Journal of Cluster Science, Vol. 8, No. 1, 1997
New Electron-Deficient Alkene and Alkyne
Derivatives of Rus(ltt5-C)(CO)l 5" The Syntheses and
Crystal Structure Analyses of Rus(las-C)(CO)13
[C2 H2(CO2Me)2] and Rus(p:C)(CO)15
[C2(CO2Me)2]
Ching-Juh Way, I Yun Chi, 1"3 lpe J. Mavunkal, l Sue-Lein Wang, 1 Fen-Ling Liao, ~ Shie-Ming Peng, 2 and Gene-Hsiang Lee 2
Received August 12, 1996
Treatment of carbido cluster Rus(/zs-C)(CO)~5 with Me3NO in acetonitrile solution followed by addition of dimethyl maleate or dimethyl acetylene dicar- boxylate affords new clusters Rus(/zs-C)(CO)~3[C2H2(CO2Me)2] (1) and Rus(/zs-C)(CO)Is[C2(CO2Me)2] (2), respectively. Single crystal X-ray struc- tural studies reveal that both complexes contain a wingtip-bridged butterfly pen- tametallic skeleton. In complex 1 the maleate fragment is coordinated to one wingtip Ru atom through its carbon-carbon double bond and to the adjacent Ru atom by the formation of two O ~ Ru dative bonding interactions, while the acetylene dicarboxylate fragment in 2 is best considered as a cis-dimetaUated alkene, linking one hinge Ru atom and the nearby Ru atom at the bridged posi- tion. Crystal data for 1: space group P 42/n; a=20.199(6), c = t3.941(3)A, Z = 8 ; final Rr=0.025, R,,.=0.026 for 3963 reflections with l>2a(1). Crystal data for 2: space group P2~/n; a = 9.634(3), b = 20.062(6), c = 17.372(5) A, fl=90.62(2) °, Z = 4 ; final R r = 0 033, R,,,=0.036 for 4683 reflections with I > 3a(I).
KEY WORDS: Ruthenium carbide; carbonyl; alkyne; alkene; dimethyl maleate; dimethyl acetylene dicarboxylate.
I N T R O D U C T I O N
The ruthenium carbido cluster Rus(/t:C)(CO)15 was first prepared in trace amount from the reaction of Ru4(p-H)4(CO)I 2 with ethylene [1]. 1 Department of Chemistry, National Tsing Hua University, Hsinchu 30043, Taiwan. 2 Department of Chemistry and Instrumentation Center, National Taiwan University~ Taipei
10764, Taiwan, Republic of China.
3 To whom all correspondence should be addressed. 87
88 Way ~ aL Subsequently, an indirect procedure involving the high pressure carbonyla- tion of R u 6 ( / 1 6 - C ) ( C O ) l 7 w a s selected to produce this medium-nuclearity carbido cluster compound in large quantity [2]. Much chemistry of Ru5(/15-C)(CO)15 has been reported since then. Thus,. the further addition of carbon monoxide or weakly coordinated acetonitrile under mild condi- tions afforded the wingtip-bridged butterfly clusters R u 5 ( f l 5 - C ) ( C O ) l 6 and Rus(/xs-C)(CO)]5(NCMe), respectively [ 3 ]. The addition of Au(PPh3)CI to R%(/15-C)(CO)Is formed Rus(/zs-C)(CO)]a(/x-C1)(/~-AuPPh3) which then eliminated one mole of CO to form Rus(/~5-C)(CO)13(/2-C1)(/~-AuPPh3) where bridging chlorine ligand functions as a three-electron donor [4]. Coordination of H2S, H2Se and HSR, R = Me or Et, led to the formation of the clusters Rus(/xs-C)(CO)]4(p-H)(/~-SH), Rus(/2:C)(CO)14(/~-H) (/~-SeH), and Rus(/~s-C)(CO)14(/z-H)(p-S), respectively [5]. In these molecules, the Ru5 frameworks resemble that of the bridged-butterfly geometry in R%(/15-C)(CO)Is(NCMe) with the hydride associated with the hinge Ru-Ru bond and the thiolate group bridging across the hinge and the bridged ruthenium atoms. The nucleophiles, such as with LiMe, NaCsH5 and [ P P N ] [ N O 2 ] , reacted with Rus(/xs-C)(CO)15 to afford anionic cluster complexes, while upon addition of the aurated cation [AuPR3] ÷, R = Ph or Et, giving the acyl cluster R%(ps-C)(CO)14(p-MeCO) (p-AuPPh3), the cyclopentadienyl cluster CpRus(/15-C)(CO)]a(/~-AuPPh3) and the nitrosyl cluster Rus(/~5-C)(CO)13(NO)(/x-AuPEt3) [6]. In most cases, the reactions are clean and produce only one major product. This novel reac- tivity pattern has encouraged us to investigate the subsequent reactions of Rus(/~5-C)(CO)x5 with unsaturated hydrocarbons except for the diene molecules, as the latter are known to produce a wide variety of structurally characterized arene clusters [ 7 ]. In this paper wc describe the reactions of Ru5(/~5-C)(CO)~5 with electron-deficient dimeth~,l maleate and dimethyl acetylene dicarboxylate, and will emphasize on the X-ray structure of the alkene and alkyne derivatives Rus(/15-C)(CO)la[C:H2(CO2Me)2] (1) and R%(/15-C)(CO)]5[ C2(CO2Me)2] (2) obtained.
