Running Head: Anti-inflammatory activities of Cinnamomum cassia Constituents 1
2
Anti-inflammatory Activities of Cinnamomum cassia Constituents in vitro
3
and in vivo
4 5
Jung-Chun Liaoa, Jeng-Shyan Dengb, Chuan-Sung Chic, d, Wen-Chi Houe, Shyh-Shyun 6
Huanga, Pei-Hsin Shiec, Guan-Jhong Huangc,* 7
8 a
School of Pharmacy, College of Pharmacy, China Medical University, Taichung 404, 9
Taiwan 10
b
Department of Health and Nutrition Biotechnology, Asia University, Taichung 413, 11
Taiwan 12
c
School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, College 13
of Pharmacy, China Medical University, Taichung 404, Taiwan. 14
d
Nursing Department, Hsin Sheng College of Medical Care and Management, Taoyuan 325, 15
Taiwan. 16
e
Graduate Institute of Pharmacognosy, Taipei Medical University, Taipei 250, Taiwan. 17 18 * Corresponding author: 19 Guan-Jhong Huang 20
School of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, College of 21
Pharmacy, China Medical University, Taichung 404, Taiwan. 22
Tel: +886- 4- 2205-3366. Ext: 5508. Fax: +886- 4-2208-3362, 23
E-mail address: gjhuang@mail.cmu.edu.tw 24
25 26
Abstract
27
In this study, we have investigated the anti-inflammatory effects of Cinnamomum 28
cassia constituents (cinnamic aldehyde, cinnamic alcohol, cinnamic acid, and coumarin) 29
using lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW264.7) in vitro and 30
carrageenan (Carr)-induced mouse paw edema model in vivo. When RAW264.7
31
macrophages were treated with cinnamic aldehyde together with LPS, a significant
32
concentration-dependent inhibition of nitric oxide (NO), tumor necrosis factor (TNF-α),
33
and prostaglandin E2 (PGE2) levels productions were detected. Western blotting 34
revealed that cinnamic aldehyde blocked protein expression of inducible nitric oxide
35
synthase (iNOS), cyclooxygenase-2 (COX-2), nuclear transcription factor kappa B
36
(NF-B), and IB, significantly.
37
In the anti-inflammatory test, cinnamic aldehyde decreased the paw edema at the 4th 38
and the 5th h after -carrageenin (Carr) administration, and increased the activities of 39
catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in the 40
paw tissue. We also demonstrated cinnamic aldehyde significantly attenuated the 41
malondialdehyde (MDA) level and myeloperoxidase (MPO) activity in the edema paw at 42
the 5th h after Carr injection. Cinnamic aldehyde decreased the NO, TNF-α, and PGE2 43
levels on the serum level at the 5th h after Carr injection. Western blotting revealed that 44
cinnamic aldehyde decreased Carr-induced iNOS, COX-2, and NF-B expressions at the 45
5th h in the edema paw. An intraperitoneal (i.p.) injection treatment with cinnamic 46
aldehyde also diminished neutrophil infiltration into sites of inflammation as did 47
indomethacin (Indo). The anti-inflammatory mechanisms of cinnamic aldehyde might be 48
related to the decrease in the level of MDA, MPO, iNOS, and COX-2 via increasing the 49
activities of CAT, SOD, and GPx in the edema paw through the suppression of NO, 50
TNF-, and PGE2. These findings demonstrated that cinnamic aldehyde has excellent 51
anti-inflammatory activities in vitro and in vivo and thus have great potential to be used 52
as a source for natural health products. 53
54
KEY WORDS: Chinese medicine; Cinnamic aldehyde; Anti-inflammation; NO; TNF-α.
55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72
INTRODUCTION
73
Inflammation is recognized as a biological process in response to tissue injury. At the 74
injury site, an increase in blood vessel wall permeability followed by migration of 75
immune cells can lead edema formation during inflammation [1]. Inflammation leads to 76
the up-regulation of a series of enzymes and signaling proteins in affected cells and 77
tissues. Inducible nitric oxide synthase (iNOS), a member of the NOS protein family, 78
catalyzes the formation of nitric oxide (NO) from L-arginine [2]. Low concentration of 79
NO produced by iNOS is likely to contribute to the antimicrobial activity of macrophages 80
against certain bacterial pathogens. Lipopolysaccharide (LPS) is an endotoxin and a 81
constituent of the outer membrane of gram-negative bacteria. LPS stimulates innate 82
immunity, by regulating the productions of inflammatory mediators, like, NO, TNF-α, 83
and Interleukin-6 [3]. And in the animal the inflammation model of a carrageenan (Carr) 84
induced edema is usually used to assess the contributionof natural products in resisting 85
the biochemical changes associated with acute inflammation. Carr can induce acute 86
inflammation beginning with infiltration of phagocytes, the production of free radicals as 87
wellas the release of inflammatory mediators [4]. The resulting inflammation has been 88
shown to be associated with a number of chronic diseases, including asthma, rheumatoid 89
arthritis, inflammatory bowel disease, atherosclerosis, and Alzheimer’s disease, and also 90
has a role in various human cancers [5]. 91
Intracellular antioxidant mechanisms against these inflammatory stresses involve 92
antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and 93
glutathione peroxidase (GPx) in tissues. Recently, it has been shown that faulty cellular 94
antioxidant systems cause organisms to develop a series of inflammatory and cancer 95
diseases [6]. However, it appears that the various roles of enzymatic antioxidants help to 96
protect organisms from excessive generation of oxidative stress in the inflammatory 97
process, which has triggered studies focusing on the role of natural products in 98
suppressing the production of oxidation by increasing enzymatic antioxidants in tissues 99
[7]. 100
Cinnamomum cassia (C. cassia), bark is the outer skin of an evergreen tall tree 101
belonging to the family Lauraceae. It is commonly used as traditional Chinese medicine 102
for treating dyspepsia, gastritis, blood circulation disturbances, and inflammatory 103
diseases. Its extracts contain several active components such as essential oils (cinnamic 104
aldehyde, cinnamic alcohol, cinnamic acid, and coumarin), tannin, mucus and 105
carbohydrates [8]. C. cassia has been shown to have many pharmacological properties, 106
such as antiulcerogenic, anti-inflammatory, antipyretic, antimicrobial, antidiabetic and 107
anti-tumor activity [9, 10]. However, in this paper we examined that cinnamic aldehyde 108
was the most potent anti-inflammatory constituent of C. cassia on LPS-induced in 109
RAW264.7 cells and Carr-induced on paw edema in mice. And we detected the levels of 110
iNOS, COX-2, and NF-B in either RAW264.7 cell or paw edema. Also, the activities of 111
CAT, SOD, and GPx in the paw tissue at the 5th h after Carr injection were measured to 112
understand the relationship between the anti-inflammatory mechanism of cinnamic 113
aldehyde and antioxidant enzymes. 114
115
Materials and methods
116
Chemicals
LPS (endotoxin from Escherichia coli, serotype 0127:B8), Carr, indomethacin, 118
cinnamic aldehyde (≥ 98%), cinnamic alcohol (≥ 98%), cinnamic acid (≥ 99%), 119
coumarin (≥ 99%) (Fig. 1A) and other chemicals were purchased from Sigma Chemical 120
Co. (St. Louis, USA). TNF-α and PGE2 were purchased from Biosource International Inc. 121
(Camarillo, CA, USA). Anti-iNOS, anti-COX-2, anti-NF-B, anti-IB, and anti-β-actin 122
antibody (Santa Cruz, USA) and a protein assay kit (Bio-Rad Laboratories Ltd., Watford, 123
Herts, U.K.) were obtained as indicated. Poly-(vinylidene fluoride) membrane 124
(Immobilon-P) was obtained from Millipore Corp. (Bedford, MA, USA). 125
126
Animals
127
6-8 weeks male imprinting control region (ICR) mice were obtained from the 128
BioLASCO Taiwan Co., Ltd. The animals were kept in plexiglass cages at a constant 129
temperature of 22 ±1°C, and relative humidity of 55 ± 5 % with 12 h dark-light cycle for 130
at least 2 week before the experiment. They were given food and water ad libitum. All 131
experimental procedures were performed according to the National Institutes of Health 132
(NIH) Guide for the Care and Use of Laboratory Animals. In addition, all tests were 133
conducted under the guidelines of the International Association for the Study of Pain. 134
After a 2-week adaptation period, male ICR mice (18-25 g) were randomly assigned 135
to four groups (n=6) of the animals in the study. The control group receives normal saline 136
(i.p.). The other three groups include a Carr-treated, a positive control (Carr + Indo) and 137
cinnamic aldehyde administered groups (Carr + cinnamic aldehyde). 138
Cell culture
140
A murine macrophage cell line RAW264.7 (BCRC No. 60001) was purchased from
141
the Bioresources Collection and Research Center (BCRC) of the Food Industry Research 142
and Development Institute (Hsinchu, Taiwan). Cells were cultured in plastic dishes 143
containing Dulbecco’s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) 144
supplemented with 10% fetal bovine serum (FBS, Sigma, USA) in a CO2 incubator (5% 145
CO2 in air) at 37°C and subcultured every 3 days at a dilution of 1:5 using 0.05% 146
trypsin–0.02% EDTA in Ca2+-, Mg2+- free phosphate-buffered saline (DPBS). 147
148
Cell viability
149
Cells (2 x 105) were cultured in 96-well plate containing DMEM supplemented with 150
