行政院國家科學委員會專題研究計畫 成果報告
與大腸直腸癌細胞在轉移過程中進入血管可能有關的因子
計畫類別: 個別型計畫 計畫編號: NSC92-2311-B-002-099- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院外科 計畫主持人: 田郁文 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 10 月 5 日
Intravasation-Related Metastatic Factors in Colorectal
Cancer
Yu-Wen Tien,a Yung-Ming Jeng, b Rey-Heng Hu,a King-Jen Chang,a Su-Ming Hsu,b
Po-Huang Leea
Department of Surgerya and Pathologyb, National Taiwan University Hospital and
National Taiwan University College of Medicine, Taiwan, R.O.C.
Short Title: Intravasation-Related Metastatic Factors
Key words: Matrix metalloproteinase, E-cadherin, α-catenin, β-catenin,
metastasis
Corresponding Author
:
Address: Department of Surgery, National Taiwan University Hospital, 7
Chung-Shan South Rd. Taipei 10002, Taiwan
Telephone number: 011-886-2-23123456 ext: 5330
Fax number: 011-886-2-23568810
Abstract
Alterations in adhesion molecules, angiogenesis, and matrix metalloproteinases
have been associated with metastasis and intravasation. The present study
investigated the role of these metastatic factors in the context of primary colorectal
tumor. Intravasated colorectal epithelial cells were detected by an RT-PCR assay, and
expression of E-cadherin, α-catenin, or β-catenin as well as the vascularity of
tumor was assessed by immunohistochemical staining. Activity of matrix
metalloproteinase was assessed by gelatin zymography. The tumor venous blood was
positive for GCC mRNA expression in 40 of 68 patients, but alteration in expression
of E-cadherin, α-catenin, or β-catenin was not significantly associated with the
presence of colorectal epithelial cells in paired portal venous blood. Further, matrix
metalloproteinase activity did not correlate with the presence of intravasated
colorectal epithelial cells. Multivariate analysis demonstrated that the only factor
associated with intravasated colorectal tumor cells was vascularity of the tumor. Thus,
metastasis of colon cancer may result from passive entry into the circulation
secondary to angiogenic factors and does not appear to involve other metastatic
Introduction
The metastatic spread of cancer cells from a primary tumor to distant sites in the
body is responsible for most morbidity and mortality in cancer patients. Metastasis
involves a cascade of linked, sequential steps, including invasion of extracellular
matrix, neovascularization, invasion of blood vessel wall (intravasation), exit from
the circulation (extravasation), and establishment of secondary growth (1, 2). While
most aspects of cancer dissemination have been extensively studied, very little
biochemical information related to the process of intravasation is available. This may
be due to the paucity of experimental models capable of mimicking the cellular and
molecular interactions required for a successful completion of intravasation. Further,
most “experimental metastases” in xenogenic hosts are produced after intravascular
delivery of a large number of cancer cells, a route that bypasses the need of
intravasation. Another obstacle in the study of intravasation is the nature of most
metastatic assays. These assays tend to involve end-point factors in which nature of
the primary tumor and outcome (survival rate and ratio of metastasis) are known
while mechanistic properties are based on inference.
Postulated intravasation-related metastatic factors include neovascularization,
mutation of adhesion molecules and matrix metalloproteases (3-5). Although many
postulated intravasation-facilitating factors (3-5), these associations are based on
inferential, end point assay rather than by direct observation.
The current study attempts to more directly investigate intravasation by assessing
for the presence of tumor cells in the blood. PCR-based assays of mutated DNA or
tissue-specific RNA are highly sensitive methods for detecting circulating tumor cells.
However, DNA-targeted PCR assay may detect DNA derived from degraded tissue
instead of viable tumor cells (6). In contrast, RNA identification implies that RNA is
intact (extracellular RNA is rapidly degraded if it is not within an intact cell) and
functional (only viable cells produce the protein). Therefore, an RNA-targeted PCR
assay was selected to detect intravasated viable tumor cells. Detection of cytokeratin
(CK4)- 20 mRNA (7), carcinoembryonic antigen (CEA4) mRNA (8), and guanylyl
cyclase C (GCC4 ) mRNA (9) have been used for the detection of circulating viable
colorectal tumor cells. Of note, GCC transcripts have greater specificity than
transcripts of CK -20 or of CEA in detecting circulating colorectal tumor cells (10).