\ I / O ~ c ~ O M e - ..,..,.. / x ~ / \ I '"~ /-..-C / Id U : " ' \ - - I ' - - - - ~ l d U \ ~ \ 1 / I \ H ~.Rul
.I
/ ~ R u , ~ /C02Me/ \ " C
/ \ (I) (2)Electron-Deficient Alkene and Alkyne Derivatives 89
E X P E R I M E N T A L P R O C E D U R E
General Information and Materials. Infrared spectra were recorded on
a Perkin Elmer 2000 FT-IR spectrometer. ~H and ~3C NMR spectra were recorded on a Bruker AM-400 (400.13 MHz) or a AMX-300 (300.6 Mhz) instrument. Chemical shifts are quoted with respect to internal standard tetramethylsilane. Mass spectra were obtained on a JEOL-HXII0 spec- trometer operating in fast atom bombardment (FAB) mode. All reactions were performed under a nitrogen atmosphere using deoxygenated solvents dried with an appropriate reagent. Reactions were monitored by analytical thin-layer chromatography (5735 Kieselgel 60 F254, E. Merck) and the products were separated on commercially available preparative thin-layer chromatographic plates (Kieselgel 60 Fzs 4, E. Merck). The elemental analyses were performed at the NSC Regional Instrument Center at National Cheng Kung University, Tainan, Taiwan.
Reaction of Rus(lls-C)( CO) 5 with Dimethyl Maleate. An acetonitrile
solution (10ml) of freshly sublimed Me3NO (8.8mg, 0.12mmol) was added dropwise into a CHzC12 solution (25ml) of Rus(/~5-C)(CO)I5 (50 mg, 0.053 mmol) within 30 min. After the addition of Me3NO solution was completed, the color of solution faded from red to light red. Dimethyl maleate (60 pl, 0.504 mmol) was added into the flask using a microsyringe. The solvents were removed under vacuum and the oily residue was redissolved into a mixture of CH2C12 (10m l) and heptane (30m 1). The heating was continued for 10 min until the color changed to dark red. Then the solvent was removed and the residue was redissolved in the minimum of CH2C12 and separated by thin-layer chromatography. Development with a 1:2 mixture of dichloromethane and hexane produced an orange band, which was extracted from silica gel to yield 28 mg of Rus(/~5-C) (CO)13 [C2H2(CO2Me)2] (1, 0.027 mmol, 51%) after recrystallization.
Spectral data for 1: MS spectrum (FAB, ~°2Ru), m/z 1029(M+). IR(CH2C12): v(CO), 2082 (m), 2046 (vs), 2036 (s), 2023 (s), 1998 (br, m), 1969 (br, m ) c m - 1 ; v(ester-CO), 1610 (br, m ) c m -~ 1H NMR (CD2C12, 294 K): 6 4.12 (d, 1H, JH-H =9.4 HZ), 3.96 (s, 3H), 3.68 (d, 1H, Jr~-H = 9.4 Hz), 3.62 (S, 3H). 13C NMR (CD2C12, 294 K): fi 463.6 (ps-C), 200.1 (CO), 199.1 (CO), 197.0 (2CO), 196.2 (br, 2CO), 193.1 (CO), 190.9 (CO), 189.0 (CO2Me), 182.5 (_CO2Me), 55.4 (OCH3) , 55.1 (OCH3) , 40.9 (_CH), 37.8 (_CH). Elemental analysis for C20HsO17Rus: Calcd.: C, 23.42; H, 0.79. Found: C, 23.25; H. 0.85.
Reaction of Rus(ps-C)(CO)13[C2He(CO:Me)2 ] with CO. To a
50ml reaction flask, 18rag of Rus(kts-C)(CO)Ia[CEH2(CO2Me)2 ] (0.017 mmol), 10 ml of CH2C12 and 15 ml of heptane were added. The resulting
90 Way et a L
solution was stirred at reflux temperature under a CO atmosphere for 40 min during which time the color changed from orange to dark red slowly. The solvent was removed and the residue was separated by thin- layer chromatography (dichloromethane:hexane= 1:2), affording 8mg of