10% FBS for 1 day to become nearly confluent. Then cells were cultured with cinnamic 151
aldehyde, cinnamic alcohol, cinnamic acid, and coumarin in the presence of 100 ng/mL 152
LPS (lipopolysaccharide) for 24 h. After that, the cells were washed twice with DPBS 153
and incubated with 100 L of 0.5 mg/mL MTT for 2 h at 37°C testing for cell viability 154
{MTT, (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide)}. The medium 155
was then discarded and 100 L dimethyl sulfoxide (DMSO) was added. After 30-min 156
incubation, absorbance at 570 nm was read using a microplate reader. 157
158
Measurement of Nitric oxide/Nitrite
159
NO production was indirectly assessed by measuring the nitrite levels in the cultured
160
media and serum determined by a colorimetric method based on the Griess reaction [4]. 161
The cells were incubated with cinnamic aldehyde, cinnamic alcohol, cinnamic acid, 162
coumarin (0, 6.25, 12.5, 25, and 50 M) in the presence of LPS (100 ng/mL) at 370C for 163
24 h. Then, cells were dispensed into 96-well plates, and 100 mL of each supernatant was 164
mixed with the same volume of Griess reagent (1% sulfanilamide, 0.1% naphthyl 165
ethylenediamine dihydrochloride and 5% phosphoric acid) and incubated at room 166
temperature for 10 min, the absorbance was measured at 540 nm with a Micro-Reader 167
(Molecular Devices, Orleans Drive, Sunnyvale, CA). Serum samples were diluted four 168
times with distilled water and deproteinized by adding 1/20 volume of zinc sulfate (300 169
g/L) to a final concentration of 15 g/L. After centrifugation at 10,000×g for 5 min at room 170
temperature, 100 μL supernatant was applied to a microtiter plate well, followed by 100 171
μL of Griess reagent. After 10 min of color development at room temperature, the 172
absorbance was measured at 540 nm with a Micro-Reader. By using sodium nitrite to 173
generate a standard curve, the concentration of nitrite was measured by absorbance at 540 174 nm. 175 176 Carr-induced Edema 177
The Carr-induced hind paw edema model was used for determination of
178
anti-inflammatory activity [1]. Animals were i.p. treated with cinnamic aldehyde (1.25, 179
2.5 and 5 mg/kg), Indo or normal saline, 30 min prior to injection of 1% Carr (50 μL) in 180
the plantar side of right hind paws of the mice. The paw volume was measured after Carr 181
injection and at 1, 2, 3, 4, and 5 h intervals after the administration of the edematogenic 182
agent using a plethysmometer (model 7159, Ugo Basile, Varese, Italy). The degree of 183
swelling induced was evaluated by the ratio a/b, where a is the volume of the right hind 184
paw after Carr treatment, and b is the volume of the right hind paw before Carr treatment. 185
Indo was used as a positive control. After 5 h, the animals were sacrificed and the 186
Carr-induced edema feet were dissected and stored at -80 ºC. Also, blood were 187
withdrawn and kept at -80 ºC. The protein concentration of the sample was determined by 188
the Bradford dye-binding assay (Bio-Rad, Hercules, CA). 189
190
MDA Assay
191
MDA from Carr-induced edema foot was evaluated by the thiobarbituric acid reacting
192
substance (TRARS) method [1]. Briefly, MDA reacted with thiobarbituric acid in the 193
acidic high temperature and formed a red-complex TBARS. The absorbance of TBARS 194
was determined at 532 nm. 195
196
Myeloperoxidase Activity Assay
197
The activity of tissue MPO was assessed at the 5th h after injection of Carr into
198
the mouse right hind paw according to the method of Bani et al. [11] with some
199
modifications. Samples were placed in 0.75 mL of 80 mM phosphate-buffered
200
saline (PBS), pH 5.4, and then homogenized in a motor-driven homogenizer. The
201
homogenate was centrifuged at 12,000 × g at 4 °C for 15 min. Triplicate 0.1 mL of
202
supernatant with 2.9 mL of potassium phosphate buffer (50 mM, pH 6) containing 0.19
203
mg/mL of o-dianisidine chloride and 0.0005% H2O2 was a substrate for myeloperoxidase. 204
Oxidized o-dianisidine formed a soluble chromophore and absorbance (OD460) was 205
determined by spectrophotometry (Molecular Devices, Orleans Drive, Sunnyvale, CA)
over 2 min. Myeloperoxidase activity ( OD460) was calculated by subtracting the value 207
of OD460 at time 0 min from that at 2 min for each sample. 208
209
Measurement of TNF-α and PGE2 by an Enzyme-Linked Immunosorbent Assay
210
(ELISA). The levels of TNF- and PGE2 were determined using a commercially 211
available ELISA kit (Biosource International Inc., Camarillo, CA) according to the 212
manufacturer’s instruction. TNF- and PGE2were determined from a standard curve. 213
214
Antioxidant Enzyme Activity Measurements
215
The following biochemical parameters were analyzed to check the paw tissues 216
activity of cinnamic aldehyde by the methods given below. 217
Total SOD activity was determined by the inhibition of cytochromec reduction [12].
218
The reduction of cytochrome c was mediated by superoxide anions generated by the 219
xanthine/xanthine oxidase system and monitored at 550 nm. One unit of SOD was 220
defined as the amount of enzyme requiredto inhibit the rate of cytochrome c reduction by 221
50%. Total CAT activity was based on that of Aebi [13]. In brief, the reduction of 10mM 222
H2O2 in 20 mM of phosphate buffer (pH 7.0) was monitored by measuring the absorbance 223
at 240 nm. The activity was calculated using a molar absorption coefficient, and the 224
enzyme activities were defined as nanomoles of dissipating hydrogen peroxide per 225
milligram protein per minute. Total GPx activity in cytosol was determined according to 226
Paglia and Valentine’s method [14]. The enzyme solution was added to a mixture 227
containing hydrogen peroxide and glutathione in 0.1 mM Tris buffer (pH 7.2) and the 228
absorbance at 340 nm was measured. Activity was evaluated from a calibration curve, 229
and the enzyme activities were defined as nanomoles of NADPH oxidized per milligram 230
protein per minute. 231
232
Protein Lysate Preparation and Western blot Analysis of iNOS, COX-2, IB, and
233
NF-B
234
The stimulated murine macrophage cell line RAW264.7 cells were washed with 235
PBS and lysed in an ice-cold lysis buffer [10% glycerol, 1% Triton X-100, 1mM Na3VO4, 236
1mM EGTA, 10mM NaF, 1mM Na4P2O7, 20 mM Tris buffer (pH 7.9), 100 mM 237
-glycerophosphate, 137 mM NaCl, 5 mM EDTA, and one protease inhibitor cocktail 238
tablet (Roche, Indianapolis, IN, USA)] on ice for 1 h, followed by centrifugation at 239
12,000 rpm for 30 min at 4°C. Soft tissues were removed from individual mice paws and 240
homogenized in a solution containing 10 mM CHAPS, 1 mM phenylmethylsulphonyl 241
fluoride (PMSF), 5 g/mL, aprotinin, 1 M pepstatin and 10 M leupeptin. The 242
homogenates were centrifuged at 12,000g for 20 min, and 30 g of protein from the 243
supernatants was then separated on 10% sodium dodecylsulphate–polyacrylamide gel 244
(SDS-PAGE) and transferred to polyvinylidene difluoride membranes. After transfer, the 245
membrane was blocked for 2 h at room temperature with 5% skim milk in Tris-buffered 246
saline-Tween (TBST; 20 mM Tris, 500 mM NaCl, pH 7.5, 0.1% Tween 20). The 247
membranes were then incubated with mouse monoclonal anti-iNOS, anti-COX-2, 248
anti-IB, or anti-NF-B antibody in 5% skim milk in TBST for 2 h at room temperature. 249
The membranes were washed three times with TBST at room temperature and then 250
incubated with a 1 : 2000 dilution of anti-mouse IgG secondary antibody conjugated to 251
horseradish peroxidase (Sigma, St Louis, MO, U.S.A.) in 2.5% skim milk in TBST for 1 252
h at room temperature. The membranes were washed three times and the immunoreactive 253
proteins were detected by enhanced chemiluminescence (ECL) using hyperfilm and ECL 254
reagent (Amersham International plc., Buckinghamshire, U.K.). The results of Western 255
blot analysis were quantified by measuring the relative intensity compared to the control 256
using Kodak Molecular Imaging Software (Version 4.0.5, Eastman Kodak Company, 257
Rochester, NY) and represented in the relative intensities. 258
259
Histological Examination. For histological examination, biopsies of paws were taken 5
260
h following the interplanetary injection of Carr. The tissue slices were fixed in a solution 261
(1.85% formaldehyde, 1% acetic acid) for 1 week at room temperature, dehydrated by 262
graded ethanol and embedded in Paraffin (Sherwood Medical). Sections (thickness 5 μm) 263
were deparaffinized with xylene and stained with hematoxylin and eosin (H&E) stain. All 264
samples were observed and photographed with BH-2 Olympus microscopy. Every 3~5 265
tissue slices were randomly chosen from Carr, Indo and cinnamic aldehyde-treated (5 266
mg/kg) groups. Histological examination of these tissue slices revealed an excessive 267
inflammatory response with massive infiltration of neutrophils [ploymorphonuclear 268
leukocytes (PMNs)] by microscopy. The numbers of neutrophils were counted in each 269
scope (400 x) and thereafter obtain their average count from 5 scopes of every tissue slice 270
[15]. 271
272
Statistical Analysis. Data are expressed as mean ± standard error of the mean (SEM).
273
Statistical evaluation was carried out by one-way analysis of variance (ANOVA followed 274
by Scheffe's multiple range test). Statistical significance is expressed as *p < 0.05, **p < 275 0.01, ***p < 0.001. 276 277 Results 278
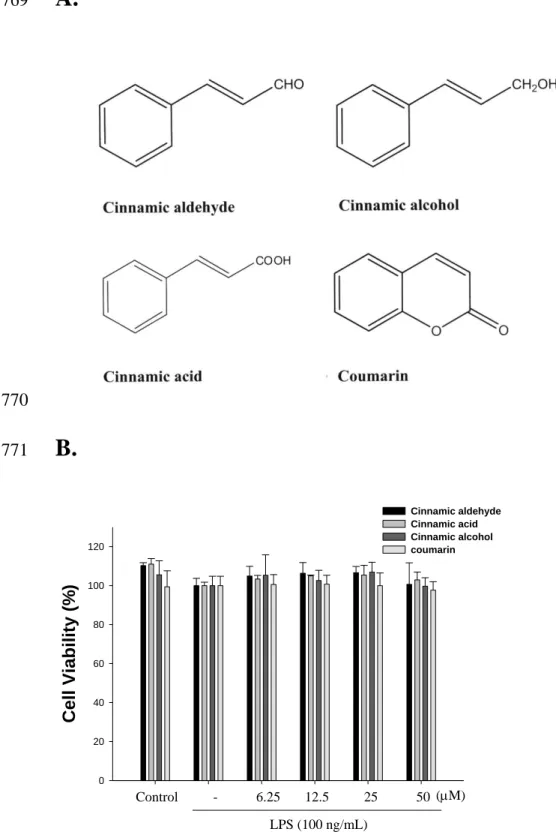
Cell Viability. The effect of C. cassia constituents (cinnamic aldehyde, cinnamic alcohol,
279
cinnamic acid, and coumarin) on RAW264.7 cell viability was determined by a MTT 280
assay. Cells cultured with samples at the concentrations (0, 6.25, 12.5, 25, and 50 M) 281
used in the presence of 100 ng/mL LPS for 24 h did not change cell viability (Fig. 1B). 282
283
Effect of Cinnamic aldehyde, Cinnamic alcohol, Cinnamic acid, and Coumarin on
284
LPS-induced NO Production in Macrophages. In the present study, effects of cinnamic
285
aldehyde, cinnamic alcohol, cinnamic acid, and coumarin on LPS-induced NO production 286
in RAW264.7 macrophages were investigated. Nitrite accumulated in the culture medium 287
was estimated by the Griess reaction as an index for NO release from the cells. After 288
treatment with LPS (100 ng/mL) for 24 h, the nitrite concentration increased in the 289
medium. When RAW264.7 macrophages were treated with different concentrations of 290
cinnamic aldehyde together with LPS for 24 h, the cinnamic aldehyde inhibited nitrite 291
production significantly (Fig. 2). Cinnamic aldehyde did not interfere with the reaction 292
between nitrite and Griess reagents at 50 M (data not shown). Unstimulated 293
macrophages, after 24 h of incubation in culture medium produced background levels of 294
nitrite. When RAW264.7 macrophages were treated with different concentrations of 295
cinnamic aldehyde (0, 6.25, 12.5, 25, and 50 M) together with LPS (100 ng/mL) for 24 296
h, a significant concentration-dependent inhibition of nitrite production was detected. 297
There was either a significant decrease in the nitrite production of group treated with 12.5 298
M cinnamic aldehyde (p < 0.05), or very or highly significant decrease of groups treated 299
respectively with 25 or 50 M of cinnamic aldehyde when compared with the LPS-alone 300
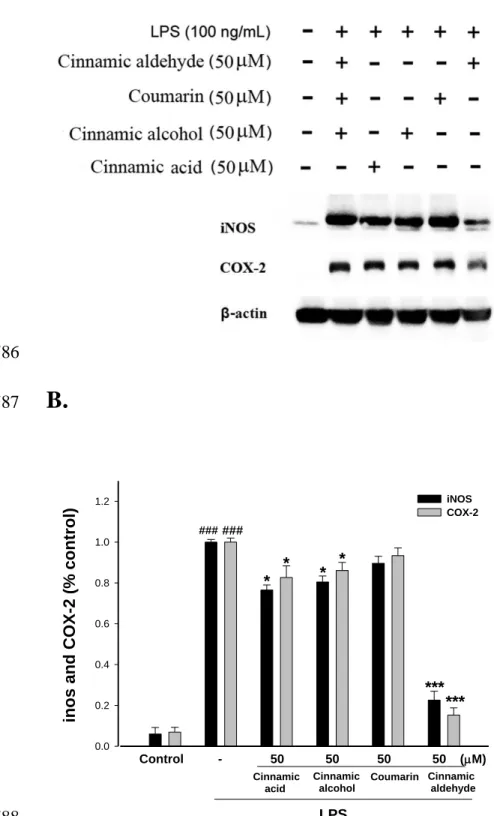
group (p < 0.01 or p < 0.001). The IC50 value for inhibition of nitrite production of 301
cinnamic aldehyde was about 45.56 ± 1.36 M 302
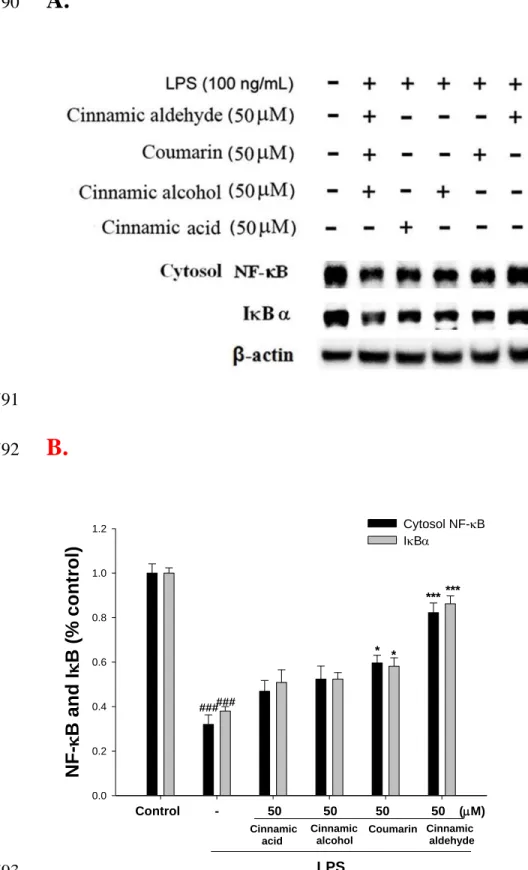
303
Inhibition of LPS-induced iNOS, COX-2, IB, and NF-B Protein by Cinnamic
304
aldehyde, Cinnamic alcohol, Cinnamic acid, and Coumarin. In order to investigate
305
whether the inhibition of NO production was due to a decreased iNOS, COX-2, IB,
306
and NF-B protein level, the effect of cinnamic aldehyde, cinnamic alcohol, cinnamic 307
acid, and coumarin was studied by immunoblot. The results showed the incubation with
308
cinnamic aldehyde (50 M) in the presence of LPS (100 ng/mL) for 24 h or 1h inhibited
309
iNOS, COX-2, IB, and NF-B proteins expression in mouse macrophage RAW264.7
310
cells in the cytosol (Fig. 3A and Fig. 4A). The detection of β-actin was also performed in
311
the same blot as an internal control. The intensity of protein bands was analyzed by using
312
Kodak Quantity software in three independent experiments and it showed an average of
313
77.4% and 84.8% down-regulation of iNOS and COX-2proteins, respectively, after
314
treatment with cinnamic aldehyde at 50 M compared with the LPS-alone (Fig. 3B). And
315
the intensity of protein bands showed an average of 82.6% and 86.2% up-regulation of
316
NF-BandIBprotein (p<0.001) (Fig.4B).
318
Inhibition of LPS-induced the level of TNF-and PGE2 by Cinnamic aldehyde.
319
TNF- mediates the production of many other cytokines during inflammation, in
320
particular, the production of interleukin-1 beta (IL-1 and interleukin-6 (IL-6) [16]. We
321
examined the effect of cinnamic aldehyde on LPS induced up-regulation of TNF-. A
322
very low amount of TNF- protein was detected by a specific ELISA for TNF- in
323
controls (Fig. 5A). When RAW264.7 macrophages were treated with different
324
concentrations of cinnamic aldehyde (12.5, 25, and 50 ) together with LPS (100
325
ng/mL) for 24 h, a significant concentration-dependent inhibition of TNF-production
326
was detected. There was either a significant decrease in the TNF-production of group
327
treated with 12.5 cinnamic aldehyde (p < 0.05), or highly significant decrease of
328
groups treated respectively with 25 and 50 of cinnamic aldehyde when compared
329
with the LPS-alone group (p < 0.01 or p < 0.001). The IC50 value for inhibition of TNF- 330
production of cinnamic aldehyde was about 29.58 ± 0.34 M.
331
PGE2 represents the most important inflammatory product of COX-2 activity and it 332
was quantified in cell-free culture supernatant [16]. As shown in Fig. 5B, cells were
333
stimulated with LPS alone raised significant amount of PGE2 in RAW264.7 macrophages.
334
When RAW264.7 macrophages were treated with different concentrations of cinnamic
335
aldehyde (12.5, 25, and 50 ) together with LPS (100 ng/mL) for 24 h, a significant
336
concentration-dependent inhibition of PGE2 production was detected. The IC50 value for 337
inhibition of PGE2 production of cinnamic aldehyde was about 37.67 ± 0.58 M. 338
339
Effects of Cinnamic aldehyde on Carr-induced Mice Paw Edema. In this study, we
340
used Carr-induced edema because this model is widely employed for screening the effects 341
of anti-inflammatory drugs. Carr-induced paw edema is shown in Fig. 6A. Cinnamic 342
aldehyde (5 mg/kg) inhibited (p < 0.001) the development of paw edema induced by Carr 343
after the 4th and the 5th h of treatment, significantly. Indo (10 mg/kg) significantly 344
decreased the Carr induced paw edema after the 4th and the 5th h of treatment (p < 0.001). 345
346
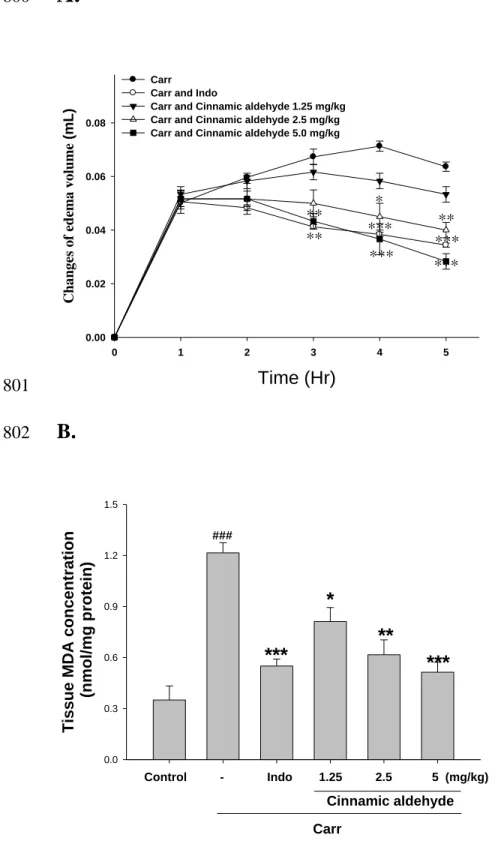
Effects of Cinnamic aldehyde on the MDA level. The MDA level increased
347
significantly in the edema paw at the 5th h after Carr injection (p < 0.001). However, the 348
MDA level was decreased significantly by treatment with cinnamic aldehyde (5 mg/kg) 349
( p < 0.001), as well as 10 mg/kg Indo (Fig. 6B). 350
351
Effects of Cinnamic aldehyde on the MPO activity. The MPO activity increased
352
significantly in the edema paw at the 5th h after Carr injection (p < 0.001). However, the
353
MPO activity was decreased significantly by the treatment with cinnamic aldehyde (5
354
mg/kg) (p < 0.001), as well as 10 mg/kg Indo (Fig. 6C).
355 356
Effects of Cinnamic aldehyde on the NO Level. In Fig. 6D, the NO level increased
357
significantly in the edema serum at the 5th h after Carr injection (p < 0.001). Cinnamic 358
aldehyde (5 mg/kg) significantly decreased the serum NO level (p < 0.001). The 359
inhibitory potency was similar to that of Indo (10 mg/kg) at the 5th h after induction. 360
361
Effects of Cinnamic aldehyde on the TNF-α and PGE2 Level. The TNF-α and PGE2 362
level increased significantly in serum at the 5th h after Carr injection (p < 0.001).
363
However, cinnamic aldehyde (1.25 or 2.5 mg/kg) decreased the TNF-α and PGE2 level in
364
serum at the 5th h after Carr injection (p < 0.05 or p < 0.01), as well as 10 mg/kg Indo (Fig.
365
6E and 6F).
366 367
Effects of Cinnamic aldehyde on activities of Antioxidant Enzymes. At the 5th h after 368
the intrapaw injection of Carr, paw tissues were also analyzed for the biochemical 369
parameters such as CAT, SOD, and GPx activities. CAT, SOD, and GPx activities in paw 370
tissue were decreased significantly by Carr administration. CAT, SOD, and GPx activity 371
were increased significantly after treated with 5 mg/kg cinnamic aldehyde and 10 mg/kg 372
Indo (P<0.01 or P<0.001) (Table 1). 373
374
Effects of Cinnamic aldehyde on Carr-induced iNOS, COX-2, and NF-B protein
375
expressions in Mice Paw Edema. To investigate whether the inhibition of NO
376
production was due to a decreased iNOS, COX-2, and NF-B protein level, the effect of 377
cinnamic aldehyde on iNOS, COX-2, and NF-B proteins expression were studied by 378
western blot. The results showed that injection of cinnamic aldehyde (5 mg/kg) on 379
Carr-induced for 5 h inhibited iNOS, COX-2, and NF-B proteins expression in mouse 380
paw edema (Fig. 7A). The detection of β-actin was also performed in the same blot as an 381
internal control. The intensity of protein bands was analyzed by using Kodak Quantity 382
software in three independent experiments and showed an average of 76.1% and 63.3% 383
down-regulation of iNOS and COX-2 protein respectively after treatment with cinnamic 384
aldehyde at 5 mg/kg compared with the Carr-induced alone (Fig. 7B). In addition, the 385
protein expression showed an average of 57.1% and 45.1% down-regulation of iNOS, 386
and COX-2 protein after treatment with Indo at 10 mg/kg compared with the 387
Carr-induced alone (Fig. 7B). And the intensity of protein bands showed an average of
388
87.6% up-regulation of NF-Bprotein (p<0.001) (Fig.7B). The down-regulation of 389
iNOS, COX-2, and NF-B activity of the cinnamic aldehyde (5 mg/kg) was better than 390
Indo (10 mg/kg). 391
392
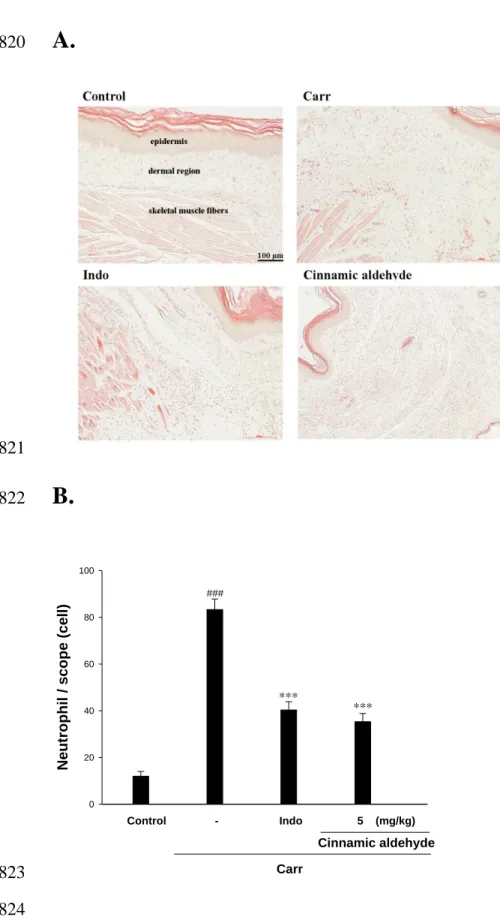
Histological Examination. Paw biopsies of Carr model animals showed marked cellular
393
infiltration in the connective tissue. The infiltrates accumulated between collagen fibers 394
and into intercellular spaces. Paw biopsies of animals treated with cinnamic aldehyde (5 395
mg/kg) showed a reduction in Carr-induced inflammatory response. Actually 396
inflammatory cells were reduced in number and confined to near the vascular areas. 397
Intercellular spaces did not show any cellular infiltrations. Collagen fibers were regular in 398
shape and showed a reduction of intercellular spaces. Moreover, the hypoderm 399
connective tissue was not damaged (Fig. 8A). Neutrophils increased with Carr treatment 400
(P < 0.01). As Indo and cinnamic aldehyde (5 mg/kg) could significantly decrease the 401
neutrophils numbers as compared to the Carr-treated group (P < 0.001) (Fig. 8B). 402
403
Discussion
404
In the present study, we demonstrated anti-inflammatory activities of C. cassia 405
constituents (cinnamic aldehyde, cinnamic alcohol, cinnamic acid, and coumarin) in both 406
in in vitro and in vivo experimental systems, using LPS-stimulated RAW264.7 407
macrophages and a mouse model of topical inflammation respectively. Dual inhibitory 408
activities against iNOS, COX-2, and NF-B as shown in in vitro assays appear to confer 409
on cinnamic aldehyde a potent in vivo efficacy in mouse, Carr-induced, paw edema, 410
comparable with a potent COX inhibitor, indomethacin, suggesting its potential 411
therapeutic usage as a novel topical anti-inflammatory source of health food. 412
The pathology of inflammation is initiated by complex processes triggered by 413
microbial pathogens such as LPS, which is a prototypical endotoxin. LPS can directly 414
activate macrophages, which trigger the production of inflammatory mediators, such as 415
NO and TNF-α [17]. The pharmacological reduction of LPS-inducible inflammatory 416
mediators is regarded as one of the essential conditions to alleviate a variety of disorders 417
caused by activation of macrophages. Thus, RAW264.7 macrophages provide us with an 418
good model for anti-inflammatory drug screening and for subsequently evaluating the 419
inhibitors of the signal pathways that lead to the induction of pro-inflammatory enzymes 420
and to the production of pro-inflammatory cytokines. 421
Cinnamic aldehyde, the major constituent of leaf essential oil from C. cassia. 422
Cinnamic aldehyde has been demonstrated to exhibit anti-tumor activities, anti-bacteria 423
activities, anti LPS-induced NF-B transcriptional activities [18, 19]. Cinnamic aldehyde 424
which has unsaturated carbonyl moiety exerted suppressive effect on toll-like 425
receptor 4 (TLR4)-mediated signaling [20]. And in this paper, we first evaluated that 426
cinnamic alcohol, cinnamic acid, and coumarin only little or less anti-inflammatory 427
activities in LPS-inducible inflammatory model in vitro. Our current results provided a 428
potential medical application in modulating inflammatory diseases. 429
As many of these conditions exhibit rapid onset and development, often resulting in 430
the failure of conventional anti-inflammatory therapies and extremely high mortality rates, 431
a simultaneous suppression of NO production pathways, as shown by cinnamic aldehyde, 432
may satisfy the so far unmet need for control of the rapid progression of the inflammatory 433
process. In vitro models such as macrophage cells or other cell lines are useful materials 434
with a steady high-level production of NO. The mechanisms by which cinnamic aldehyde 435
inhibits macrophage functions have not been elucidated. Results in vitro showed that 436
cinnamic aldehyde suppressed LPS-induced production of NO, the expression of 437
inflammatory protein products such as iNOS, COX-2, IB, and NF-B. Examination of 438
the cytotoxicity of cinnamic aldehyde in RAW264.7 macrophages using MTT assay has 439
indicated that cinnamic aldehyde even at 50 M did not affect the viability of RAW264.7 440
cells. Therefore, inhibition of LPS-induced nitrite production by cinnamic aldehyde was 441
not the result of a possible cytotoxic effect on these cells. 442
Excess amounts of NO and PGE2 play a critical role in the aggravation of chronic 443
inflammatory diseases, such as hepatic dysfunction and pulmonary disease. Recently, in
444
vitro and in vivo have indicated an existing cross talk between the release of NO and 445
prostaglandins (PGs) in the modulation of molecular mechanisms that regulate PGs
446
generating pathway [21]. Scientific papers were observed that while the production of
447
both NO and PGE2 was blocked by the NOS inhibitors in mouse macrophages
448
RAW264.7 cells, these inhibitory effects were reversed by co-incubation with the
449
precursor of NO synthesis, L-Arginine. Furthermore, inhibition of iNOS activity by
450
nonselective NOS inhibitors attenuated the release of NO and PGs simultaneously in
451
LPS-activated macrophages, which suggested that endogenously released NO from
macrophages exerted a stimulatory action on enhancing the PGs production. Conversely,
453
it has been shown that COX activation in turn modulates L-arginine-NO pathway,
454
whereas COX inhibition decreases NOS activity in human platelets [22]. These results
455
are indicative of the cross-talk between NO and PGs pathways.
456
The Carr-induced mice paw edema is a suitable test for evaluating anti-inflammatory 457
drugs and has frequently been used to assess the anti-edematous effect of natural products 458
[23]. The degree of swelling of the Carr-injected paws was maximal 3th h after injection. 459
Cinnamic aldehyde and Indo significantly inhibited the development of edema the 4th and 460
the 5th h after treatment (p<0.001). They both showed anti-inflammatory effects in 461
Carr-induced mice edema paw. It is well known that the third phase of the edema-induced 462
by Carr, in which the edema reaches its highest volume, is characterized by the presence 463
of prostaglandins and other compounds of slow reaction found that the injection of Carr 464
into the rat paw induces the liberation of bradykinin, which later induces the biosynthesis 465
of prostaglandin and other autacoids, which are responsible for the formation of the 466
inflammatory exudates [24]. 467
In the studies of the mechanism on the inflammation, NO plays an important role in 468
the Carr-induced inflammatory response [25]. Our present results confirm that 469
Carr-induced paw edema model results in the production of NO. The expression of the 470
inducible isoform of NO synthase has been proposed as an important mediator of 471
inflammation. In our study, the level of NO was decreased significantly by treatment with 472
1.25, 2.5, and 5 mg/kg cinnamic aldehyde. We suggest the anti-inflammatory mechanism 473
of cinnamic aldehyde may be through the L-arginine–NO pathway because cinnamic 474
aldehyde significantly inhibits the NO production. 475
The proinflammatory cytokines such as TNF-α and IL-1 are small secreted proteins, 476
which mediate and regulate immunity and inflammation. The production of TNF- is 477
crucial for the synergistic induction of NO synthesis in LPS-stimulated macrophages. 478
TNF-α induces a number of physiological effects including septic shock, inflammation, 479
and cytotoxicity [26]. Also, TNF- is a mediator of Carr-induced inflammatory 480
incapacitation, and is able to induce the further release of kinins and leukotrienes, which 481
is suggested to have an important role in the maintenance of long-lasting nociceptive 482
response [27]. In this study, we found that cinnamic aldehyde decreased the TNF-α level 483
in serum after Carr injection by treatment with 1.25, 2.5, and 5 mg/kg, significantly. 484
Neutrophils and macrophages are critical to the pathogenesis of acute injury, 485
rheumatoid arthritis and other inflammatory diseases [15]. The Carr-induced 486
inflammatory response has been linked to neutrophils infiltration and the production of 487
neutrophils-derived free radicals, such as hydrogen peroxide as well as the release of 488
other neutrophils-derived mediators [1]. Some researches demonstrate that inflammatory 489
effect induced by Carr is associated with free radical. Free radical, prostaglandin and NO 490
will be released when administrating with Carr for 1 ~ 6 h. ROS play an important role in 491
modulating the extent of inflammatory response and consequent tissue and cell injury. 492
MDA is a metabolic product of lipid peroxidation, the level of which is raised in 493
oxidative stress. MDA production is due to free radical attack plasma membrane. 494
Increasing evidence regarding free radical-generating agents and inflammatory processes 495
suggests that accumulation of reactive oxygen species can cause tissue injury [28]. Thus, 496
inflammatory effect would result in the accumulation of MDA. In this study, there is 497
significantly increased in CAT, SOD, and GPx activities with cinnamic aldehyde 498
treatment. Furthermore, there are significantly decreases in MDA level with cinnamic 499
aldehyde treatment. We assume the suppression of MDA production is probably due to 500
the increases of CAT, SOD, and GPx activities. 501
Activation of polymorphonuclear neutrophils (PMNs) reflects a primary
502
immunological response to invading pathogens [29]. In Carr-induced inflammation,
503
cinnamic aldehyde significantly inhibited cellular infiltration
504
(neutrophils and granulocytes) into the air-pouch fluid. Also, MPO from the neutrophil's
505
azurophilic granules is responsible for invoking tissue injury [30]. Results indicate that
506
cinnamic aldehyde has considerable potential as a therapeutic inhibitor of MPO-mediated
507
tissue damage.
508
NF-B is known to be a major transcription factor to regulate the expressions of
509
pro-inflammatory enzymes and cytokines, such as iNOS, COX-2, and TNF- [31].
510
NF-B subunits (p65 and/or p50) are normally sequestered in the cytosol as an inactive
511
complex by binding to inhibitory factor IB- in un-stimulated cells. Upon stimulation of
512
pro-inflammatory signals including LPS, IB- is phosphorylated by IB kinase (IKK)
513
and inactivated through ubiquitin-mediated degradation [32]. The resulting free NF-B is
514
translocated into the nucleus and it acts as a transcription factor. As shown in Fig. 4A, the
515
treatment with cinnamic aldehyde blocks the degradation of NF-B in LPS-induced
516
macrophage and Carr-induced paw edema. Therefore, these results suggest that cinnamic
517
aldehyde inhibits the expression of iNOS and COX-2, and thus NO production through 518
inactivation of NF-B activation. 519
The anti-inflammatory properties of cinnamic aldehyde would appear to be similar 520
to the anti-inflammatory properties of certain other essential oils deriving from certain 521
other plants. Hyptis pectinata essential oil exhibits antinociceptive 522
and anti-inflammatory activity through the inhibition of NO and PGE2 production 523
after Carr injection [33]. And, the essential oil of Cordia verbenacea significantly 524
decreased TNF-a production in Carr-injected rat paws [34]. 525
In conclusion, these results suggested that cinnamic aldehyde possessed 526
anti-inflammatory effects. The anti-inflammatory mechanism of cinnamic aldehyde may 527
be related to iNOS and it is associated with the increase in the activities of antioxidant 528
enzymes (CAT, SOD, and GPx). Cinnamic aldehyde may be used as a pharmacological 529
agent in the prevention or treatment of disease in which free radical formation in a 530 pathogenic factor. 531 532 Acknowledgement 533
The authors want to thank the financial supports from the National Science Council 534
(NSC100-2313-B-039-004- and NSC 100-2320-B-039-033-), China Medical University 535
(CMU) (CMU99-TC-35, CMU99-COL-10, and CMU100-TC-11) and Taiwan 536
Department of Heath Clinical Trial and Research Center of Excellence 537 (DOH101-TD-B-111-004). 538 539 Reference 540
(1) S. S. Huang, C. S. Chiu, H. J. Chen, et al. ―Antinociceptive activities and the 541
mechanisms of anti-inflammation of asiatic acid in mice,‖ Evidence-Based 542
Complementary and Alternative Medicine, vol. 2011, pp. 895857, 2011. 543
(2) H. Y. Chang, M. J. Sheu, C. H. et al. ―Analgesic effects and the mechanisms of 544
anti-inflammation of hispolon in mice,‖ Evidence-Based Complementary and 545
Alternative Medicine, vol. 2011, pp. 478246, 2011. 546
(3) S. C. Fang, C. L. Hsu, and G. C. Yen. ―Anti-inflammatory effects of phenolic 547
compounds isolated from the fruits of Artocarpus heterophyllus,― Journal of 548
Agricultural and Food Chemistry, vol. 56, no. 12, pp. 4463-4468, 2008. 549
(4) M. H. Huang, B. S. Wang, C. S. Chiu, et al. ―Antioxidant, antinociceptive, and 550
anti-inflammatory activities of Xanthii Fructus extract,― Journal of 551
Ethnopharmacology, vol. 135, no. 2, pp. 545-552, 2011. 552
(5) C. T. Chang, S. S. Huang, S. S. Lin, et al., ―Anti-inflammatory activities of tormentic 553
acid from suspension cells of Eriobotrya Japonica ex vivo and in vivo,― Food 554
Chemistry, vol. 127, no. 3, pp. 1131-1137, 2011. 555
(6) G. J. Huang, S. S. Huang, S. S. Lin, et al. ―Analgesic effects and the mechanisms of 556
anti-inflammation of ergostatrien-3-ol from Antrodia camphorata submerged whole 557
broth in mice,― Journal of Agricultural and Food Chemistry, vol. 58, no. 12, pp. 558
7445-7452, 2010. 559
(7) C. Tohda, N. Nakayama, F. Hatanaka, and K. Komatsu. ―Comparison of 560
anti-inflammatory activities of six Curcuma rhizomes: a possible 561
curcuminoid-independent pathway mediated by Curcuma phaeocaulis 562
extract,― Evidence-Based Complementary and Alternative Medicine, vol. 3, no. 2, pp. 563
255-260, 2006. 564
(8) Z. D. He, C. F. Qiao, Q. B. Han, et al.
565
―Authentication and quantitative analysis onthe chemical profile of cassia bark (corte 566
xcinnamomi) by high-pressure liquid chromatography,― Journal of Agricultural and 567
Food Chemistry, vol. 53, no. 7, pp. 2424-2428, 2005. 568
(9) Y. Y. Sung, T. Yoon, J. Y. Jang, S. J. Park, G. H. Jeong, and H. K. Kim. ―Inhibitory 569
effects of Cinnamomum cassia extract on atopic dermatitis-like skin lesions induced 570
by mite antigen in NC/Nga mice, ― Journal of Ethnopharmacolog, vol. 133, no. 2, pp. 571
621-628, 2011. 572
(10) H. K. Kwon, J. S. Hwang, J. S. So, et al. ―Cinnamon extract induces tumor cell 573
death through inhibition of NFkappaB and AP1,― BMC Cancer, vol. 10, 392-401, 574
2010. 575
(11) D. Bani, E. Masini, M. G. Bello, M. Bigazzi, and T. B. Sacchi. ―Relaxin protects
576
against myocardial injury caused by ischaemia and reperfusion in rat
577
heart,― American Journal of Pathology, vol. 152, no.5, pp. 1367-1376, 1998.
578
(12) L. Flohe, and F. Otting. Superoxide dismutase assays. ―Methods of Enzymology,― vol, 579
105, pp. 93–104, 1984. 580
(13) H. Aebi. Catalase in vitro. ―Methods of Enzymology,― vol, 105. pp.121–126, 1984. 581
(14) E. D. Paglia, and W. N. Valentine. ―Studies on the quantitative and qualitative 582
characterization of erythrocytes glutathione peroxidase,― Journal of Laboratory and 583
clnical medicine, vol, 70, no, 1, pp. 158–169, 1967. 584
(15) M. J. Sheu, P. Y. Chou, H. C. Cheng, et al. ―Analgesic 585
and anti-inflammatory activities of a water extract of Trachelospermum jasminoides 586
(Apocynaceae),― Journal of Ethnopharmacology, vol, 126, no. 2; pp. 332-338, 587
2009. 588
(16) C. S. Lai, J. H. Lee, C. T. Ho, et al. ―Rosmanol potently inhibits
589
lipopolysaccharide-Induced iNOS and COX-2 expression through downregulating
590
MAPK, NF-κB, STAT3 and C/EBP signaling pathways,― Journal of Agricultural
591
and Food Chemistry, vol. 57, no. 22, pp. 10990–10998, 2009. 592
(17) B. Saad, B. S. Abouatta, W. Basha, et al. ―Hypericum triquetrifolium—Derived 593
factors downregulate the production levels of LPS-Induced nitric oxide and tumor 594
necrosis factor-a in THP-1 Cells,― Evidence-Based Complementary and Alternative 595
Medicine, vol. 4, no, .pp. 425–430, 2007. 596
(18) B. Huang, H. D. Yuan, do Y. Kim, H. Y. Quan, and S. H. Chung. 597
―Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation 598
ofperoxisome proliferator-activated receptor-γ (PPARγ) and AMP- 599
activated protein kinase (AMPK) pathways,― Journal of Agricultural and Food 600
Chemistry, vol. 59, pp. 3666-3673, 2011. 601
(19) A. H. Reddy, J. H. Seo, S. Y. Ryu, et al. ―Cinnamaldehyde and 602
2-methoxycinnamaldehyde as NF-B inhibitors from Cinnamomum 603
cassia,― Planta Medica, vol.70, no. 9, pp. 823–827, 2004. 604
(20) H. S. Youn, J. K. Lee, Y. J. Choi, et al.
605
―Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through 606
the inhibition of receptor oligomerization,― Biochemical Pharmacology, vol. 75, no. 607
2, pp. 494-502, 2008. 608
(21) I. N. Hsieh, A. S. Chang, C. M. Teng, C. C. Chen, and C. R. Yang, ―Aciculatin
609
inhibits lipopolysaccharide-mediated inducible nitric oxide synthase and
cyclooxygenase-2 expression via suppressing NF-κB and JNK/p38 MAPK activation
611
pathways,―Journal of Biomedical Science, vol. 18, pp. 28, 2011. 612
(22) R. L. Handy, and P. K. Moore, ―A comparison of the effects of L-NAME, 7-NI and
613
L-NIL on carrageenan-induced hindpaw oedema and NOS activity,― British Journal
614
of Pharmacology, vol. 123, no. 6, 1119-1126, 1998. 615
(23) A. F. Viana, I. S. Maciel, E. M. Motta, et al. ―Antinociceptive Activity of Trichilia 616
catigua Hydroalcoholic extract: new evidence on its Dopaminergic 617
Effects,― Evidence-Based Complementary and Alternative Medicine, vol. 2011, pp. 618
120820,2011. 619
(24) C. Tohda, N. Nakayama, F. Hatanaka, and K. Komatsu. ―Comparison of 620
anti-inflammatory activities of six Curcuma rhizomes: a possible 621
curcuminoid-independent pathway mediated by Curcuma phaeocaulis 622
extract,― Evidence-Based Complementary and Alternative Medicine, vol. 3, pp. 623
255–260, 2006. 624
(25) D. Salvemini, Z. Wang, D. M. Bourdon, M. K. Stern, M. G. Curne, P. T. Manning. 625
―Evidence of peroxynitrite involvement in the carrageenan induced rat paw 626
edema,― European Journal of Clinical Pharmacology, vol. 303, no. 3, pp. 217–220, 627
1996. 628
(26) K. J. Yun, D. J. Koh, S. H. Kim, et al. ―Anti-inflammatory effects of sinapic 629
acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and 630
proinflammatory cytokines expressions via nuclear factor-kappaB 631
inactivation,― Journal of Agricultural and Food Chemistry, vol. 56, no. 21, pp. 632
10265-10272, 2008. 633
(27) C. R. Tonussi, and S. H. Ferreira. ―Tumour necrosis factor-alpha mediates carrageen 634
in-induced knee-joint incapacitation and also triggers overt nociception in previously 635
inflamed rat knee-joints,― Pain, vol. 82, no. 1, 81-87, 1999, 636
(28) W. L. Lin, C. J. Wang, Y. Y. Tsai, C. L. Liu, J. M. Hwang, T. H. Tseng. 637
―Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide 638
in rat liver,― Archives of Toxicology, vol. 74, no. 8, pp. 467-472, 2000. 639
(29) V. Wittamer, B. Bondue, A. Guillabert, G. Vassart, M. Parmentier, and D. Communi.
640
―Neutrophil-mediated maturation of chemerin: a link between innate and adaptive
641
immunity,― Journal of Immunoogyl. Vol. 175, no. 1, pp. 487-493, 2005.
642
(30) T. C. Busnardo, C. Padoani, T. C. Mora, et al., ―Anti-inflammatory evaluation of
643
Coronopus didymus in the pleurisy and paw oedema models in mice,― Journal of
644
Ethnopharmacology. Vol.128, no. 2, pp. 519-525, 2010. 645
(31) J. S. Deng, C. S. Chiu, S. S. Huang, P. H. Shie, T. H. Lin, and G. J. Huang.
646
―Antioxidant, Analgesic, and Anti-inflammatory activities of the ethanolic extracts of
647
Taxillus liquidambaricola,― Journal of Ethnopharmacology. vol. 137, no. 3, pp. 648
1161-1171, 2011.
649
(32) Karin M, and Ben-Neriah Y. ―Phosphorylation meets ubiquitination: the control of 650
NF-B activity,― Annual Review of Immunology, vol. 18, pp. 621–63, 2000. 651
(33) L. J. Raymundo, C. C. Guilhon, D. S. Alviano, et al. ―Characterisation of 652
the anti-inflammatory and antinociceptive activities of the Hyptis pectinata (L.) 653
Poitessential oil,―Journal of Ethnopharmacology. vol. 34, no.3, pp. 725-32, 2011. 654
(34) E. S. Fernandes, G. F. Passos, R. Medeiros, et al. ―Anti-inflammatory effects of 655
compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential 656
oil of Cordia verbenacea,― European Journal of Pharmacology, vol. 569, no.5, pp. 657 228-236, 2007. 658 659 660 661 662 663 664 665 666 667 668 669 670 671 672 673 674 675 676 677
Figure Legends
678Figure 1. Chemical structure of cinnamomum cassia constituents (cinnamic aldehyde, 679
cinnamic alcohol, cinnamic acid, and coumarin) (A) and cytotoxic effects of 680
cinnamomum cassia constituents in RAW264.7 cells (B). Cells were incubated for 24 h 681
with 100 ng/mL of LPS in the absence or presence of samples (0, 6.25, 12.5, 25, and 50 682
M). Samples were added 1 h before incubation with LPS (lipopolysaccharide). Cell 683
viability assay was performed using MTT assay. The data were presented as mean ± 684
S.D. for three different experiments performed in triplicate. 685
686
Figure 2. Effects of cinnamomum cassia constituents (cinnamic aldehyde, cinnamic 687
alcohol, cinnamic acid, and coumarin) on LPS-induced NO production of RAW264.7 688
macrophages. Cells were incubated for 24 h with 100 ng/mL of LPS in the absence or 689
presence of samples (0, 6.25, 12.5, 25, and 50 M). Samples were added 1 h before 690
incubation with LPS. Nitrite concentration in the medium was determined using Griess 691
reagent. The data were presented as mean ± S.D. for three different experiments 692
performed in triplicate. ###compared with sample of control group. *p < 0.05, **p < 0.01, 693
and ***p < 0.001 were compared with LPS-alone group. 694
695
Figure 3. Inhibition of iNOS and COX-2 protein expression by cinnamomum cassia 696
constituents ((cinnamic aldehyde, cinnamic alcohol, cinnamic acid, and coumarin) in 697
LPS-stimulated RAW264.7 cells. Cells were incubated for 24 h with 100 ng/mL of LPS 698
in the absence or the presence of samples (50 M). Samples were added 1 h before 699
incubation with LPS. Lysed cells were then prepared and subjected to western blotting 700
using an antibody specific for iNOS and COX-2. β-actin was used as an internal control. 701
(A) A representative western blot from two separate experiments is shown. (B) Relative 702
iNOS and COX-2 protein levels were calculated with reference to a LPS-stimulated 703
culture. ###compared with sample of control group. The data were presented as mean ± 704
S.D. for three different experiments performed in triplicate. *p < 0.05 and ***p < 0.001 705
were compared with LPS-alone group. 706
707
Figure 4. Inhibition of NF-B and IB (A) protein expressions by cinnamomum cassia
708
constituents (cinnamic aldehyde, cinnamic alcohol, cinnamic acid, and coumarin) in
709
LPS-stimulated RAW264.7 cells. Samples (50 M) were added into cells 1 h before LPS
710
(100 ng/mL) stimulation and protein samples were prepared for 1 h after LPS stimulation.
711
Activations of signaling molecules were then evaluated by Western blot analysis. Lysed 712
cells were then prepared and subjected to western blotting using an antibody specific for 713
NF-B (P65) and IB in the cytosol. β-actin was used as an internal control. A 714
representative western blot from two separate experiments is shown. Relative NF-B and
715
IB protein levels were calculated with reference to a LPS-stimulated culture (B). 716
###
compared with sample of control group. The data were presented as mean ± S.D. for 717
three different experiments performed in triplicate. *p < 0.05 and ***p < 0.001 were 718
compared with LPS-alone group. 719
720
Figure 5. The effects of cinnamic aldehyde on lipopolysaccharide (LPS)-induced TNF-
721
(A) and PGE2 (B) in LPS-stimulated RAW264.7 cells. Cells were incubated for 24 h with 722
100 ng/mL of LPS in the absence or in the presence of cinnamic aldehyde (0, 12.5, 25,
and 50 M). Cinnamic aldehyde was added 1 h before the incubation with LPS.
724
TNF-and PGE2concentrations in the medium were determined using ELISA kit. The 725
data were presented as mean ± S.D. for three different experiments performed in
726
triplicate. ###p < 0.001 compared with sample of control group.*p < 0.05, **p < 0.01, and 727
***
p < 0.001 were compared with LPS-alone group. 728
729
Figure 6. Effects of cinnamic aldehyde and Indo on hind paw edema induced by Carr in
730
mice (A), the tissue MDA (B) and MPO (C) concentrations of foot in mice, Carr-induced
731
NO (D), TNF- (E) and PGE2 (F) concentrations of serum at the 5th hr in mice. The 732
values are averaged, obtained in individual animals (n=6). Each value represents as mean 733
± S.E.M. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared with the Carr group. 734
735
Figure 7. Inhibition of iNOS, COX-2, and NF-B protein expressions by cinnamic 736
aldehyde induced by Carr of foot at the 5th h in mice. Tissue suspended were then 737
prepared and subjected to western blotting using an antibody specific for iNOS, COX-2, 738
and NF-B. β-actin was used as an internal control. (A) A representative western blot 739
from two separate experiments is shown. (B) Relative iNOS, COX-2, and NF-B protein 740
levels were calculated with reference to a Carr-injected mouse. ###compared with sample 741
of control group. The data were presented as mean ± S.D. for three different 742
experiments performed in triplicate. **p < 0.01 and ***p < 0.001 were compared with 743
Carr-alone group. 744
745
Figure 8. Representative light micrographs of mouse hind footpad H&E stained to reveal 746
hemorrhage, edema and inflammatory cell infiltration in control mice (A), Carr-treated 747
mice demonstrates hemorrhage with moderately extravascular red blood cell and large 748
amount of inflammatory leukocyte mainly neutrophils infiltration in the subdermis 749
interstitial tissue of mice (B), and mice given indomethacin (Indo) (10 mg/kg) before 750
Carr. (C). Cinnamic aldehyde (5 mg/kg) significantly show morphological alterations 751
(100×) (D) and the numbers of neutrophils in each scope (400x) (E) compared to 752
subcutaneous injection of Carr only. ###p < 0.001 as compared with the control group. 753
***p < 0.001 compared with Carr group. Scale bar = 100 μm. 754 755 756 757 758 759 760 761 762 763 764 765 766 767
Figure 1.
768A.
769 770B.
771 Cell Vi abil ity (% ) 0 20 40 60 80 100 120 Cinnamic aldehyde Cinnamic acid Cinnamic alcohol coumarin Control - 6.25 12.5 25 50 LPS (100 ng/mL) (M) 772773
Figure 2.
774Nitri
te (
M)
0 2 4 6 8 10 12 14 16 18 Cinnamic aldehyde Cinnamic acid Cinnamic alcohol Courmarin Control - 6.25 12.5 25 50 LPS (100 ng/mL) (M) ### ###### ### *** ** * 775 776 777 778 779 780 781 782 783Figure 3.
784A.
785 786B.
787 *** Control - 50 50 50 50 (M) ** **inos and COX-2
(% control) 0.0 0.2 0.4 0.6 0.8 1.0 1.2 iNOS COX-2 LPS ###### *** * *** * * * Cinnamic acid Cinnamic alcohol Coumarin Cinnamic aldehyde 788
Figure 4.
789A.
790 791B.
792 *** Control - 50 50 50 50 (M) ** ** LPS ### *** Cinnamic acid Cinnamic alcohol Coumarin Cinnamic aldehyde *** ** NF- B and I B (% contro l) 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Cytosol NF-B IB ** *** ###### *** * * 793Figure 5.
794A.
795 TNF- (ng/ mL) 0 20 40 60 80 100 120 Control - 12.5 25 50 LPS (100 ng/mL) *** * ### ** Cinnamic aldehyde (M) 796B.
797 PGE 2 (p g/m L) 0 200 400 600 800 ### ** * Control - 12.5 25 50 LPS (100 ng/mL) Cinnamic aldehyde (M) *** 798Figure 6.
799A.
800 Time (Hr) 0 1 2 3 4 5 Changes of edema vol ume (mL) 0.00 0.02 0.04 0.06 0.08 Carr Carr and IndoCarr and Cinnamic aldehyde 1.25 mg/kg Carr and Cinnamic aldehyde 2.5 mg/kg Carr and Cinnamic aldehyde 5.0 mg/kg
*** *** ** * *** *** ** ** 801
B.
802 Ti ssue MDA con cent ratio n (n mol /mg p rotei n) 0.0 0.3 0.6 0.9 1.2 1.5 Control - Indo 1.25 2.5 5 (mg/kg) Carr**
***
### Cinnamic aldehyde***
*
803
C.
804 My el opero xidase (MP O) acti vity (%) 0 20 40 60 80 100 ###**
***
**
*
Control - Indo 1.25 2.5 5 (mg/kg) Carr Cinnamic aldehyde 805D.
806 *** Control - Indo 1.25 2.5 5 (mg/kg) ** ** *** *** ** ** Carr ### Nit ri te ( M) 0 2 4 6 8 10 12 14 16 ### ** *** Cinnamic aldehyde * ** 807E.
808 T NF - ( p g /m L ) 0 100 200 300 400 500 600 Carr ### Control - Indo 1.25 2.5 5 (mg/kg)***
***
**
Cinnamic aldehyde*
809F.
810 PGE 2 ( pg/mL) 0 20 40 60 80 100 120 ###***
***
**
*
Control - Indo 1.25 2.5 5 (mg/kg) Carr Cinnamic aldehyde 811812
Figure 7.
813A.
814 815B.
816 *** ** ** *** Control - 10 5 (mg/kg) Indo Cinnamic aldehydeCarr ** ** *** iNO S , CO X -2, an d NF - B (% of co ntr ol ) 0.0 0.2 0.4 0.6 0.8 1.0 1.2 iNOS COX-2 Cytosol NF-kB ### ### ### *** *** *** ** ** ** 817 818