In our previous study, vascularity of primary colorectal tumor was a valuable
predictor of the presence of circulating colorectal epithelial cells in portal venous
blood (11). Thus, we investigated whether vascularity and the presence of other
postulated intravasation-related factors (MMP2, MMP9, E-cadherin, α-catenin, and
in the portal venous blood from patients with colorectal carcinomas.
Patients and Methods:
Patients
Informed consent was obtained from all patients, and the ethics committee of the
National Taiwan University approved the study protocol.
From January 1997 to June 1998, 68 patients (35 males and 33 females; ages
28–89 years; mean age of 65 years) with histologically confirmed colorectal
adenocarcinoma (32 patients with colon carcinoma, and 36 patients with rectal
carcinoma) were treated at our institution. Tumor stage and grading were classified as
per the Astler-Coller system. Potential curative resections were performed in 53
patients; and palliative resections of the colorectal tumor due to multiple liver
metastases were elected in 15 patients.
Blood and Tumor Sample Collection
Immediately after entering the peritoneal cavity and prior to manipulation of the
tumor, 10 cc of blood was collected from the drainage vein of the tumor-bearing
colorectal segment and used as portal venous blood sample. A piece consisting of at
least 2 cm3 of freshly harvested tumor tissue from each resected specimen was snap
frozen in liquid nitrogen at the time of operation and stored at –70°C.
In addition, peripheral blood samples from 11 healthy volunteers were obtained
RNA Extraction and Nested Duplex RT-PCR
RNA extraction (from peripheral mononuclear blood cells and frozen tissue) and
GCC RT-PCR was performed as previously described (11). In brief, cDNA was
synthesized in a 20-µl reaction mixture containing 2 µg of total RNA. For the first round of the nested polymerase chain reaction (PCR), 20-µl reactions were prepared with 4 µl of the cDNA preparation and primer (antisense, nucleotides 1197-1218; and sense, nucleotides 685-708). The second PCR was performed with 10 µl of this reaction mixture and antisense (nucleotides 1000-1021) and sense (nucleotides
759-781) primers. RT-PCR products were analyzed by agarose gel (2.5%)
electrophoresis and visualized by UV transillumination after staining with ethidium
bromide (0.5 µg/ml). The nested GCC PCR yielded a 262-bp product. The amplified products were sequenced using the ABI Model 373A DNA Sequencer (Perkin Elmer
Biosystems, U-S-A) as specified by the manufacturer. The DNA sequences were
aligned and analyzed using an Acer computer.
Sensitivity of GCC RT-PCR
The sensitivity of the GCC RT-PCR assay was determined in cell spiking
experiments as previously described, allowing the detection of 10 CCL 220 cells in
Gelatinase Zymography
Up to four 10-µm sections (between 10 and 20 mg of tissue) from each cryopreserved tumor were homogenized in protein extraction buffer (500 µl). Ten minutes later, the sample was centrifuged at 4°C at maximum r.p.m. for 10 min, and the supernatant was collected and stored in -20°C until protein assay was performed. Using a Bio-Rad protein assay reagent, the protein content of each sample was
measured against bovine serum albumin standards.
Gelatin zymography was performed according to the method described by Parsons
et al. (12). Briefly, each sample (20 µg of extracted protein) was run in parallel with a molecular weight marker, and 20 µg of extracted protein from patient 1 was included as an internal standard on SDS-polyacrylamide gels (7.5%) containing 0.1% gelatin
as the substrate. This method can detect the inactive proforms of collagenases,
because SDS causes activation of the enzymes without proteolytic cleavage of the
inhibitory N-terminal sequence (13).Western blotting using monoclonal antibodies for
latent MMP-9, active MMP9, latent MMP-2, and active MMP-2 was performed to verify
that the bands seen on zymography were as described.