Rus(ps-C)(CO)~5 (0.008 mmol, 49 %)
as the only isolable product.Reaction of Rus(I~:C)(CO). with Dimethyl Acetylene Dicarboxylate.
An acetonitrile solution (15ml) of freshly sublimed Me3NO (17.6mg, 0.23 mmol) was added dropwise into a CH2C12 solution (50 ml) of Ru5 (/t5-C)(CO)~5 (200 mg, 0.212mmol) within 30 min. After the addition of Me3NO solution was completed, the color faded from red to light red. Dimethyl acetylene dicarboxylate (151pl, 1.23 mmol) was then added into the reaction flask. The mixture was then stirred at room temperature for 20 min. and the color changed to dark red. The solvent was removed under vacuum and the residue was separated by thin-layer chromatography usingTable I. Experimental Data for the X-ray Diffraction Studies"
Compound 1 2
Empirical formula RusC20HsOt7 RtlsC22HrOt9" CH2C12
Crystal system tetragonal monoclinic
Space group P 42/n P 21/n a (A) 20.199(6) 9.634(3) b (A) 20.062(6) c (A) 13.941(3) 17.372(5) /~ (°) 90.62(2) Volume (A 3) 5688(2) 3358(2) Mol. wt. 1025.62 1164.5
Crystal size, ram, 0.40 x 0 5 0 x 0.60 0.44 × 0.41 × 0.40
Z 8 4
diffractometer used Nonius CAD-4 Siemens R3m/V
D,. (/cm 3) 2.395 2.304
F(000) 3872 2208
h, k, l ranges 0 23, 0 23, 0 16 - 11 11, 0 23, 0 20 / l ( M o - K , ) cm - ~ 26.29 24.35
Transmission factors 1.00, 0.924 0.96, 0.82
No. of unique data 4987 5950
No. of observed data 3963 ( I > 2a(1)) 4683 ( I > 3a(I))
No. of parameters 380 443
Weight modifier 0.00004 0.0006
RF; R..; G.O.F. 0.025; 0.026; 1.56 0.033; 0.036;'1.34
Maximum A / a ratio 0.006 0.008
Residual electron, e A -3 0 . 6 5 / - 0 . 3 9 1.08/-0.78
"Features common to all determinations: ) , ( M o - K ~ ) = 0 . 7 1 0 7 A ; minimize function:
5~(WlFo-F,12),
weighting scheme:w-'=~(Fo)+lglF2o;
G.O.F.=[ZwlFo--F,.[2/ (No - N,,)] ,/2 (No = number of observations; N,, = number of variables).Electron-Deficient Alkene and Alkyne Derivatives 91
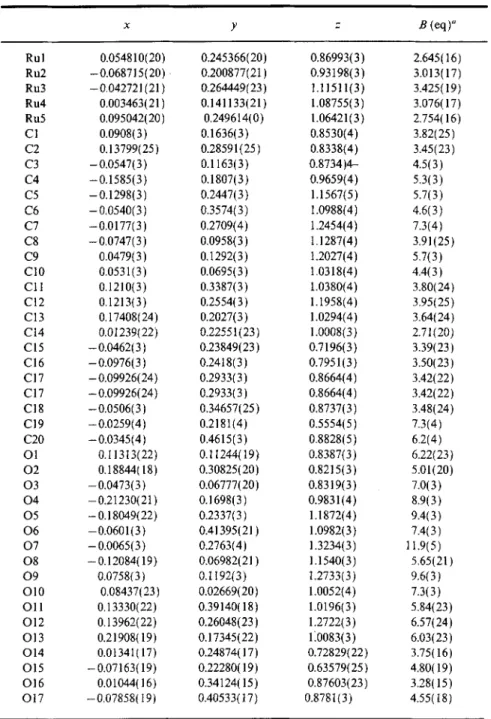
Table II. Atomic Coordinates and Equivalent Isotropic Displacement Coefficients for 1
x y z B (eq)" Rul 0.054810(20) 0.245366(20) 0.86993(3) 2.645(16) Ru2 -0.068715(20) 0.200877(21) 0.93198(3) 3.013(17) Ru3 -0.042721(21) 0.264449(23) 1.11511(3) 3.425(19) Ru4 0.003463(21) 0.141133(21) 1.08755(3) 3.076(17) Ru5 0.095042( 20 ) 0.249614(0 ) 1.06421 ( 3 ) 2.754(16) C 1 0.0908( 3 ) 0.1636( 3 ) 0.8530(4) 3.82( 25 ) C2 0.13799( 25 ) 0.28591 ( 25 ) 0.8338(4 ) 3.45 ( 23 ) C3 -0.0547(3) 0.1163(3) 0.8734)4- 4.5(3) C4 -0.1585(3) 0.1807(3) 0.9659(4) 5.3(3) C5 -0.1298(3) 0.2447(3) 1.1567(5) 5.7(3) C6 - 0.0540( 3 ) 0.3574(3) 1.0988(4) 4.6( 3 ) C7 -0.0177(3) 0.2709(4) 1.2454(4) 7.3(4) C8 - 0.0747(3) 0.0958(3) 1.1287(4) 3.91(25) C9 0.0479( 3 ) 0.1292( 3 ) 1.2027( 4 ) 5.7( 3 ) C10 0.0531(3) 0.0695(3) 1.0318(4) 4.4(3) C11 0.1210(3) 0.3387(3) 1.0380(4) 3.80(24) C12 0.1213(3) 0.2554(3) 1.1958(4) 3.95(25) C13 0.17408(24) 0.2027(3) 1.0294(4) 3.64(24) C14 0.01239(22) 0.22551(23) 1.0008(3) 2.71(20) C 15 - 0.0462( 3 ) 0.23849( 23 ) 0.7196( 3 ) 3.39( 23 ) C16 -0.0976(3) 0.2418(3) 0.7951(3) 3.50(23) C17 -0.09926(24) 0.2933(3) 0.8664(4) 3.42(22) C17 -0.09926(24) 