Po-Ling Chang1 Tai-Chia Chiu2 Huan-Tsung Chang1 1Department of Chemistry,
National Taiwan University, Taipei, Taiwan
2Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei, Taiwan Received April 26, 2005 Revised December 7, 2005 Accepted December 7, 2005
Research Article
Stacking, derivatization, and separation by
capillary electrophoresis of amino acids from
cerebrospinal fluids
This paper describes the in-column derivatization, stacking, and separation of amino acids by CE in conjunction with light-emitting diode-induced fluorescence using naphthalene-2,3-dicarboxaldehyde (NDA). According to the relative electrophoretic mobilities and the migration direction in tetraborate solution (pH 9.3), the injection order is cyanide, then amino acids, then NDA. Once poly(ethylene oxide) (PEO) migrates through the capillary under EOF, the amino acid?NDA derivatives, amino acids, and CN2ions migrating against the EOF enter the PEO zone. As a result of
increases in viscosity and possible interactions with PEO molecules, the reagents/ analytes slow down such that they become stacked at the boundary. In comparison with the off-column approach to the analysis of amino acids, our proposed method provides a lower degree of interference from polymeric NDA compounds and other side products. As a result, the plot of the peak height as a function of g-aminobutyric acid (GABA) concentration is linear over the range from 1025to 1028M, with the LOD
being 4 nM. We demonstrate the diagnostic potential of this approach for the deter-mination of amino acids, including GABA and glutamine, in biological samples through the analysis of large volumes of cerebral spinal fluids without the need for sample pretreatment.
Keywords: Amino acids / In-column derivatization / Light-emitting diodes / Naphtha-lene-2,3-dicarboxaldehyde / Stacking DOI 10.1002/elps.200500496
1 Introduction
Amino acids play essential roles in the metabolic pro-cesses of living organisms and, thus, their determination is important for clinical diagnosis and treatment of her-editary diseases, kidney diseases, liver diseases, and neuropathy [1]. The importance of quantitatively analyzing the amino acids present in cerebral spinal fluids (CSFs) is highlighted by efforts to understand neuron activity, cell– cell communication, and cell dysfunction [2–4]. g-Amino-butyric acid (GABA) is the main inhibitory amino acid
neurotransmitter in the mammalian central nervous sys-tem; in addition, GABAergic dysfunctions are thought to be present in various neurological and psychiatric dis-orders, including epilepsy, anxiety, and depression [5, 6]. Thus, rapid and sensitive approaches to the determina-tion of GABA and other amino acids, such as glutamine and glutamate, in CSF remain in high demand.
CE in conjunction with LIF has become one of the most powerful analytical tools for the determination of amino acids in biological samples because it offers the advan-tages of rapidity, high resolution, sensitivity, and flexibility, all while requiring very small sample sizes [7–11]. Be-cause of the small separation channels and narrow detection area, a bright and coherent laser is usually used – although it is one of the most expensive parts, especially in the case of UV-lasers – in a typical CE-LIF system. To reduce the cost of CE systems, while seeking to offer comparable sensitivity, light-emitting diodes (LEDs) have been tested as replacements for lasers [12–14]. LEDs offer a number of advantages over lasers, including small
Correspondence: Professor Huan-Tsung Chang, Department of
Chemistry, National Taiwan University, 1, Section 4, Roosevelt Road, Taipei 106, Taiwan
E-mail: changht@ntu.edu.tw Fax: 111-886-2-33661171
Abbreviations: CSF, cerebral spinal fluid; GABA, g-aminobutyric
acid; LED, light-emitting diode; LEDIF, LED-induced fluorescence;
NDA, naphthalene-2,3-dicarboxaldehyde; PEO, poly(ethylene oxide); TB, Tris–borate
size, low cost, ease of operation, and a wider range (UV and visible) of excitation wavelengths. The light from LEDs, however, is usually divergent and not pure. As a result, it is difficult to focus this light onto a small capillary, which leads to a relatively high fluorescence background. Several attempts have been made to overcome these drawbacks, including the so-called pigtailing approach [14] and the use of an iris and lens [15], a miniaturized liquid-core waveguide [16], and a time-discrimination and average acquisition system [17]. Recently, a focusing system consisting of a plastic lens and an objective was constructed to allow LED light to be focused accurately onto small capillaries [14].
Other than aromatic amino acids, such as tryptophan, most amino acids are not readily detectable in their native forms and, thus, pre-, in-, or postcolumn derivatization using chromogenic or fluorescent compounds is often required [18–20]. The most common fluorophores used for the derivatization of amino acids are o-phthalaldehyde [21], naphthalene-2,3-dicarboxaldehyde (NDA) [22], FITC [23], fluorescamine [24], and 5-furoylquinoline-3-carboxyaldehyde [25]. NDA reacts with primary amines in the presence of cyanide to form cyano[f]benzoisoindole (CBI) products that are thermally stable and highly fluo-rescent (lex= 420 nm; lem= 490 nm) [26–29]. Other
fea-tures of NDA that make it suited to the analysis of amino acids are that the derivatization is fast (,15 min) and complete (ca. 100%) [26]; these characteristics are essential when performing in-column derivatization. Despite the advantages of using NDA and LEDs, the sensitivity of the CE/LED-induced fluorescence detec-tion (CE-LEDIF) systems remains relatively poor when compared with that of CE-LIF systems. We have recently discovered that using a laboratory-made CE-LEDIF system in conjunction with NDA provides a LOD (at S/N = 3) for tryptophan that is ca. 20 times higher than that obtained by CE-LIF using a Nd:YAG UV laser at 266 nm (native fluorescence). The LOD at S/N 3 of the CE-LEDIF system for GABA is 0.8 mM, indicating its limitations for detecting GABA in CSF samples, which ranges in concentration from sub- to several micro-moles. A number of stacking techniques, including field amplification, ITP, pH mediation, pH junctions, and sweeping, have been applied to CE with great improvements in sensitivity [30–32]. For example, up to 1.46105-fold sensitivity improvements have been
demonstrated by sweeping in CE [33]. In addition to these methods, stacking of small solutes has been demonstrated using poly(ethylene oxide) (PEO) solution [34]. After sample injection, PEO (neutral) solution is introduced via EOF at the inlet end of the capillary. The analytes possess negative charges and thus migrate
toward PEO zone at high voltage. Because of the increased viscosity, changes in electric field, and their interactions with PEO, the analytes slow down so that they become stacked at the boundary between the sample zone and the PEO zone.