Control Gels for MMPs
Control gels contained the MMP inhibitor EDTA in the MMP incubation buffer to
Quantification of the gels
Quantification was performed using laser densitometry and Quantity One software
(Discovery Series, Pharmacia Biotech, UK). The relative gelatinolytic activity was
determined for each proteinase by multiplying the area of each band by its optical
density. The following four lysis bands were observed on the gelatin zymography in
all the patients’ samples: 92 kDa, corresponding to latent MMP-9 (Gelatinase B);
82kD, active MMP-9; 72 kDa, latent MMP-2 (Gelatinase A); and 62 kDa, active
MMP-2. The total gelatinolytic activity (expressed in arbitrary units / 20μg of
protein) was obtained by summing the activities of the 92-kDa latent MMP-9, 82-kD
active MMP-9, 72-kDa latent MMP-2 and 62-kDa active MMP-2. To correct the
variation in background staining of the gel (intergel variation), the total gelatinolytic
activity of 20 µg of protein from patient 1 on each gel was defined as 20 arbitrary units of gelatinolytic activity and served as internal standard. Separate (latent or
active MMP2 or MMP9) and total gelatinolytic activity of each specimen on the same
gel was then expressed in arbitrary units / 20µg of protein.
Immunohistochemistry
The indirect avidin-biotin immunoperoxidase method was used for
immunostaining. All tissue samples were fixed in 10% buffered formalin and
xylene and dehydrated in ethanol. The sections were pre-digested with protease for 20
min at 37oC and then immersed in 3% hydrogen peroxide (H2O2) for 30 min to inhibit
endogenous peroxidase. After washing with PBS, sections were incubated in normal
rabbit serum for 30 min, followed by incubation overnight with either anti-E-cadherin
monoclonal mouse antibody (R & D System Europe, Abingdon, UK) diluted 1:200 in
Tris-buffered saline pH 7.6 (TBS); anti-β-catenin monoclonal IgG (Transduction
Laboratories, Lexington, KY, USA ) diluted 1: 100 in TBS; anti-α-catenin
monoclonal IgG (Zymed Laboratories Inc., South San Francisco, CA, USA) diluted
1:20 in TBS; or anti-CD31 monoclonal antibody (Union Biotech Inc.) at a 1:50
dilution. The sections were then thoroughly washed with TBS followed by addition of
a biotinylated rabbit anti-mouse immunoglobulin G for 15 minutes (Amersham Life
Science, UK). After incubation with ABC reagent (Dako, UK), the slides were
developed by immersion into 0.01% H2O2 and 0.05% diaminobenzidine
tetrahydrochloride (DAB) for 2 minutes. Normal mouse IgG was substituted for the
primary antibody in the negative control. The sections were counterstained with
hematoxylin.
After staining, blood vessels appeared intensely brown in color, which facilitated
identification and quantification. Expression of E-cadherin and catenins in cancer
always expressed these molecules. Two observers without knowledge of the clinical
and histological parameters evaluated these slides independently. Slides were scored
as ‘normal’ when more than 80% of the tumor epithelial cells showed linear
intercellular staining, and ‘reduced’ when less than 80% of the tumor cells expressed
intercellular staining (Fig.1).
Vascular Counting
Vascular counting was performed as previously described (11). In brief, slides
were examined at low power magnification (X40 and X100) to identify the areas of
highest vessel density. For each slide, the three most vascular areas within the tumor
mass were chosen. A 200X field in each of these three areas was counted. The
average counts of the three fields were recorded. Two pathologists without knowledge
of the corresponding clinicopathologic data counted all of the immunostained slides.
Statistical Analysis
Because intravasation was either present or absent, logistic regression was used to
analyze our data. Associations between the presence or the absence of GCC mRNA
expression in portal venous blood and potential prognostic factors, such as tumor size,
tumor grade, tumor stage, or those postulated intravasation-related metastatic factors
were determined. This method provided odds ratios or estimates of the relative risk of
investigated to determine which factors were related to intravasation, and stepwise
multivariate logistic regression was used to determine whether some combination of
variables provided a better estimate of the relative risk of intravasation than any
single variable.