0.2933(3) 0.8664(4) 3.42(22) C I 8 - 0.0506(3) 0.34657(25) 0.8737(3) 3.48(24) C19 -0.0259(4) 0.2181(4) 0.5554(5) 7.3(4) C20 -0.0345(4) 0.4615(3) 0.8828(5) 6.2(4) Ol 0.11313(22) 0.11244(I9) 0.8387(3) 6.22(23) 02 0.18844(18) 0.30825(20) 0.8215(3) 5.01(20) 03 -0.0473(3) 0.06777(20) 0.8319(3) 7.0(3) 04 -0.21230(21) 0.1698(3) 0.9831(4) 8.9(3) 05 -0.18049(22) 0.2337(3) 1.1872(4) 9.4(3) 06 -0.0601(3) 0.41395(21) 1.0982(3) 7.4(3) 07 -0.0065(3) 0.2763(4) 1.3234(3) 11.9(5) 08 -0.12084(19) 0.06982(21) 1.1540(3) 5.65(21) 09 0.0758(3) 0.1192(3) 1.2733(3) 9.6(3) O10 0.08437(23) 0.02669(20) 1.0052(4) 7.3(3) O l l 0.13330(22) 0.39140(18) 1.0196(3) 5.84(23) O12 0.13962(22) 0.26048(23) 1.2722(3) 6.57(24) O 13 0.21908( 19 ) 0.17345( 22 ) 110083(3 ) 6.03( 23 ) O14 0.01341(17) 0.24874(17) 0.72829(22) 3.75(16) O15 -0.07163(19) 0.22280(19) 0.63579(25) 4.80(19) O16 0.01044(16) 0.34124(15) 0.87603(23) 3.28(15) O17 -0.07858(19) 0.40533(17) 0.8781(3) 4.55(18)
92 Way et aL
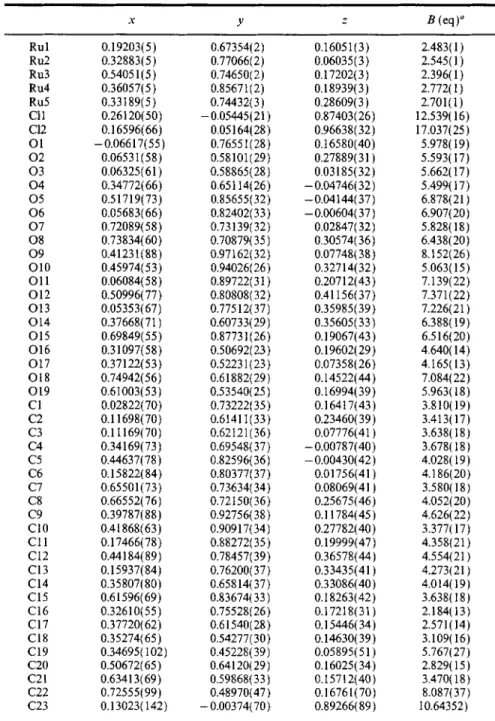
Table III. Atomic Coordinates and Equivalent Isotropic Displacement Coefficients for 2
x y z B (eq) u Rul 0.19203(5) 0.67354(2) 0.16051(3) 2.483(1) Ru2 0.32883(5) 0.77066(2) 0.06035(3) 2.545(1) Ru3 0.54051 ( 5 ) 0.74650( 2 ) 0.17202( 3 ) 2.396( 1 ) Ru4 0.36057(5) 0.85671(2) 0.18939(3) 2.772(1) Ru5 0.33189(5) 0.74432(3 ) 0.28609(3 ) 2.701( 1 ) CI1 0.26120(50) -0.05445(21 ) 0.87403(26) 12.539(16) C12 0.16596(66) 0.05164(28) 0.96638(32) 17.037(25) O1 -0.06617(55) 0.76551(28) 0.16580(40) 5.978(19) 02 0.06531(58) 0.58101(29) 0.27889(31) 5.593(17) 03 0.06325(61) 0.58865(28) 0.03185(32) 5.662(17) 0 4 0.34772(66) 0.65114(26) -0.04746(32) 5.499(17) 05 0.51719(73) 0.85655(32) -0.04144(37) 6.878(21) 06 0.05683(66) 0.82402(33) -0.00604(37) 6.907(20) 07 0.72089(58) 0.73139(32) 0.02847(32) 5.828(18) 08 0.73834(60) 0.70879(35) 0.30574(36) 6.438(20) O9 0.41231 ( 88 ) 0.97162( 32 ) 0.07748( 38 ) 8.152( 26 ) O10 0.45974(53) 0.94026(26) 0.32714(32) 5.063(15) O 11 0.06084(58) 0.89722( 31 ) 0.20712( 43 ) 7.139(22) O12 0.50996(77) 0.80808(32) 0.41156(37) 7.371(22) O 13 0.05353(67) 0.77512( 37 ) 0.35985( 39 ) 7.226(21 ) O 14 0.37668(71 ) 0.60733(29) 0.35605(33) 6.388(19) O15 0.69849(55) 0.87731(26) 0.19067(43) 6.516(20) O16 0.31097(58) 0.50692(23) 0.19602(29) 4.640(14) O17 0.37122(53) 0.52231(23) 0.07358(26) 4.165(13) O18 0.74942(56) 0.61882(29) 0.14522(44) 7.084(22) O19 0.61003(53) 0.53540(25) 0.16994(39) 5.963(18) CI 0.02822(70) 0.73222(35) 0.16417(43) 3.810(19) C2 0.11698(70) 0.61411(33) 0.23460(39) 3.413(17) C3 0.11169(70) 0.62121(36) 0.07776(41) 3.638(18) C4 0.34169(73) 0.69548(37) -0.00787(40) 3.678(18) C5 0.44637(78) 0.82596(36) -0.00430(42) 4.028(19) C6 0.15822(84) 0.80377(37) 0.01756(41 ) 4.186(20) C7 0.65501(73) 0.73634(34) 0.08069(41 ) 3.580(18) C8 0.66552( 76 ) 0.72150( 36 ) 0.25675( 46 ) 4.052( 20 ) C9 0.39787( 88 ) 0.92756( 38 ) 0.11784( 45 ) 4.626( 22 ) C 10 0.41868( 63 ) 0.90917( 34 ) 0.27782( 40 ) 3.377( 17 ) C 11 0.17466( 78 ) 0.88272( 35 ) 0.19999( 47 ) 4.358( 21 ) C12 0.44184(89) 0.78457(39) 0.36578(44) 4.554(21 ) C13 0.15937(84) 0.76200(37) 0.33435(41 ) 4.273(21 ) C 14 0.35807(80) 0.65814( 