The aim of this study was to demonstrate the stacking, derivatization, and separation of NDA derivatives of amino acids using a laboratory-made CE-LEDIF system. After injecting the samples into the capillary, the amino acids react with CN2 ions and NDA when a voltage is
applied. After derivatization, the amino acid?NDA deriva-tives migrate to the PEO zone, where they stack upon applying a high voltage. We have carefully explored the effects that a number of factors – such as the concentra-tion of PEO, the plug lengths of the sample and deriva-tized mixture, the compositions of the derivaderiva-tized mixture, and the reaction times – have on the sensitivity and reso-lution of the analysis of these amino acids. Our ability to identify GABA, glutamine (Gln), serine (Ser), alanine (Ala), and glycine (Gly) in CSF samples highlights the practical potential of this method in diagnostic applications.
2 Materials and methods
2.1 ChemicalsThe amino acids, NaCN, and all of the chemicals required to prepare the BGEs were obtained from Sigma (St. Louis, MO, USA), except for PEO, methanol, ACN, and NDA. PEO (MW 8 000 000 g/mol) and (3-aminopropyl)triethoxy-silane were obtained from Aldrich (Milwaukee, WI, USA). Methanol and ACN were obtained from J. T. Baker (Phil-lipsburg, NJ, USA), while NDA was obtained from Tokyo Chemical Industry (Tokyo, Japan). Tris–borate (TB) solu-tions were prepared from Tris by using suitable amounts of boric acid to adjust the pH to 8.0 and 10.0. In this study, the molarity of Tris represents the concentration of the TB solutions. PEO solutions were prepared either in 10 mM tetraborate (pH 9.3) or 400 mM TB buffer (pH 8.0) [35]. NDA (10 mM) was dissolved in methanol according to lit-erature [27] and the amino acid solutions were prepared in 1.0 mM sodium tetraborate (pH 9.3). The stock solution of CN2 (100 mM) was prepared in deionized water. All the
prepared solutions were stored at 47C and used within a week.
2.2 CSF
CSF samples were obtained from the lower part of the lumbar spinal column while the patients were placed in a sitting position. The patients who were suspected to have bacterial meningitis were diagnosed in the Clinical
Laboratory Department of Mackay Memorial Hospital (Taipei, Taiwan). The CSF samples were stored in a freezer at 47C.
2.3 CE-LEDIF
The basic design of the CE-LEDIF system has been described previously [14]. Briefly, a high-voltage power supply (Gamma High Voltage Research, Ormond Beach, FL, USA) was used to drive electrophoresis. The entire system was placed in a black box possessing a high-voltage interlock. For safety, the high-high-voltage end of the separation system was placed in a laboratory-made plexiglass box. A violet LED (InGaN; maximum output at 405 nm in the range 390–420 nm), obtained from Kwang-Hwa Electronic Material (Taichung, Taiwan), was used for excitation. A laboratory-made power supply, which allowed the potentials to be adjusted up to 5.0 V, was used to drive the LED. In this study, the applied voltage was set at 3.9 V. The excitation light was focused on the capillary through a plastic lens (Raise Electro-Optics, Tai-pei, Taiwan) and a 406 objective (numerical aperture: 0.65). The fluorescence was collected through a 106 objective (numerical aperture: 0.25). One inter-ference filter (488 nm) was used to block scattered light before the emitted light reached the photomultiplier tube (R928, Hamamatsu Photonics K. K., Shizuoka-Ken, Japan). The fluorescence signal was transferred directly through a 10-kO resistor to a 24-bit A/D interface at 10 Hz (Borwin, JMBS Developments, Le Fontanil, France) and stored in a personal computer. Fused-silica capillaries (Polymicro Technologies, Phoenix, AZ, USA), having id of 75 mm and od of 365 mm, were 50 cm long (effective length: 40 cm).
2.4 Off-column derivatization
Derivatization of amino acids with NDA was conducted in 1.5-mL centrifuge tubes using a procedure modified from a previous report [26]. Appropriate volumes (5 mL) of amino acid stock solutions (1023M) were added to
solu-tions (485 mL) composed of sodium tetraborate (pH 9.3) and ACN. NaCN and NDA were then added to the mix-tures up to a final volume of 0.5 mL. The concentrations of the amino acids, tetraborate, ACN, NaCN, and NDA in the final mixture were 10 mM, 1 mM, 40%, 0.1 mM, and 0.1 mM, respectively. For the analysis of CSF, 50-mL samples that had not been pretreated were mixed with 10.0 mM sodium tetraborate (pH 9.3, 50 mL), 0.01 M NaCN (5 mL), 0.01 M NDA (5 mL), and 99.8% ACN (200 mL). The solutions were diluted with deionized H2O to
final volumes of 500 mL and were then left to react for 30 min prior to analysis.