Probability values < 0.05 were considered significant; all reported p values are
Results
Expression of GCC mRNA in portal venous blood
Peripheral blood samples from 11 all healthy volunteer were negative for GCC
mRNA. In contrast, GCC transcripts were detected in portal venous blood from 40 of
68 patients (59%) with colon caner. As shown in Table 1, tumor stage was the only
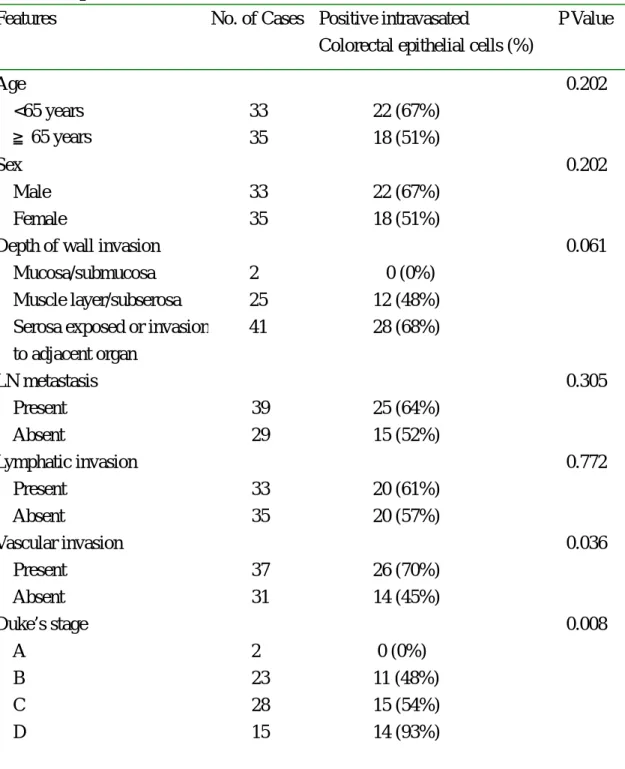
predictor of the presence of intravasated colorectal epithelial cells.
Gelatinolytic activity in primary colorectal tumor tissue
Patients were classified into one of two groups according to low or high
individual type gelatinolytic activity of their colorectal cancer tissue. For example, in
analyzing active MMP2, patients were classified according to low or high active
MMP2 gelatinolytic activity. The cutoff level corresponded to the median value of the
entire population of each type MMP for this classification scheme. However, none of
any individual type or total gelatinolytic activity was significantly related to the
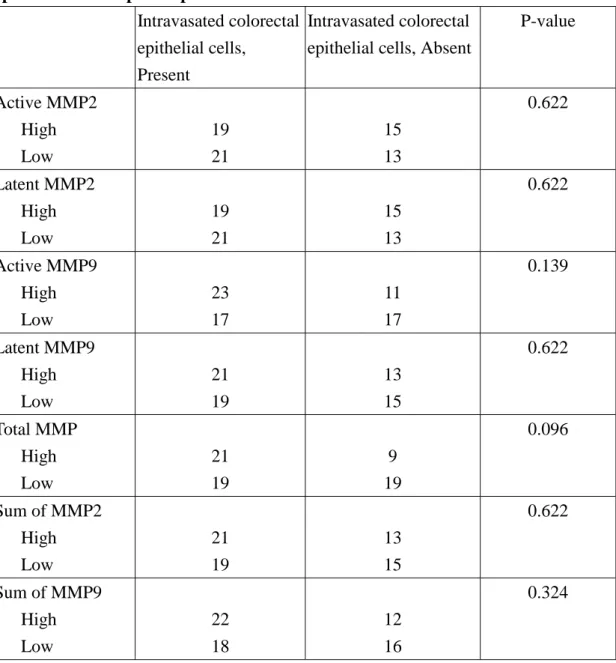
presence of intravasated colorectal epithelial cells (Table 2).