37 ) 0.33086(40) 4.014( 19 ) C15 0.61596(69) 0.83674(33) 0.18263(42) 3.638(18) Ct6 0.32610(55) 0.75528(26) 0.17218(31) 2.184(13) C17 0.37720(62) 0.61540(28) 0.15446(34) 2.571(14) C18 0.35274(65) 0.54277(30) 0.14630(39) 3.109(16) C19 0.34695(102) 0.45228(39) 0.05895(51) 5.767(27) C20 0.50672( 65 ) 0.64120( 29 ) 0.16025 ( 34 ) 2.829( 15 ) C21 0.63413( 69 ) 0.59868( 33 ) 0.15712(40 ) 3 A70( 18 ) C22 0.72555( 99 ) 0.48970( 47 ) 0.16761 ( 70 ) 8.087(37) C23 0.13023(142) -0.00374(70) 0.89266(89) 10.64352)
Electron-Deficient Alkene and Alkyne Derivatives 93
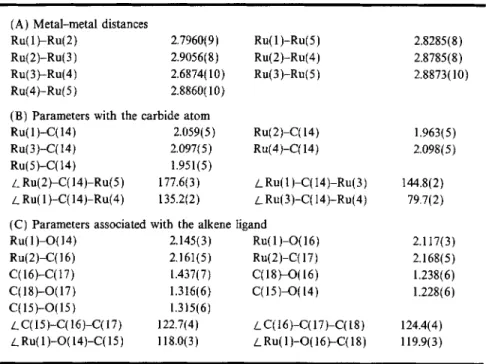
Table IV. Selected Bond Distances (A) and Bond Angles (Deg.) of 1 (esd in Parentheses)
(A) Metal-metal distances
Ru(1)-Ru(2) 2.7960(9) Ru(1)-Ru(5) 2.8285(8)
Ru(2)-Ru(3) 2.9056(8) Ru(2)-Ru(4) 2.8785(8)
Ru(3)-Ru(4) 2.6874(10) Ru(3)-Ru(5) 2.8873(10)
Ru(4)-Ru(5 ) 2.8860(10)
(B) Parameters with the carbide atom
Ru(1)-C(14) 2.059(5) Ru(2)-C(14) 1.963(5)
Ru(3)-C(14) 2.097(5) Ru(4)-C(14) 2.098(5)
Ru(5)-C(14) 1.951(5)
LRu(2)-C(14)-Ru(5) 1 7 7 . 6 ( 3 ) LRu(1)-C(14)-Ru(3) 144.8(2)
LRu(1)-C(14)-Ru(4) 1 3 5 . 2 ( 2 ) /Ru(3)-C(14)-Ru(4) 79.7(2)
(C) Parameters associated with the alkene ligand
Ru(1)-O(14) 2.145(3) Ru(2)-C(16) 2.161(5) C(16)-C(17) 1.437(7) C(18)-O(17) 1.316(6) C(15)-O(15) 1.315(6) LC(15)-C(16)-C(17) 122.7(4) LRu(1)-O(14)-C(15) 118.0(3) Ru(1)-O(16) 2.117(3) Ru(2)-C(17) 2.168(5) C(18)-O(16) 1.238(6) C(15)-O(14) 1.228(6) LC(16)-C(17)-C(18) 124.4(4) /_ Ru(1)-O(16)-C(18) 119.9(3)
Table V. Selected Bond Distances (A) and Bond Angles (Deg.) of 2 (esd in Parentheses)
(A) Metal-metal distances
Ru(1)-Ru(2) 2.934(I) Ru(1)-Ru(5) 2.920(1)
Ru(2)-Ru(3) 2.841(1) Ru(2)-Ru(4) 2.843(1)
Ru(3)-Ru(4) 2.828(1) Ru(3)-Ru(5) 2.838(1)
Ru(4)-Ru(5 ) 2.827( 1 )
(B) Parameters associated with the carbide atom
Ru(1)-C(16) 2.096(5) Ru(2)-C(16) 1.967(5)
Ru(3)-C(16) 2.073(5) Ru(4)-C(16) 2.083(5)
Ru(5)-C(16) 1.99(5)
/._ Ru(2)-C(16)-Ru(5) 176.4(3) L Ru(1)-C(16)-Ra(3) 123.1(3)
L Ru(I)-C(16)-Ru(4) 151.1(3) L Ru(3)-C(16)-Ru(4) 85.7(2)
(C) Parameters associated with the alkyne ligand
Ru(1)-C(17) 2.135(5) Ru(3)-C(20) 2.147(6)
C(17)-C(20) 1.354(9)
L C(18)-C(17)~C(20) 122.7(4) /_ C(17)-C(20)-C(21 ) 124.4(4)
94 Way e t aL pure dichloromethane as eluent, affording yellow orange Rus(ps-C)(CO)15 [C2(CO2Me)2] (2, 17.6 mg, 0.016 mmol, 8 %) as the major isolable cluster product. Single crystals suitable for X-ray diffraction study were obtained from a mixture of CH2Clz and methanol at -20°C.
Spectral data for 2: MS spectrum (FAB, ~°2Ru), m/z 1038(M÷). IR(CH2C12): v(CO), 2111 (w), 2081 (s), 2073 (vs), 2068 (s), 2051 (s), 2024 (br, m), 1946 (br, v w ) c m - l ; v(ester-CO), 1690 (br, w ) c m -1. IH NMR (CDzC1 z, 294 K): fi 3.52 (s, 3H), 3.48 (s, 3H). (d, ~H, JH-H = 9.4 HZ), 3.96 (S, 3H), I3C N M R "(CD2C12, 294 K): fi 440.7 (/~s-C), 194.9 (2CO), 193.6 (2CO), 192.9 (2CO), 192.6 (CO), 190.9 (3CO), 190.6 (2CO), 189.2 (2CO), 188.3 (CO), 18t.7 (_CO2Me), 171.4 (_CO2Me), 170.2 (_C2), 160.4 (_C2), 189.0 (_CO2Me), 182.5 (_CO2Me), 51.7 (O_CH3), 51.5 (O_CH3). Elemental analysis for C22H6019Rus: Calcd.: C, 24.48, H, 0.56. Found: C, 24.11; H. 0.72.
X-Ray Crystallography. Diffraction measurements were carried out on a Nonius CAD-4 or a Siemens R3m/V diffractometer. Lattice param- eters of 1 were determined from 25 randomly selected high angle reflections with 20 angles in the range 18.50-23.32, whereas the corresponding cell dimensions of complex 2 were determined from 23 reflections with 20 angle in the range of 13.21-27.55. All reflections were corrected for Lorentz, polarization, and absorption effects. All data reduction and refinement were performed using the NRCC-SDP-VAX and Siemens SHELXTL PLUS (VMS) packages. The structures were refined by full-matrix least squares, all nonhydrogen atoms were refined with anisotropic thermal parameters and the hydrogen atoms on organic ligands were calculated in idealized positions and included in the structure factor calculation. The combined data collection and refinement parameters are given in Table I. Atomic positional parameters for complexes 1 and 2 are found in Tables II and III, whereas the selected bond angles and lengths appear in Tables IV and V, respectively.
RESULTS AND DISCUSSION
Synthesis and Characterization of 1. The carbido cluster Rus(ps-C) (CO),5 reacts with two equiv, of the oxidative decarbonylation reagent Me3NO in acetonitrile solution at room temperature to afford an unstable light red complex which is tentatively assigned to have an empirical for- mula Rus(ps-C)(CO)I3(NCMe)2. No attempt is made to isolate and characterize this material. However, upon the addition of excess of dimethyl maleate, it was converted to an orange cluster Rus(/~ 5-C)(CO) ~ 3 [C2H2(CO2Me)2] (1) in 51% yield by the incorporation of one dimethyl maleate molecule. The direct reaction of Rus(/15-C)(CO)~ 5 with excess dimethyl maleate in dichloromethane containing two equiv, of Me3NO
Electron-Deficient Alkene and Alkyne Derivatives 95
also affords the maleate cluster 1, but the yield is substantially lower. These employed conditions differ from that utilized for the reactions of
Ru6(~6-C )
(CO)L7 with dienes in producing the arene clusters, where no acetonitrile solvent was added in stabilizing the intermediate [8].
On the contrary, treatment of Rus(ps-C)(CO)15 with dimethyl fumarate, in which the CO2Me functional groups adopt
trans-disposition
at the carbon-carbon double bond, does not produce the corresponding fumarate complex but cluster decomposition. This dramatic diversity in reactivity provides the first indication to the possible involvement of bothC02
Me functional groups in stabilizing the maleate complex 1.The maleate cluster 1 is characterized by spectroscopic methods and X-ray diffraction study. The FAB mass spectrum displays a parent molecular ion at
m/z
1029, showing the existence of 13 CO ligands and one ligated olefin fragment. The ~H NMR spectrum is very simple, showing two doublets at ~ 4.12 and 3.68 with a coupling constant 3JH_H=9.4 Hz and two singlet signals at fi 3.96 and 3.62, an indicative of a dimethyl maleate ligand. From these spectroscopic data we can assume that the vacant coor- dination sites generated by the elimination of two CO ligands is filled by the maleate ligand, which is coordinated to the ruthenium atoms through the carbon-carbon double bond and the oxygen atoms of the carbonyl ligands.The molecular structure of 1 is shown in Fig. 1 together with the atomic numbering scheme. The selective bond angles and distances are presented in Table IV. The metal core structure is closely related to the "wingtip-bridged butterfly" structure adopted by several analogous pen- taruthenium and osmium carbido derivatives [9]. In this molecule, all carbonyl ligands adopt the terminal bonding mode with almost linear Ru-CO angles in the range 173.4-179.0 °. The Ru-Ru distances are of three types. The shortest is the Ru(hinge)-Ru(hinge) bond (Ru(3)-Ru(4)= 2.6874(10) A), then the Ru(bridge)-Ru(wingtip) bonds (Ru(1)-Ru(2)= 2.7960(9)A and Ru(1)-Ru(5)=2.8285(8)A and the longest are the Ru( wingtip)-Ru (hinge) bonds at 2.8860(10)-2.9056(8) A. The Ru(wingtip)- C(14)-Ru(wingtip) angle is nearly linear with angle 177.6(3) °. The carbide carbon distances to the wingtip ruthenium atoms (Ru(2)-C(14) = 1.963(5)/~ and Ru(5)-C(14)= 1.951(6),~ are significantly shorter than those to the hinge and bridged ruthenium atoms (2.059(5)/~-2.098(5)A).
The most striking feature is the bonding of the dimethyl maleate ligand. The alkene portion is coordinated to the Ru(2) atom with distance C(16)-C(17)= 1.438(7)A, which is similar to that observed in the Mo maleate complexes [10]. The carbonyl functional groups are extended across the Ru(1)-Ru(2) bond and coordinated to the adjacent Ru(1) atom with distances Ru(1)-O(14)=2.145(3) A and Ru(1)-O(16)=2.117(3) A.
96 Way et al. 102 012 013 016 C18 014 07 u2 C16 09 011 05 03 04 08
Fig. 1. Molecular structure of Rus(#5-C)(COh3[C2H2(CO2Me)2] (1) showing the crys- tallographic labeling scheme with thermal ellipsoids at the 30 % probability level.
These values are typical for the O --+ Ru dative bond in triruthenium com- plexes containing such r/2-carbonyl group [11], but are slightly shorter than that observed in dinuclear (CsMes)zRu2(kt-H)[C2Hz(CO2Me)2 ] [C2H(CO2Me):] (2.23(1) A) [ 12] and mononuclear (Ph3P)3RuH[CH = CMe(CO2Bu)] (2.246(7)A) [13], in which the carbonyl oxygen is also coordinated to ruthenium atom. Therefore, the structure of 1 represents the first example in which the dimethyl maleate ligand serves as a six-electron donor through the coordination of its carbon--carbon double bond and both carbonyl fragments.