2.5 Electrophoresis procedure
Prior to analysis, the capillaries were treated overnight with 0.5 M NaOH [36]. The derivatized standard samples were injected by hydrodynamic injection (30 cm height; 10 s) into a capillary filled with 10 mM tetraborate (pH 9.3). After sample injection, the two ends of the cap-illary were immersed in 0.6% PEO (prepared in tetra-borate, pH 9.3) solution and the separation was con-ducted at 15 kV. We note that it is not required to fill the outlet vial with 0.6% PEO solution. Although the separa-tion is shorter at high-electric fields, spikes due to arcing are more apparent in humid and dusty environments. In order to monitor the repeatability of EOF, 10 mM (3-ami-nopropyl)triethoxysilane?NDA derivative was analyzed in the absence of amino acid?NDA derivatives by CE-LEDIF. When using PEO, the peak for the derivative was detect-ed at the time that the baseline shiftdetect-ed (only can be seen in small scales). Thus, the migration times for the (3-ami-nopropyl)triethoxysilane?NDA derivative or the times when the baseline shifted were used to calculate the EOF mobilities under different conditions. After each run, the capillary was equilibrated with 0.5 M NaOH at 1 kV for 10 min – to remove PEO molecules and to refresh the capillary wall – and then filled with 10 mM tetraborate (pH 9.3). This treatment process was quite successful at generating a constant bulk EOF mobility; the RSD from five consecutive runs was below 1.5%.
For the analysis of CSF samples, the same electrophore-sis process for the analyelectrophore-sis of standard samples was applied with slight differences. The capillary was filled with 1.5 M TB (pH 10.0) instead of 10 mM tetraborate (pH 9.3) and 0.6% PEO solution was prepared in 400 mM TB (pH 8.0) instead of 10 mM tetraborate (pH 9.3). In this study, the molarity of Tris was used for that of the TB solutions. The adsorption of proteins on the capillary wall is reduced when using these conditions; this phenomenon leads to better resolution and reproducibil-ity. During the separations, the currents changed and eventually reached a maximum of 25 mA, suggesting small Joule heats generated.
2.6 In-column derivatization, stacking, and separation procedure
PEO (1.5%) was prepared in 10 mM tetraborate solution (pH 9.3). NaCN solution, the standard sample, and NDA were injected sequentially – by hydrodynamic means at 30 cm height for certain times – into a capillary filled with 10 mM tetraborate (pH 9.3); for more details, see Sec-tion 3. We point out that PEO soluSec-tion was introduced to the inlet of the capillary after NaCN, sample, and NDA. Then, a voltage (0.25 or 1.2 kV) was applied for 15 min,
unless otherwise noted, to allow mixing and reaction be-tween the reagents and the analytes. The separation voltage was either 15 or 20 kV (large sample volumes). Once the amino acid?NDA derivatives migrated to the PEO zone, they were stacked at the boundary. In order to estimate the sample length, we conducted hydrodynamic injection of the GABA?NDA derivative at 30 cm height while monitoring the baseline shift. The sample length was estimated to be 0.4 cm per 10 s.
For stacking of the derivatized CSF samples, 0.6% PEO solution was prepared in 400 mM TB (pH 8.0) instead of 10 mM tetraborate (pH 9.3). NaCN solution, the CSF sample, and NDA were injected sequentially – by hydro-dynamic means at 30 cm height for certain times – into a capillary filled with 1.5 M TB solution (pH 10.0); for more details, see Section 3. In order to achieve efficient stack-ing, a low-pH plug (5.0 mM phosphate containing 40% ACN, pH 5.0) was injected at 30 cm height for 30 s after injection of NDA. We point out that PEO solution was introduced to the inlet of the capillary after NaCN, sample, and NDA (and a low-pH plug for the analysis of CSF sample). Then, a voltage (0.25 or 1.2 kV) was applied for 15 min, unless otherwise noted, to allow mixing and reaction between the reagents and the analytes. The separation voltage was 20 kV.
3 Results and discussion
3.1 Effect of polymer solution on resolution
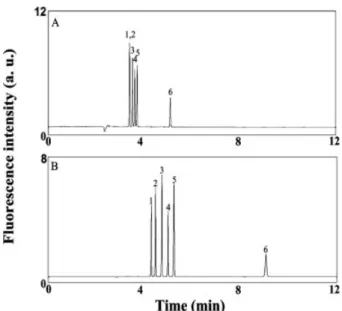
The concentration factor (sensitivity improvement) and resolution for amines and amino acids both increase upon increasing the PEO concentration [34], but the separation is slow when using high concentrations of PEO, mainly because of the small EOF, which results from increases in viscosity and the greater adsorption of PEO molecules onto the capillary wall. To test the role that PEO plays in separating the amino acid?NDA derivatives, we con-ducted the separation of six amino acid?NDA derivatives – those of Ala, GABA, glutamic acid (Glu), Gly, histidine (His), and phenylalanine (Phe) – in the absence and pres-ence of 0.5% PEO (pH 9.3) at 15 kV. We point out that the sensitivity is optimized at pH around 9.3 according to lit-erature [28]. The electropherogram depicted in Fig. 1A indicates that only the Phe and His derivatives were not separated when using a capillary filled with tetraborate solution (pH 9.3). In contrast, all six of the amino acid?-NDA derivatives were well resolved in the presence of 0.5% PEO (Fig. 1B). The fact that the migration times for the amino acid?NDA derivatives were longer than the time at which the baseline shifted (only can be seen in a ver-tical scale less than 1.0) indicates that they all bear
net-Figure 1. Separation of six amino acids (1025M) in the
absence and presence of PEO. A 50-cm capillary (effec-tive length: 40 cm) was filled with 10 mM tetraborate (pH 9.3) prior to separation. Separation voltages were 15 kV in both (A) and (B). Anodic and cathodic vials con-tained 10 mM tetraborate (pH 9.3) in (A) and concon-tained 0.5% PEO prepared in 10 mM tetraborate (pH 9.3) in (B). Hydrodynamic injection was conducted at 30 cm height for 10 s. Peak identities (NDA derivatives): (1) Phe; (2) His; (3) GABA; (4) Ala; (5) Gly; (6) Glu.