E-cadherin, α-catenin, and β-catenin Expression in colorectal tumor
Noncancerous epithelium of the large bowel expressed E-cadherin, α-catenin,
and β-catenin at cell-cell boundaries. As summarized in Table 3, the expression of
E-cadherin was maintained in 39 (57%) of the 68 tumors; the expression of α
-catenin was maintained in 39 (57%) of the 68 tumors. However, decreased
expression of any of these proteins was not significantly associated with the presence
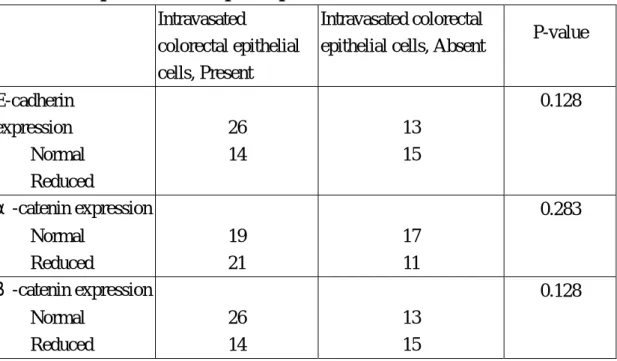
of intravasated colorectal epithelial cells (table 3).
Microvessel density in colorectal tumor
Considerable intratumor heterogeneity was observed in the distribution of
stained microvessels. Mean microvessel density (MVD) for anti-CD 31 antibody was
75.79 ± 24. In analysis of angiogenesis, patients were classified into one of two groups according to low or high microvessel density in their colorectal cancer tissue.
The cutoff level corresponded to the median value of the entire population of
microvessel density for this classification scheme.
Correlation between postulated intravasation-related factors and the presence of
intravasated colorectal epithelial cells
The relation of each postulated intravasation factor to the presence of intravasated
colorectal epithelial cells is summarized in Table 4. Only high MVD in the primary
tumor was positively related to the presence of intravasated colorectal epithelial cells.
High MVD in the primary tumor was associated with a nearly four fold increase in
detection of intravasated colorectal tumor cells (hazard ratio 3.52; 95% confidence
interval [CI] 1.3-9.750, Table 4). Multivariate analysis of postulated
Discussion
Previous studies using animal models have demonstrated that radiolabelled tumor
cells injected intravenously were entrapped in the capillary beds of the first target
organ and only a few were detectable in the peripheral blood (14). Theoretically,
intravasated colorectal tumor cells are detected more frequently in portal venous
blood than in peripheral venous blood. Thus, detection of intravasated tumor cells in
the drainage veins of tumor-bearing colorectal segments may be a better reflection of
the presence of intravasated colorectal tumor cells.
The use of RT-PCR system for detecting intravasated tumor cells may result in the
detection of tissue-specific mRNA instead of tumor cell specific mRNA. However,
normal colon epithelia or liver cells rapidly underwent apoptosis after having been
released into the circulation (15). We further minimized contamination by direct
puncture of drainage vein of tumor bearing segment. The metastatic cascade is
postulated to start with a breakdown of the epithelial integrity, thereby enabling tumor
cells to leave epithelial structures and invade the surrounding stroma. The loss of
E-cadherin expression has been associated with tumor cell de-differentiation and
correlates with an increased likelihood of distant metastases (16, 17). Presumably,
down-regulation of E-cadherin and/or associated catenins reduces the ability of cells
advancement into the surrounding tissue and vessels. However, these presumptions
were also obtained by end point assay and have not been definitely proven.
Among various adhesion molecules, the cadherin family of transmembrane
glycoproteins (responsible for calcium-dependent intercellular adhesion by
homophilic interaction) is of particular importance (18).The family includes many
subtypes, including E-cadherin (E-cad), P-cadherin, and N-cadherin, which are
distinct in immunologic specificity and distribution. Among these molecules,
E-cadherin is especially noteworthy as it maintains the epithelial structure (19). The
cytoplasmic domain of E-cadherin interacts with intracellular proteins called α-,β-,
and γ-catenins, which make contact with the microfilament network (18). The
interaction of these molecules is the prerequisite for the proper formation of
functionally intact adherens junctions. Thus, in tumors with normal expression of
E-cadherin, perturbations of the cadherin cell adhesion system may be due to
abnormal expression or function of the associated catenins (20). However, the present
study showed no association of down regulation of E-cadherin, α or β catenin
with increased risk of colorectal epithelial cell intravasation.
