. Seventh, one study revealed that about 2% of acute pancreatitis cases could be associated with the procedure of iatrogenic endoscopic retrograde cholangiopancreatography (ERCP). Whether patients included were admitted for undergoing therapeutic ERCP to treat biliary-related causes or were admitted for other causes but complicated by iatrogenic ERCP can not be completely determined due to no detailed records in this database. Therefore, the cause-effect relationship between ERCP and acute pancreatitis can not be definitely revealed in this study. That is why ERCP was not included for analysis.
We conclude that current use of meloxicam is associated with increased odds of acute pancreatitis. Clinicians should consider the potential risk of acute pancreatitis when prescribing meloxicam.
Acknowledgement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Tseng-Lien Lin Foundation in Taichung in Taiwan, Taiwan Brain Disease Foundation in Taipei in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Specific author contributions
Shih-Wei Lai substantially contributed to the conception of the article. He planned and conducted this study. He initiated the draft of the article and critically revised this article.
Cheng-Li Lin conducted the data analysis and critically revised the article. Kuan-Fu Liao planned and conducted this study. He participated in the data interpretation and also critically revised the article.
REFERENCES
1. Barner A. Review of clinical trials and benefit/risk ratio of meloxicam. Scand J Rheumatol Suppl. 1996;102:29-37.
2. Fleischmann R, Iqbal I, Slobodin G. Meloxicam. Expert Opin Pharmacother. 2002 Oct;3(10):1501-12.
3. eHealthMe study from FDA and social media reports. Review: could meloxicam cause pancreatitis? http://www.ehealthme.com/print/ds15323511. [cited in 2014 December].
4. Sorensen HT, Jacobsen J, Norgaard M, Pedersen L, Johnsen SP, Baron JA. Newer cyclo-oxygenase-2 selective inhibitors, other non-steroidal anti-inflammatory drugs and the risk of acute pancreatitis. Aliment Pharmacol Ther. 2006 Jul
1;24(1):111-6.
5. National Health Insurance Research Database. Taiwan.
http://nhird.nhri.org.tw/en/Background.html [cited in 2014 December].
6. Lai SW, Muo CH, Liao KF, Sung FC, Chen PC. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol. 2011 Sep;106(9):1697-704.
7. Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012 Apr;27(4):709-13.
8. Lai SW, Liao KF, Lin CL, Chen PC. Pyogenic liver abscess correlates with increased risk of acute pancreatitis: a population-based cohort study. J Epidemiol. 2015;25(3):246-53.
9. Lai SW, Lai HC, Lin CL, Liao KF, Tseng CH. Chronic osteomyelitis correlates with increased risk of acute pancreatitis in a case-control study in Taiwan. Eur J Intern Med. 2015 Jun 7.
10. Nitsche C, Maertin S, Scheiber J, Ritter CA, Lerch MM, Mayerle J. Drug-induced pancreatitis. Curr Gastroenterol Rep. 2012 Apr;14(2):131-8.
11. Bugdaci MS, Oztekin E, Kara E, Koker I, Tufan A. Prognostic value of increased B type natriuretic peptide in cases with acute pancreatitis. Eur J Intern Med. 2012 Jun;23(4):e97-e100.
Table 1. Characteristic profiles of cases of acute pancreatitis and control subjects Acute pancreatitis Controls N= 21393 Cases N= 6780 Variable n (%) n (%) P value a Sex 0.93 Female 6591 30.8 2085 30.8 Male 14802 69.2 4695 69.2
Age group (year) 0.01
20-39 12979 60.7 3989 58.8
40-64 4992 23.3 1611 23.8
65-84 3422 16.0 1180 17.4
Age (year), mean (standard deviation) b 48.0 14.9 48.6 15.1 0.01
Meloxicam <0.001
Never use 21218 99.2 6682 98.6
Current use 113 0.53 69 1.02
Late use 62 0.29 29 0.43
Other cyclooxygenase-2 inhibitors <0.001
Never use 18380 85.9 5661 83.5
Ever use 3013 14.1 1119 16.5
Comorbidities before index date
Alcohol-related disease 1436 6.71 586 8.64 <0.001
Biliary stone 1830 8.55 793 11.7 <0.001
Cardiovascular disease 4065 19.0 1397 20.6 0.004
Chronic kidney disease 338 1.58 215 3.17 <0.001
Chronic obstructive pulmonary disease 2537 11.9 879 13.0 0.02 Diabetes mellitus 2961 13.8 1133 16.7 <0.001 Hepatitis B 405 1.89 221 3.26 <0.001 Hepatitis C 139 0.65 130 1.92 <0.001 Hypercalcemia 0 - 1 0.01 -Hypertriglyceridemia 2955 13.8 1027 15.2 0.006
Data are presented as the number of subjects in each group, with percentages given in parentheses, or mean with standard deviation given in parentheses.
Table 2. Crude and adjusted odds ratio and 95% confidence interval of acute pancreatitis associated with meloxicam use
Crude Adjusted a
Variable OR (95%CI) OR (95%CI)
Meloxicam (never use as a reference)
Current use 1.94 (1.44, 2.62) 1.76 (1.30, 2.40)
late use 1.49 (0.96, 2.31) 1.29 (0.82, 2.03)
a Variables found to be significant in the univariable unconditional logistic regression model
were then included in the multivariable unconditional logistic regression model.
Additionally controlling for age, other cyclooxygenase-2 inhibitors, alcohol-related disease, biliary stone, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, and hypertriglyceridemia
Table 3. Association of acute pancreatitis stratified by current use of meloxicam and alcohol-related disease and biliary stone
Variable Adjusted OR a (95% CI)
Meloxicam Presence of alcohol-related disease or biliary stone Case number/ control number
Never use No 5356/ 17985 1.00 (Reference)
Never use Yes 1326/3233 1.40 (1.30, 1.50)
Current use No 55/99 1.67 (1.19, 2.33)
Current use Yes 14/14 3.19 (1.51, 6.74)
a Controlling for controlling for age, other cyclooxygenase-2 inhibitors, cardiovascular
disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hepatitis B, hepatitis C, and hypertriglyceridemia