negative charges. We point out that the shift is due to detection of PEO (neutral) and the shift time is the same as the migration time for the neutral marker ((3-amino-propyl)triethoxysilane?NDA derivative) (not shown). The longer separation occurred because of a smaller EOF mobility in the presence of PEO, which occurred as a result of PEO adsorption and an increase in viscosity [37]. The EOF mobilities were 6.461024(RSD = 1.7%, n = 5)
and 9.161024 (RSD = 1.1%, n = 5) cm2V21s21 in the
presence and absence of PEO, respectively. Because of PEO adsorption, EOF gradually decreased during the separation. The analytes detected earlier experienced a higher bulk EOF compared to that detected later. As a result of greater apparent mobilities of any two adjacent analytes increased, a greater resolving power was achieved. The resolution values for the pairs of Phe/His, Ala/Gly, and Gly/Glu are 2.0, 2.6, and 27.2, respectively, in the presence of PEO, while they are 0, 1.6, and 18.3, respectively, in the absence of PEO. The improved re-solving power might possibly also be due to the interac-tions of the analytes with PEO molecules, mainly through hydrogen bonding and hydrophobic interactions [34]. In order to achieve run-to-run repeatability (RSD for EOF,1.5%), it is extremely important to treat the capillary with 0.5 M NaOH at 1 kV for 10 min.
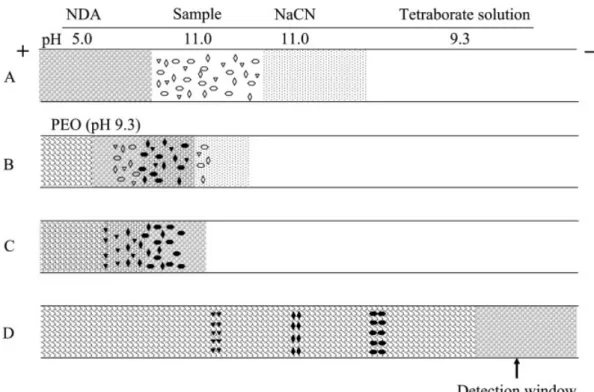
Figure 2. Representative mechanism of the process of in-column derivatization, stacking, and separation of amino acids. (A) Hydrodynamic injections at 30 cm height of NaCN (120 s, 4.8 cm), a standard amino acids solution or CSF (120 s, 4.8 cm), and NDA (120 s, 3.2 cm), respectively. (B) After injection, a lower voltage is applied to migrate CN2and the amino acids into the NDA zone (pH 5.0).
(C) Reagents, amino acids, and amino acid?NDA derivatives migrate to the PEO zone, where they then stack. (D) NDA derivatives are separated and detected at the cathodic side. NaCN and amino acids were prepared in a solution (pH 11.0) consisting of 1.0 mM tetraborate, 10 mM NaCl, 40% ACN, and NaOH (used to adjust pH). NDA was prepared in a solution (pH 5.0) of 1.0 mM tetra-borate, 10.0 mM NaCl, 40% ACN, and HCl (use to adjust pH). When CSF sample was analyzed, a low-pH plug of 5.0 mM phosphate solution containing 40% ACN (pH 5.0) was injected at 30 cm height for 30 s after NDA injection.
3.2 In-column derivatization, stacking, and separation under discontinuous conditions
To minimize the problems associated with the instability of amino acid?NDA derivatives and to simplify the CE system, we conducted in-column derivatization at room temperature using a slight modification of the approach described above. Based on their mobility and migration direction, cyanide, the amino acid sample, and NDA were injected sequentially into the capillary through hydro-dynamic means as indicated in Fig. 2A. For the analysis of CSF samples, a low-pH plug of phosphate solution was injected after NDA. Once the voltage (1.2 kV) was applied, CN2 and the amino acids (negative charges) migrated
against the EOF toward the anodic end and were mixed with NDA (neutral) as shown in Fig. 2B. During derivatiza-tion, CN2, the amino acids, and the negatively charged
amino acid?NDA derivatives migrated to the PEO zone. They slowed down and stacked at the boundary as
depicted in Fig. 2C. Finally, the applied voltage was increased to 15 kV (or 20 kV) to accelerate the separation process (Fig. 2D).