After detachment from the primary tumor mass, tumor cells must create
passageways for migration via enzymatic degradation of the ECM components. The
consists of type IV collagen and gelatin. Type IV collagenase is a metalloproteinase
that cleaves type IV collagen of epithelial and vascular basement membranes and that
is involved in tumor cell invasion (21). The present data failed to show an association
between high individual or high total gelatinolytic activity and presence of
intravasated colorectal epithelial cells. In fact, microvessel density was the only
predictor of the presence of intravasated colorectal epithelial cells.
Metastasis results from selective competition that favors survival of a
subpopulation of metastatic tumor cells that preexist within the heterogeneous
primary tumor (22). Kerbel determined that the metastatic subpopulation dominates
the primary tumor mass early in its growth (23), while many studies establish
metastasis as the final stage in tumor progression from a normal cell to a fully
malignant cell (24).
Some animal studies have demonstrated that the primary tumor may actually
suppress the growth of metastatic lesions (25). This is consistent with the
evolutionary viewpoint that the primary tumor’s main goal is to assure its own growth
rather than establish secondary growth in other organ. Promotion of primary tumor
growth is greatly dependent on angiogenesis, which results from the net balance
between positive and negative regulators of neovascularization (26). Endothelial cells
cell growth factor , secrete metalloproteinase-2 (gelatinase A) which contributes to
degradation of basement membrane in microvessel walls (27). This breakdown in the
vascular basement membrane may facilitate extravasation of endothelial cells during
formation of neovascular sprouts, as well as intravasation of tumor cells into the
lumen (28).If tumor vessel formation is rapid and haphazard and endothelial
proliferation is insufficient or endothelial junctions are unstable, cancer cells may be
exposed to the lumen and passively enter the circulation during the angiogenic
process (29). This presumption is compatible with our data that angiogenesis was the
only predictor of the presence of intravasated colorectal epithelial cells. Supporting
evidence is provided by studies of mosaic blood vessels in tumors that demonstrated a
subpopulation of tumor cells that coexist with endothelial cells and are progressively
shed into the circulation (42).
In conclusion, vascularity of the tumor was a significant predictor of the presence
of intravasated colorectal epithelial cells. In contrast, expression or activity of
E-cadherin, α-catenin, β-catenin, MMP-2, and MMP9 had no significant
association with the presence of intravasated colorectal epithelial cells. These data
may indicate that tumor cells enter the circulation passively during the process of
References
1. Nicolson GL: Molecular mechanisms of cancer metastasis: tumor and host
properties and the role of oncogen and suppressor genes. Curr Opin Oncol 1991;3: 75-92.
2. Chambers AF, Matrisian LM: Changing views of the role of matrix
metalloproteinases in metastasis. J Natl Cancer Inst 1997;89:1260-1270.
3. Folkman J: Tumor angiogenesis. In. Mendelson J, Howley PM, Israel MA, Liotta
LA, editors: The molecular basis of cancer. Philadelphia: Saunders 1995:206-32.
4. Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D,
Birchmeier W: E-cadherin-mediated cell-cell adhesion prevents invasiveness of
human carcinoma cells. J Cell Biol 1991;113:173-185.
5. Ambiru S, Miyazaki M, Ito H: A prospective study of prgnostic value of type IV
collagenase activity in colorectal cancer tissue. Dig Dis Sci 1997;42:1660-1665.
6. Bustin SA, Dorudi S: Molecular assessment of tumor stage and disease recurrence
using PCR-based assay. Mole Med Today 1998;4:389-396.
7. Weitz J, Kienle P, Lacroix J, Willeke F, Benner A, Lehnert T, Herfarth C, von
Knebel Doeberitz M: Dissemination of tumor cells in patients undergoing surgery
for colorectal cancer. Clin Cancer Res 1998;4:343-348.
8. Gerhard M, Juhl M, Kalthoff H, Schreiber HW, Wagener C, Neumaier M: Specific
9. Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD,
Waldman SA: Guanylyl cyclase C is a selective marker for metastatic colorectal
tumors in human extraintestinal tissues. Proc Natl Acad Sci USA 1996;93:
14827-14832.
10. Bustin SA, Gyselman VG, Williams NS, Dorudi S. Detection of cytokeratins 19
/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. British
J Cancer 1999;79:1813-1820.