After understanding the structure of 1, the mechanism leading to the generation of such novel cluster compound can be envisioned. Basically, formation of 1 may be viewed as an initial coordination by the olefinic por- tion of the dimethyl maleate, followed by bending of both carbonyl oxygen atoms to the ligand sphere of an adjacent Ru atom. In this case, one oxygen donor replaces the second weakly coordinated acetonitrile ligand, whereas in the formation of the second oxygen to ruthenium atom donor interaction, a Ru-Ru bond is broken. The
cis-disposition
of the carbonylElectron-Deficient Alkene and Alkyne Derivatives 97
groups is important in stabilizing this particular metal framework, as the direct reaction with dimethyl fumarate, which contains two trans CO2Me functional groups, failed to produced the alkene adduct. It seems that the direct linkage with the second carbonyl group and the subsequent cleavage of the Ru-Ru bond are of importance in stabilizing the cluster.
Synthesis and Characterization of 2. Treatment of Rus(/.ts-C)(CO)15
with stoichiometric amount of Me3NO in acetonitrile followed by addition of dimethyl acetylene dicarboxylate results in the formation of pen- taruthenium compound Rus(//5-C)(CO)]5[C2(CO2Me)2 ] (2) in 8 % yield as an orange material. The IR spectrum in CH2C12 solution shows only terminal CO stretches at 2111-1946 cm-~. The ~H NMR spectrum consists of two methyl resonance signals at 5 3.52 and 3.48, suggesting the presence of one dimethyl acetylene dicarboxylate molecule.
In attempts to explore the possible reaction mechanism, we have varied the conditions by addition of two equivalents of Me3NO instead.
02 03q 01 04 p011 016 C18 05 014 C20 09 018 08 010 015
Fig. 2. Perspective drawing of Rus(/2s-C)(CO)ls[C2(CO2Me)2 ] (2) showing the crys- tallographic labeling scheme with thermal ellipsoids at the 30 % probability level.
98 Way et aL However, we were unable to isolate compound 2 during this investigation. This observation probably suggests that its formation is the consequence of the coordination of dimethyl acetylene dicarboxylate to the mono- acetonitrile cluster complex Rus(ps-C)(CO)14(NCMe) to give an inter- mediate
Rus(ps-C)(CO)I4[C2(CO2Me)2],
followed by recapture of an additional CO ligand in solution. Direct formation of 2 by addition of dimethyl acetylene dicarboxylate across the Ru-Ru bond of R u s ( p : C ) (CO)15 is precluded because no cluster compound 2 can be isolated in the absence of Me3NO reagent under similar conditions.The compound 2 was examined by single-crystal X-ray analysis to determine its molecular structure. The ORTEP diagram is presented in Fig. 2 and the selective distances and angles are given in Table V. The cluster framework is related to that of the previously reported complex 1, exhibiting the same kind of "wingtip-bridged butterfly" geometry, in which the butterfly fragment is defined the atoms Ru(2), Ru(3), Ru(4), and Ru(5). The alkyne fragment adopts the novel p-t/l, ql-bonding mode [ 14] and spans the nonbonding Ru(1) and Ru(3) atoms. The C(17)-C(20) distance (1.354(9) A), which falls in the range for a formal carbon-carbon double bond, is slightly longer than the C = C distances (1.27-1.34 A) seen for the dimetaUacyclobutene complexes [ 15], while the angles (/__Ru( 1 )-C( 17)- C(20) = 123.9(4) ° and /Ru(3)-C(.20)-C(17)= 121.6(4) °) are also consis- tent with the sp z hybridization of the alkene carbons. Thus this alkyne frag- ment is bound as the cis-dimetallated alkene [ 16]. In addition, all carbonyl ligands adopt a terminal mode, except that the carbonyl ligand C(15)O(15) which bridges the Ru(hinge)-Ru(hinge) bond asymmetrically, L R u ( 3 ) - C(15)-O(15) = 167.6(6) °. The presence of this bridging CO ligand may be responsible for the slight increase of this Ru(hinge)-Ru(hinge) distance (Ru(3)-Ru(4)=2.828(1)A) with respect to the respective hinge Ru-Ru bond in 1. Moreover, the pattern of the ruthenium--carbide distances is also akin to that of the previous discussed complex 1, with the distances to the wingtip ruthenium atoms being slightly shorter than those to the hinge and the bridged ruthenium atoms.
SUMMARY AND CONCLUSIONS
The alkene and alkyne derivatives of Rus(p:C)(CO)ts, which adopt the wingtip-bridged butterfly geometry, have been synthesized and charac- terized. Our experimental result suggests that the chemical activation of the parent cluster Ru5(ps-C)(CO)I s via addition of Me3NO in the presence of acetonitrile is critical to the successful preparation of these derivatives, although the stoichiometry for the alkyne derivative 2 implies that no prior CO dissociation is required. For the maleate derivative 1, in addition to the
Electron-Deficient Alkene and Alkyne Derivatives 99
c a r b o n - c a r b o n d o u b l e bond, b o t h o x y g e n a t o m s o f the c a r b o n y l functional g r o u p s are linked to a r u t h e n i u m a t o m to c o m p e n s a t e for the u n s a t u r a t i o n generated b y loss o f t w o C O ligands a n d cleavage o f o n e R u - R u b o n d . T h e c o m b i n e d interaction f r o m the maleate ligand to the R u s cluster core is still n o t very effective, as t r e a t m e n t o f 1 with C O regenerated the c a r b o n y l cluster R u 5(P s - C ) ( C O ) 15 in 49 % yield. I n contrast, the d i m e t h y l acetylene d i c a r b o x y l a t e derivative 2, in which the alkyne is c o o r d i n a t e d to the cluster via p-t/l, I/I-bonding, shows n o such c a r b o n y l O ~ R u dative i n t e r a c t i o n due to the u n f a v o r a b l e position o f C O 2 M e functional groups. W o r k is currently in progress to investigate the c o u p l i n g o f electron deficient alkenes a n d alkynes o n such p e n t a r u t h e n i u m platform. Full details will be presented in f o r t h c o m i n g publications.
S U P P L E M E N T A R Y M A T E R I A L S A V A I L A B L E
A complete listing o f thermal parameters, tables o f n o n e s s e n t i a l b o n d distances a n d h y d r o g e n a t o m c o o r d i n a t e s for complexes 1 a n d 2 are available f r o m the a u t h o r (Y. C.).
A C K N O W L E D G M E N T S
W e t h a n k the N a t i o n a l Science Council o f the Republic o f C h i n a ( G r a n t N o . N S C 85-2113-M007-037CC) for financial support.
R E F E R E N C E S
1. c. R. Eady, B. F. G. Johnson, J. Lewis, and T. Matheson (1973). J. Organomet. Chem. 57,
C82.
2. (a) B. F. G. Johnson, J. Lewis, S. W. Sankey, K. Wong, M. McPartlin, and W. J. H.
Nelson (1980). J. Organomet. Chem. 191, C3; (b) J. N. Nicholls and M. D. Vargas (1989).
Inorg. Synth. 26, 280.
3. B. F. G. Johnson, J. Lewis, W. J. H. Nelson, J. N. Nicholls, J. Puga, P. R. Raithby,
M. J. Rosales, M. Schr6der, and M. D. Vargas (1983). J. Chem. Soc. Dalton Trans. 2447.
4. B. F. G. Johnson, J. Lewis, J. N. Nicholls, J. Puga, and K. H. Whitmire (1983). J. Chem.
Soc. Dalton Trans. 787.
5. A. G. Cowie, B. F. G. Johnson, J. Lewis, J. N. Nicholls, P. R. Raithby, and M. J. Rosales
(1983). J. Chem. Soc. Dalton Trans. 2311.
6. (a) A. G. Cowie, B. F. G. Johnson, J. Lewis, J. N. Nicholls, P. R. Raithby, and A. G.
Swanson (1985). J. Chem. Soc. Chem. Commun. 637; (b) K. Henrick, B. F. G. Johnson,
J. Lewis, J. Mace, M. McPartlin, and J. Morris (1985). J. Chem. Soc. Chem. Commun.
1617.
7. (a) D. Braga, P. Sabatino, P. J. Dyson, A. J. Blake, and B. F. G. Johnson (1994). J. Chem.
Soc. Dalton Trans. 393; (b) D. Braga, F. Grepioni, P. Sabatino, P. J. Dyson, B. F. G.
Johnson, J. Lewis, P. J. Bailey, P. R. Raithby, and D. Stalke (1993). J. Chem. Soc. Dalton
100 Way et aL 8. P. J. Dyson, B. F. G. Johnson, J. Lewis, M. Martinelli, D. Braga, and F. Grepioni (1993).
J. Am. Chem. Soc. 115, 9062.
9. (a) B. F. G. Johnson, J. Lewis, W. J. H. Nelson, J. N. Nicholl, and M. D. Vargas (1983). J. Organomet. Chem. 249, 255; (b) B. F. G. Johnson, J. Lewis, P. R. Raithby, M. J. Rosales, and D. A. Welch (1986). J. Chem. Soc. Dalton Trans. 453; (c) B. F. G. Johnson, J. Lewis, W. P. R. Raithby, V. P. Saharan, and W. T. Wong (1992). J. Organomet. Chem. 434, C10.
10. C. H. Lai, C. H. Cheng, W. C. Chou, and S. L. Wang (1993). Organometallics 12, 3418. 11. (a) M. I. Bruce, P. A. Humphrey, H. Miyamae B. W. Skelton, and A. H. White (1992).
J. Organomet. Chem. 429, 187; (b) U. Bodensieck, J. Santiago, H. Stoeckli-Evans and G. Sfiss-Fink (1992). J. Organomet. Chem. 433, 141; (c) D. S. Bohle, A. N. Christensen and P. A. Goodson (1993). lnog. Chem. 32, 4173.
12. H. Suzuki, H. Omori, D. H. Lee, Y. Yoshida, M. Fukushima, M. Tanaka, and Y. Moro-oka (1994). Organometallics 13, 1129.
13. S. Komiya, T. Ito, M. Cowie, A. Yamamoto, and J. Ibers (1976). J. Am. Chem. Soc. 98, 3874.
14. C. P. Casey, R. S. Carino, R. K. Hayashi, and K. D. Schladetzky (1996). J. Am. Chem. Soc. 118, 1617.
15. (a) J. Holton, M. F. Lappert, R. Pearce, and P. I. Yarrow (1983). Chem. Rev. 83, 135; (b) M. R. Burke and J. Takats (1986). J. Organomet. Chem. 302, C25.

![Fig. 1. Molecular structure of Rus(#5-C)(COh3[C2H2(CO2Me)2] (1) showing the crys- tallographic labeling scheme with thermal ellipsoids at the 30 % probability level](https://thumb-ap.123doks.com/thumbv2/9libinfo/8671348.196049/10.945.244.703.222.623/molecular-structure-showing-tallographic-labeling-thermal-ellipsoids-probability.webp)
![Fig. 2. Perspective drawing of Rus(/2s-C)(CO)ls[C2(CO2Me)2 ] (2) showing the crys- tallographic labeling scheme with thermal ellipsoids at the 30 % probability level](https://thumb-ap.123doks.com/thumbv2/9libinfo/8671348.196049/11.945.250.694.566.959/perspective-drawing-showing-tallographic-labeling-thermal-ellipsoids-probability.webp)