In our previous studies, we suggested that stacking of the analytes is mainly as a result of the increase in viscosity [22, 37, 38]. The viscosity of 0.5% PEO is 116 cP, which is much higher than aqueous solution (0.89 cP). We point out that the low-pH plug after NDA injection is not neces-sary when conducting stacking and separation of the off-column amino acid?NDA derivatives or when the sample is prepared in a low-conductivity solution (e.g., 1 mM tet-raborate at pH 11.0). In other words, pH changes and field amplification only play minor roles in stacking. The stacking efficiencies are comparable (less than 5%) in the absence and presence of the low-pH plug (in the pres-ence and abspres-ence of 40% ACN), supporting our reason-ing. However, it is important to prepare NDA in a solution (pH 5.0) of 1.0 mM tetraborate, 10.0 mM NaCl, 40% ACN,
and HCl (use to adjust pH). When amino acids and CN2
migrated to the NDA zone (low pH), they slow down and thus have greater time to mix together and complete reaction. By adding 10 mM NaCl to the low-conductivity solution, the adsorption problem was minimized [39]. The reasons for preparing standard samples and CN2 in
solutions at pH 11.0 are to have greater dissociation degrees of amino acids and to minimize PEO adsorption [40, 41]. When high-conductivity samples such as CSF are analyzed, the low-pH plug of phosphate solution containing 40% ACN is required to achieve great stacking efficiencies. The reason for applying the short plug of low-pH plug is to minimize salt effect on stacking when high-conductivity samples (e.g., CSF sample) are used [42]. In this case, field amplification plays a greater role in deter-mining the stacking efficiency [43] than that in low-con-ductivity samples. The maximum injection times in the presence and absence of 40% ACN in the low-pH plug were about 120 and 30 s, respectively, indicating the role of field amplification on the stacking. We also point out that adding ACN is useful to minimize the formation of the side products [26].
3.3 Reaction time
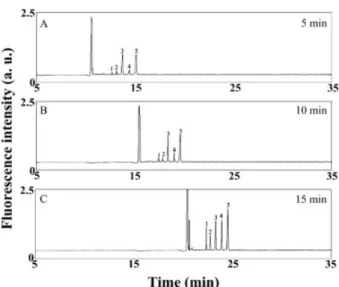
By measuring the fluorescence changes of the amino acid?NDA derivatives at different times using a fluo-rometer, we found that the reaction reached equilibrium within 15 min at room temperature under static condi-tions. Based on this result, we investigated the time de-pendence of the sensitivity and resolution over a time range from 5 to 15 min while performing in-column deri-vatization at pH 9.3 without applying a low-pH plug at 250 V. The condition was modified from those listed in Fig. 2 is to minimize the impacts of pH and field amplifi-cation on the sensitivity (stacking). The electro-pherograms depicted in Fig. 3 clearly indicate that the reaction time impacts the various amino acids in different ways. For the first 10 min, the relative magnitudes of the peaks for the Phe, His, GABA, Ala, and Gly derivatives are in good agreement with those obtained under static con-ditions. The peak heights for the derivatives of Phe, His, and Ala increased over time during a 15-min reaction pe-riod, whereas those of GABA and Gly increased less dra-matically after the first 5 min of the reaction. This situation arises mainly because GABA and Gly have greater reac-tion rates with NDA. From the data from the static experi-ments, we learned that the reactions of NDA with GABA and Gly were completed within 5 min. The peak height for Gly increased after 10 min, which is contrary to our find-ings from the static measurement results. To clarify the trend, we conducted the same experiment by injecting each individual analyte. The results (not shown) reveal
Figure 3. Impact of reaction time on in-column derivati-zation and separation of five amino acids by CE-LEDIF without a low-pH plug. (A)-(C) Derivatization was con-ducted at 250 V for 5, 10, and 15 min, respectively. Hydrodynamic injections of 1023M NaCN, 1026M amino
acids, and 1023M NDA were conducted at 30 cm height
for 30, 30, and 5 s, respectively. All solutions were pre-pared in 5 mM tetraborate (pH 9.3) containing 40% ACN. Capillary was filled with 10 mM tetraborate solution (pH 9.3); 1.5% PEO prepared in 10 mM tetraborate (pH 9.3) was introduced to the capillary through EOF dur-ing derivatization and separation. Separation voltage was 15 kV. Other conditions were the same as those provided in Fig. 1. Peak identities (NDA derivatives): (1) Phe; (2) His; (3) GABA; (4) Ala; (5) Gly.
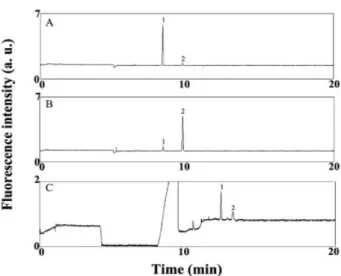
that the Gly?NDA derivative comigrated with a side prod-uct of the reaction of GABA with NDA. Figures 4A and B further suggest that the formation of the side product (dimeric or higher-order products of the GABA?NDA derivative) is dependent upon the concentrations of CN2
and NDA relative to that of GABA. At constant con-centrations of CN2and NDA, the side product is a minor
component at 1 mM GABA when conducting off-column derivatization (Fig. 4A), but it becomes a major one at 0.1 mM GABA (Fig. 4B). The peak area ratios of the GABA?NDA derivative to the major side product were 18:1 and 1:7 in Figs. 4A and B, respectively. When com-pared with off-column derivatization, the in-column deri-vatization mode provides less of the side product of GABA at 0.1 mM (Fig. 4C). We point out the peak area ratio of GABA?NDA derivative and the major side product was 3.4:1. We suggest that there is less chance of forming the side product of GABA when using the in-column derivati-zation mode because the GABA?NDA derivative is sepa-rated quite rapidly from the excess CN2 and NDA
reagents upon applying the electric field. Because the formation of the side product is slower than the formation
Figure 4. Impact of the GABA concentration on the for-mation of side products in (A and B) off-column and (C) in-column derivatization modes. GABA concentration was 1026M in (A) and 1027M in (B) and (C). Off-column
deri-vatization for 15 min: 1 mM sodium tetraborate (pH 9.3), 40% ACN, 1024M NaCN, and 1025M NDA. In-column derivatization: hydrodynamic injections of 1024M NaCN, 1027M GABA, and 1025M NDA were conducted at 30 cm height for 120, 120, and 80 s, respectively. NaCN and GABA were dissolved in 1 mM tetraborate (pH 11.0) containing 10 mM NaCl and 40% ACN; NDA was dis-solved in a solution (pH 5.0) consisting of 1.0 mM tetra-borate, 10 mM NaCl, 40% ACN, and HCl (used to adjust pH). Voltage was applied at 1.2 kV for 15 min during deri-vatization and stacking and then at 20 kV during separa-tion. Peak identities: (1) GABA-NDA derivative; (2) major side product. Other conditions were the same as those provided in Fig. 3.
of the amino acid?NDA derivatives, the interference from the side product can be minimized by conducting the reaction for a shorter period of time (,10 min) when con-ducting off-column derivatization.
3.4 Analysis of standard samples
From the off-column derivative reactions, we learned that the formation of the side products (dimeric or higher-order complexes) [26] increases upon increasing both the con-centrations of CN2and NDA and the reaction time. When
applying this in-column derivatization, stacking, and separation technique for the analysis of GABA?NDA deri-vatives, the sample plug length also affects the relative yields of the major and side products. With a shorter sample injection time, the GABA?NDA derivative has a greater chance of remaining in the zone containing the NDA and CN2. As a result, the side products are more
dominant. On the other hand, there are fewer CN2ions in
the zone containing the GABA?NDA derivative and NDA
when the length of the sample plug is longer. Because CN2(28.061024cm2V21s21) migrates faster than GABA
(23.061024cm2V21s21) and NDA (neutral) [44], the
amino acids were derivatized when CN2 (4.8 cm plug
length) migrated to NDA zone after a 120-s sample injec-tion (4.8 cm plug length) at 1.2 kV. It took about 4 min for the CN2reaching the boundary of the NDA zone and the
sample zone. The mixing of the amino acids, CN2, and
NDA (3.2 cm plug length) inside the capillary was com-pleted within ca. 10 min. At constant plug lengths of 100 mM CN2 (injection time: 120 s; 4.8 cm) and
10 mM NDA (injection time: 80 s; 3.2 cm), the peak pro-files for the GABA?NDA derivative at 0.1 mM GABA are sharp for injection times of up to 120 s; this finding indi-cates that efficient stacking of the GABA?NDA derivatives occurs. Table 1 indicates that the intensity of the peak for the GABA?NDA derivative increased upon increasing the injection time and that the side products become minor when the sample plug was injected for 120 s. When injecting the sample containing GABA for 120 s, the linear range of GABA exists from 1025to 1028M (R2= 0.99). The LOD and the LOQ at S/N = 10 for GABA were 4.2 and 14 nM, respectively. Using these same conditions, we separated the five amino acids (Phe, His, GABA, Ala, and Gly) within 13 min (Fig. 5). The RSD for the migration times and the peak areas for the five analytes were less than 2.5 and 7.0%, respectively. The LODs for Phe, His, Ala, and Gly were 41, 23, 10, 3.2 nM, respectively, while their LOQs were 137, 77, 33, 11 nM, respectively. The sharp and symmetric peak profiles again infer high stacking efficiencies. The migration time for NDA-GABA derivative is slightly shorter when compared to that in Fig. 4C, mainly because of decreases in the viscosity of PEO at higher ambient temperature. We note that there was no cooling system in our lab-made CE-LEDIF system and the ambient temperature varied between 15 and 227C. Interesting to note that the fall of the baseline before
Table 1. Effect of hydrodynamic injection time on peak heights for the GABA-NDA derivative and side products in the in-column derivatization modea)
Injection time (s)
Fluorescence intensity (mV)
GABA Minor side
product Major side product 10 70 198 1195 30 169 230 1272 60 307 114 778 90 497 106 568 120 866 53 255
a) Conditions are the same as those detailed in Fig. 4C, except for the sample injection time.
Figure 5. In-column derivatization, stacking, and separation of five amino acids (1027M) by CE-LEDIF.
Other conditions were the same as those provided in Fig. 4C. Peak identities (NDA derivatives): (1) Phe; (2) His; (3) GABA; (4) Ala; (5) Gly.
the large peak is slightly significant in the case of Fig. 4C when compared to that in Fig. 5, mainly because of a greater excess amount of NDA and smaller ionic strength in the sample zone.
3.5 Analysis of CSF samples
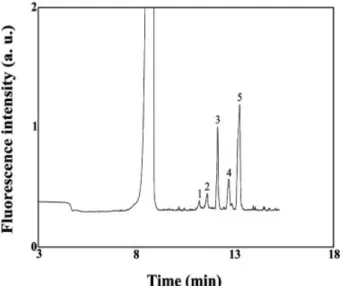
The results above suggest that our proposed method should be practical for the determination of GABA in bio-logical samples, such as CSF. To test this hypothesis, we used our in-column derivatization, stacking, and separa-tion approach to determine GABA in a CSF sample. We note that the separation was irreproducible and peak broadening occurred as a result of adsorption of the matrixes on the capillary wall when using 10 mM tetra-borate solution (pH 9.3) to fill the capillary and prepare the PEO solution. To minimize adsorption, we filled the capil-lary with 1.5 M TB (pH 10.0) and prepared 0.6% PEO in 400 mM TB (pH 9.0). We have demonstrated previously that such conditions effectively minimize protein adsorp-tion [37]. In Fig. 6A, we observe that many peaks,
includ-Figure 6. Electropherograms of a CSF sample when conducting (A) in-column and (B) off-column derivatization. For in-column derivatization, hydrodynamic injections of 1023M NaCN, diluted CSF
(1:2 v/v), and 1023M NDA were conducted at 30 cm height for 4, 2 min, and 80 s, respectively. For
off-column derivatization, hydrodynamic injections of the derivatized CSF sample (1:10 dilution) and low-pH plug (low-pH 5.0) were conducted at 30 cm height for 2 and 0.5 min, respectively. Prior to sample injection, the capillary was filled with 1.5 M TB (pH 10.0). Separations were conducted at 20 kV using 0.6% PEO prepared in 400 mM TB (pH 8.0).
ing those for the NDA derivatives of Gly, Ala, Ser, GABA, and Gln, were obtained after injecting the CSF sample, without any sample pretreatment, for 120 s. We note that it was necessary to use 400 mM TB buffer to prepare the PEO solution if we were to achieve baseline resolution of the amino acids of interest. With a high ionic strength, we expected a small bulk EOF. The adsorption of PEO is also greater at pH 8.0 than it is at pH 9.0, which leads to a small bulk EOF. To determine the concentrations of the five amino acids, the standards were spiked into the CSF samples. Based on the linear regression (each
R2 . 0.97), we determined the mean concentrations
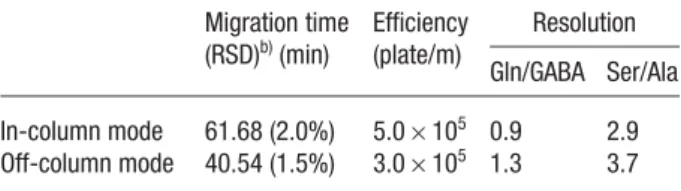
(n = 5) of Gly, Ala, Ser, GABA, and Gln in the CSF sample to be 13.6, 72.9, 62.5, 2.04, and 837 mM, respectively; these values are in good agreement with the reported results [45]. For comparison, the CSF sample derivatized with NDA in an off-column mode was injected for 120 s and separated under similar conditions. Figure 6B and Table 2 indicate that the off-column derivatization approach provides greater sensitivity and resolution. The separation time is also shorter because of the lower degree of PEO adsorption when only the CSF mixture (high salt) was injected. We note that PEO adsorption decreases upon increasing the ionic strength. The greater sen-sitivity is due mainly to the longer reaction time. Be-cause only small amounts of proteins (15–40 mg/dL) were present in the CSF samples and no protein precipitation occurred in the off-column reaction, we suggest that the greater detection sensitivity obtained in the off-column derivatization method as a result of an increased amount of amino acids released from proteins (as compared to the on-line derivatization method) should be negligible. Although the sensitivity is slightly lower and the separation time is longer, the minute sample volumes required and the lower amounts of side products formed indi-cate the merits of using our proposed in-column derivatization, stacking, and separation approach for diagnostic purposes.
Table 2. Comparison of the in-column and off-column CE approaches to the determination of GABA in CSF samplesa) Migration time (RSD)b)(min) Efficiency (plate/m) Resolution Gln/GABA Ser/Ala In-column mode 61.68 (2.0%) 5.06105 0.9 2.9 Off-column mode 40.54 (1.5%) 3.06105 1.3 3.7
a) Experimental conditions are the same as those described in Fig. 6.
b) n = 5.
4 Concluding remarks
In this article, we present an in-column derivatization, stacking, and separation approach for the determination of amino acids by CE-LEDIF. To optimize the resolution and sensitivity, it is important to control the relative con-centration and plug lengths of the injected samples of CN2, NDA, and amino acids. The approach provides an
LOD of 4 nM for GABA when injecting for 120 s and it allows the determination of GABA, Gly, and Glu in CSF samples. When compared to the off-column derivatiza-tion mode, the in-column derivatizaderivatiza-tion mode offers the advantage of providing a lower degree of interference from the side products of the reaction between GABA and NDA. As a result, the linear range spans three orders of magnitude (from 1025to 1028M); this feature makes the
proposed approach suitable for the determination of GABA in a range of biological samples. With its simplicity, low cost, rapidity, and great sensitivity, this approach displays great potential for the determination of GABA
in vivo. Thus, it is our future goal to combine this approach
with microdialysis techniques to explore the impacts that drugs have on the uptake and release of GABA in the central nervous system.
This work was supported by the National Science Council of Taiwan under contracts NSC 2113-M-002-034, 93-2113-M-002-035 and 94-2113-M-002-008. T.-C. Chiu is grateful to Academia Sinica for his postdoctoral fellow-ship at IAMS.
5 References
[1] Hamase, K., Morikawa, A., Zaitsu, K., J. Chromatogr. B 2002, 781, 73–91.
[2] Larson, A., Giovengo, S. L., Russell, I. L., Michalek, J. E.,
Pain 2002, 87, 201–211.
[3] Shah, A. J., Crespi, F., Heidbreder, C., J. Chromatogr. B 2002, 781, 151–163.
[4] Sethuraman, R., Lee, T. L., Tachibana, S., Clin. Chem. 2004,
50, 665–669.
[5] Owens, D. F., Kriegstein, A. R., Nat. Rev. Neurosci. 2002, 3, 715–727.
[6] Smith, A. J., Simpson, P. B., Anal. Bioanal. Chem. 2003, 377, 843–851.
[7] Lee, I.-H., Pinto, D., Arriaga, E. A., Zhang, Z., Dovichi, N. J.,
Anal. Chem. 1998, 70, 4546–4548.
[8] Lin, Y.-W., Chiu, T.-C., Chang, H.-T., J. Chromatogr. B 2003,
793, 37–48.
[9] Ma, Y., Liu, G., Du, M., Stayton, I., Electrophoresis 2004, 25, 1473–1484.
[10] Huck, C. W., Stecher, G., Bakry, R., Bonn, G. K.,
Electro-phoresis 2003, 24, 3977–3997.
[11] Quigley, W. W. C., Dovichi, N. J., Anal. Chem. 2004, 76, 4645–4658.
[12] King, M., Paull, B., Haddad, P. R., Macka, M., Analyst 2002,
[13] Su, A.-K., Lin, C.-H., J. Chromatogr. B 2003, 785, 39–46. [14] Chen, S.-J., Chen, M.-J., Chang, H.-T., J. Chromatogr. A
2003, 1017, 215–224.
[15] Wang, S. C., Morris, M. D., Anal. Chem. 2000, 72, 1448– 1452.
[16] Wang, S.-L., Huang, X.-J., Fang, Z.-L., Anal. Chem. 2001,
73, 4545–4549.
[17] Hillebrand, S., Schoffen, J. R., Mandaji, M., Termignoni, C.,
Electrophoresis 2002, 23, 2445–2448.
[18] Ummadi, M., Weimer, B. C., J. Chromatogr. A 2002, 964, 243–253.
[19] Ye, M., Hu, S., Quigley, W. W. C., Dovichi, N. J., J.
Chroma-togr. A 2004, 1022, 201–206.
[20] Oguri, S., Hibino, M., Mizunuma, M., Electrophoresis 2004,
25, 1810–1816.
[21] Kennedy, R. T., Thompson, J. E., Vickroy, T. W., J. Neurosci.
Methods 2002, 114, 39–49.
[22] Lu, M.-J., Chiu, T.-C., Chang, P.-L., Ho, H.-T., Chang, H.-T.,
Anal. Chim. Acta 2005, 538, 143–150.
[23] Li, H., Wang, H., Chen, J.-H., Wang, L.-H., J. Chromatogr. B 2003, 788, 93–101.
[24] Chan, K. C., Muschik, G. M., Issaq, H. J., Electrophoresis 2000, 21, 2062–2066.
[25] Wu, J., Chen, Z., Dovichi, N. J., J. Chromatogr. B 2000, 741, 85–88.
[26] Kawasaki, T., Higuchi, T., Imai, K., Wong, O. S., Anal.
Bio-chem. 1989, 180, 279–185.
[27] Roach, M. C., Harmony, M. D., Anal. Chem. 1987, 59, 411– 415.
[28] de Montigny, P., Stobaugh, J. F., Givens, R. S., Carlson, R. G., Anal. Chem. 1987, 59, 1096–1101.
[29] Robert, F., Bert, L., Denoroy, L., Renaud, B., Anal. Chem. 1995, 67, 1838–1844.
[30] Osbourn, D. M., Weiss, D. J., Lunte, C. E., Electrophoresis 2000, 21, 2768–2779.
[31] Chang, H.-T., Chiu, T.-C., G.I.T. Lab. J. 2003, 2, 64–65. [32] Urbánek, M., Krˇivánková, L., Bocˇek, P., Electrophoresis
2003, 24, 466–485.
[33] Isoo, K., Terabe, S., Anal. Chem. 2003, 75, 6789–6798. [34] Hsieh, M.-M., Hsu, C.-E., Tseng, W.-L., Chang, H.-T.,
Elec-trophoresis 2002, 23, 1633–1640.
[35] Kuo, I.-T., Chiu, T.-C., Chang, H.-T., Electrophoresis 2003,
24, 3339–3347.
[36] Ho, H.-T., Chang, P.-L., Hung, C.-C., Chang, H.-T., J. Clin.
Microbiol. 2004, 42, 3525–3531.
[37] Tseng, W.-L., Chang, H.-T., Anal. Chem. 2000, 72, 4805– 4811.
[38] Chiu, T.-C., Lin, Y.-W., Huang, C.-C., Chrambach, A., Chang, H.-T., Electrophoresis 2003, 24, 1730–1736.
[39] Huang, C.-C., Hsieh, M.-M., Chiu, T.-C., Chang, H.-T.,
Elec-trophoresis 2001, 22, 4328–4332.
[40] Preisler, J., Yeung, E. S., Anal. Chem. 1996, 68, 2885–2889. [41] Chen, H.-S., Chang, H.-T., Anal. Chem. 1999, 71, 2033–
2036.
[42] Tseng, W.-L., Chang, H.-T., J. Chromatogr. A 2001, 924, 93– 101.
[43] Choy, T. M. H., Chan, W.-H., Lee, A. W. M., Huie, C. W.,
Electrophoresis 2003, 24, 3116–3123.
[44] Veledo, M. T., de Frutos, M., Diez-Masa, J. C., J.
Chroma-togr. A 2005, 1079, 335–343.
[45] Sethuraman, R., Lee, T. L., Tachibana, S., Clin. Chem. 2004,