11. Tien YW, Chang KJ, Jeng YM, Lee PH, Wu MS, Lin JT, Hsu SM: Tumor
angiogenesis and its possible role in intravasation of colorectal epithelial cells. Clin
Cancer Res 2001;7:1627-1632.
12. Parsons SL, Watson SA, Collins HM: Gelatinase (MMP-2 and –9) expression in
gastrointestinal malignancy. Br J Cancer 1998;78:1495-1502.
13. Birkedal-Hansen H, Taylor RE: Detergent activation of latent collagenase and
resolution of its component molecules. Biochem Biophys Res Commun 1982;107:
1173-1178.
14. Fidler IJ: Metastasis:quantitative analysis of distribution and fate of tumor emboli
labeled with emboli labelled with125I-5-Iodo-2’-deoxyuridine. J Natl Cancer Inst
1970;45:773-782.
apoptosis in vitro follows disruption of β1-integrin/matrix interactions in human
colonic crypt cells. Gastroenterology 1996;110:1776-1784.
16. Dorudi S, Sheffield JP, Poulsom R, Northover JM, Hart IR: E-cadherin expression
in colorectal cancer. An immunocytochemical and in situ hybridization study. Am J
Pathol 1993;142:981-986.
17. Bringuier PP, Umbas R, Schaafsma HE, Karthaus HF, Debruyne FM, Schalken JA:
Decreased E-cadherin immunoreactivity correlates with poor survival in patients
with bladder tumors. Cancer Res 1993;53:3241-3245.
18. Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator.
Science 1991;251:1451-1455.
19. Gofuku J, Shiozaki H, Tsujinaka T, Inoue M, Tamura S, Doki Y, Matsui S,
Tssukita S, Kikkawa N, Monden M: Expression of E-cadherin and α-catenin in
patients with colorectal carcinoma-Correlation with cancer invasion and metastasis.
Am J Clin Pathol 1999;111:29-37.
20. Takeichi M: Cadherins in cancer: implications for invasion and metastasis. Curr
Opin Cell Biol 1993;5:806-811.
21. Liotta LA, Kleinerman J, Catanzaro P, Rynbrandt D: Degradation of basement
membrane by murine tumor cells. J Natl Cancer Inst 1977;58:1427-1431.
implications. Science 1982;217: 998-1001.
23. Kerbel RS: Growth dominance of the metastatic cancer cell: cellular and
molecular aspects. Adv Cancer Res 1990;55:87-131.
24. Chambers AF, Matrisian LM: Changing views of the role of matrix
metalloproteinases in metastasis. J Natl Cancer Inst 1997;89:1260-1271.
25. Sugarbaker PH, Sugarbaker SP, Pun PP: Characterization of in vivo suppression
of syngenic tumor by allogenic effector cells. J Surg Oncol 1980;15:297-308.
26. Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic
switch during tumorigenesis. Cell 1996;86:353-364.
27. Yan L, Moses MA, Huang S, Ingber DEJ: Cell Sci 2000;113:3979-3987.
28. Folkman J. Can mosaic tumor vessels facilitate molecular diagnosis of cancer? P
N A S 2001,98:398-400.
29. Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL: Mosaic
blood vessels in tumors: frequency of cancer cells in contact with flowing blood. P N
Table 1 Association of clinicopathologic variables with presence of intravasated colorectal epithelial cells
Features No. of Cases Positive intravasated
Colorectal epithelial cells (%)
P Value Age <65 years ≧65 years 33 35 22 (67%) 18 (51%) 0.202 Sex Male Female 33 35 22 (67%) 18 (51%) 0.202
Depth of wall invasion Mucosa/submucosa Muscle layer/subserosa Serosa exposed or invasion
to adjacent organ 2 25 41 0 (0%) 12 (48%) 28 (68%) 0.061 LN metastasis Present Absent 39 29 25 (64%) 15 (52%) 0.305 Lymphatic invasion Present Absent 33 35 20 (61%) 20 (57%) 0.772 Vascular invasion Present Absent 37 31 26 (70%) 14 (45%) 0.036 Duke’s stage A B C D 2 23 28 15 0 (0%) 11 (48%) 15 (54%) 14 (93%) 0.008
Table 2 Correlation of gelatinolytic activity with the presence of colorectal epithelial cells in paired portal venous blood.
Intravasated colorectal epithelial cells,
Present
Intravasated colorectal epithelial cells, Absent
P-value Active MMP2 High Low 19 21 15 13 0.622 Latent MMP2 High Low 19 21 15 13 0.622 Active MMP9 High Low 23 17 11 17 0.139 Latent MMP9 High Low 21 19 13 15 0.622 Total MMP High Low 21 19 9 19 0.096 Sum of MMP2 High Low 21 19 13 15 0.622 Sum of MMP9 High Low 22 18 12 16 0.324
Table 3 Correlation of E-cadherin, α-catenin , or β-catenin with the presence of colorectal epithelial cells in paired portal venous blood
Intravasated
colorectal epithelial cells, Present
Intravasated colorectal
epithelial cells, Absent P-value
E-cadherin expression Normal Reduced 26 14 13 15 0.128 α-catenin expression Normal Reduced 19 21 17 11 0.283 β-catenin expression Normal Reduced 26 14 13 15 0.128
Table 4 Analyses using the Multivariate Cox Proportional Hazard Regression Model
Factor Univariate Analysis Hazard ratio (95% CI) P value
Adjusted for MVD
Hazard ratio(95% CI) P value Active MMP2 0.784 (0.298~2.064) 0.622 0.968 (0.347~2.705) 0.951 Latent MMP2 0.828 (0.300~2.282) 0.715 0.828 (0.300~2.282) 0.715 Active MMP9 2.091 (0.782~5.592) 0.142 2.258 (0.798~6.390) 0.125 Latent MMP9 1.275 (0.484~3.357) 0.622 1.306 (0.473~3.605) 0.606 Sum of MMP2 1.275 (0.484~3.357) 0.622 1.545 (0.549~4.347) 0.410 Sum of MMP9 1.630 (0.615~4.315) 0.326 1.358 (0.487~3.785) 0.558 Sum of MMP 2.091 (0.782~5.592) 0.142 2.076 (0.741~5.816) 0.165 E-cadherin 2.143 (0.921~7.932) 0.130 2.703 (0.921~7.932) 0.07 α-catenin 0.585 (0.220~1.560) 0.284 0.649 (0.233~1.809) 0.409 β-catenin 2.143 (0.799~5.748) 0.130 1.533 (0.530~4.434) 0.430 MVD or mean 3.519 (1.270~9.750) 0.016
Legends for figure 1:
Figure 1. Immunostaining for α-catenin and E-Cadherin. (A) Reduced expression ofα-catenin
[left], compared with normal colorectal epithelial cells; (B) Strong and diffuseα-catenin
expression in another case; (C) Loss of E-cadherin expression in cancer cells; (D) Expression of
Dear Dr. Phil D. Rye:
It is our great pleasure to submit the paper entitled, “Intravasation-Related
Metastatic Factors in Colorectal Cancer” for consideration for publication in
Tumor Biology. Neither the submitted paper nor any similar paper, either in whole or
in part, other than an abstract or preliminary communication, has been or will be
submitted to or published in any other primary scientific journal. All of the listed
authors are aware of and agree to the content and submission of the paper. There are
no financial or other interests with regard to the submitted manuscript that might be
construed as a conflict of interest.
While most aspects of cancer dissemination have been extensively studied, very
little direct biochemical information related to the process of intravasation is available.
In the present study, we utilized the endpoint of the presence of intravasated
colorectal tumor cells detected by GCC RT-PCR rather than metastasis formation for
more direct studies of the phenomenon. Of the studied variables, only vascularity of
the tumor was a significant predictor of the presence of intravasated tumor cells. Thus,
tumor intravasation may occur in a passive process related to angiogenesis rather than
an active process related to adhesion molecules or other mechanisms. We hope you’ll
like it.
Sincerely yours,
Yu-Wen Tien
Department of Surgery, National Taiwan University Hospital, 7 Chung-Shan South
Rd. Taipei 10002, Taiwan. Phone: 011-886-2 23123456 ext 5330; Fax: