Effects of Interleukin-10 Polymorphisms and Smoking on the Risk of Gastric Cancer in Taiwan
Wu-Hsien Kuo1,2,3,*, Chung-Yu Huang4,5,*, Chun-Kai Fu1,*, Yung-Hung Hsieh5, Cheng-His Liao6, Chin-Mu Hsu6, Chia-Wen Tsai6,7, Wen-Shin Chang6,8, and Da-Tian Bau6,7,8
1 Division of Gastroenterology, Department of Internal Medicine, Kaoshiung Armed-Forces General Hospital, Kaoshiung, Taiwan. R.O.C.
2 Division of Gastroenterology, Department of Internal Medicine, Taichung Armed-Forces General Hospital, Taichung, Taiwan, R.O.C.
3 Department of Medicine, National Defense Medical Center, Taipei, Taiwan, R.O.C.
4 Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan, R.O.C.
5 Department of Pharmacy, Taichung Armed-Forces General Hospital, Taichung, Taiwan, R.O.C.
6 Terry Fox Cancer Research Laboratory, China Medical University Hospital, Taichung, Taiwan, R.O.C.
7 Graduate Institute of Basic Medical Science, China Medical University, Taichung, Taiwan, R.O.C.
8 Graduate Institute of Clinical Medical Science, China Medical University, Taichung, Taiwan, R.O.C.
* The authors contributed equally to this work
Correspondence to: Da-Tian Bau, Terry Fox Cancer Research Lab, China Medical University Hospital, 2 Yuh-Der Road, Taichung, 404 Taiwan, Tel:
+886 422052121 Ext 7534
e-mail: artbau2@gmail.com; datian@mail.cmuh.org.tw
Abstract. Gastric cancer is the second worldwide cause of death from
cancer and its prevalence and mortality rates are still very high in developed countries. Interleukin-10 (IL-10) is a pleiotropic cytokine produced by macrophages which can suppress and stimulate the immune response in tumorigenesis signaling. However, the contribution of IL-10 genomic variants to gastric cancer is still largely unknown. In this study, we aimed at investigating the role of IL-10 genotypes in gastric cancer risk. The promoter single nucleotide polymorphisms on IL-10, A-1082G (rs1800896), T-819C (rs3021097) and A-592C (rs1800872), were genotyped by PCR-RFLP method among 716 Taiwan people (358 gastric patients and 358 cancer-free controls). The results showed that there was a significant difference between the gastric cancer patient and control groups in the genotypic frequency distribution of IL-10 A-1082G genotypes (P=0.0004). In addition, people carrying G allele have a higher risk for gastric cancer compared with those of A allele (P=3.19x10-5). Furthermore, personal cigarrete smoking habits would enhance the gastric cancer risk for those IL-10 A-1082G AG and GG carriers. In conclusion, AG and GG genotype at IL-10 A-1082G, together with risky smoking lifestyle, synergistically contribute to individual susceptibility for gastric
cancer in Taiwan.
Gastric cancer is reported to be more common in male and in elder citizens aged 50 years or older (1-3). In literature, smoking, obesity, salt intake and Helicobacter pylori (H. pylori) infection are well-known factors for gastric cancer progression (4, 5). Geographically speaking, gastric cancer is prevalent in developing countries in East Asia, East Europe and South America; while the incidence is low in North America and Africa (2). Clinically the prognosis of gastric cancer is usual poor with a 5-year survival less than 20% for advanced disease (3). There are some beneficial developments in decreasing the incidence of gastric cancer in the two decades, such as the increasing use of refrigerators, the lowering dependence on salts to preserve food, the elevating availability and intake of fresh fruits and vegetables, and the effective control of chronic infection with H. pylori. However, it remains as a critical cancer threat accounting for 8% of the total cancer incidence and 10% of the total cancer death worldwide (2).
The IL-10 gene located on human chromosome 1q31-32, and is composed of five exons and four introns. IL-10 is a pleiotropic cytokine with the dual anti-cancer properties of suppression and immune-stimulation (6). The three promoter SNPs, A-1082G (rs1800896), T-819C
(rs3021097), and A-592C (rs1800872), were reported to regulate the transcription of IL-10 messenger RNA and the expression of IL-10 in
vitro (7, 8). Recently, the three promoter polymorphisms of IL-10 have
been examined of their contribution to some types of cancer, for instance, hepatocellular carcinoma (9), breast cancer (10) and renal cell carcinoma (11). As for gastric cancer, the contribution of IL-10 to gastric cancer has been investigated, but the findings remain conflicting and inconclusive (12-18). The inconsistency may possibly due to differences in study design such as sample collection, and/or ethnic differences in the populations recruited. Mechanically, increased levels of serum IL-10 were found in patients with solid and hematopoietic tumors in addition to gastric cancer (19). Since the three promoter polymorphisms IL-10 A-1082G, T-819C and A-592C were reported to influence the transcription of IL-10 messenger RNA and the expression of IL-10 in vitro (7, 8), it is very possibly that the genotypes determined the personal susceptibility for gastric cancer and could serve as an early detection biomarker. Thus, the specific aim of this study was to determine the genotypic frequency of the three promoter polymorphisms of IL-10 gene in Taiwan gastric cancer population and the feasibility of them to serve as a potential gastric
cancer biomarker.
Materials and Methods
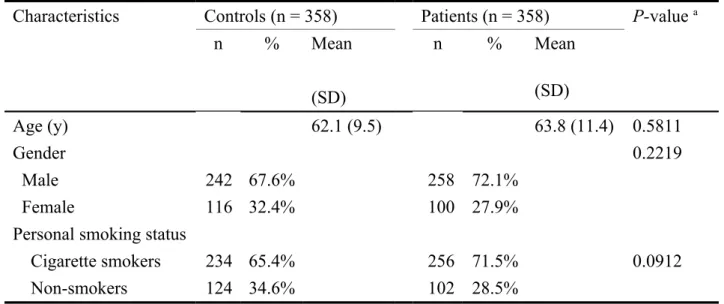
Study population and sample collection. Three hundred and fifty eight patients diagnosed with gastric cancer were recruited at the outpatient clinics of general surgery between 2001-2009 at the China Medical University Hospital, Taichung, Taiwan, Republic of China. The mean age of the gastric cancer patients and the controls were 63.8 (range = 38 to 81, SD = 11.4) and 62.1 (range = 39 to 79, SD = 9.5) years, respectively (More details please check Table I). All subjects voluntarily participated, completed a self-administered questionnaire and provided their peripheral blood samples. The equal amount of non-cancer healthy people was selected by matching for age and gender after initial random sampling from the Health Examination Cohort of the hospital as controls. Our study was approved by the Institutional Review Board of the China Medical University Hospital and written-informed consent was obtained from all participants.
Genotyping conditions. Genomic DNA of each participant was prepared
(Blossom, Taipei, Taiwan) and further processed according to our previous articles (20-22). The polymerase chain reaction (PCR) cycling conditions were: one cycle at 94°C for 5 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 10 min. The sequences of primers for PCR and the specific restriction enzymes for each DNA product are listed in Table II.
Statistical analyses. To ensure that the controls used were
representative of the general population and to exclude the possibility of genotyping error, the deviation of the genotype frequencies of IL-10 single nucleotide polymorphisms in the control subjects from those expected under the Hardy-Weinberg equilibrium was assessed using the goodness-of-fit test. Pearson’s Chi-square test or Fisher’s exact test (when the expected number in any cell was less than five) was used to compare the distribution of the IL-10 genotypes between cases and controls. The associations between the IL-10 polymorphisms and gastric cancer risk were estimated by computing odds ratios (ORs) and their 95% confidence intervals (CIs) from unconditional logistic regression analysis with the adjustment for possible confounders. P <
0.05 was considered statistically significant, and all statistical tests were two-sided.
Results
The characteristics of age, gender and personal cigarette smoking habits of all the investigated subjects are summarized and analyzed in Table I. The results showed that there was no difference in the distribution of these characteristics among gastric cancer patients and controls (Table I).
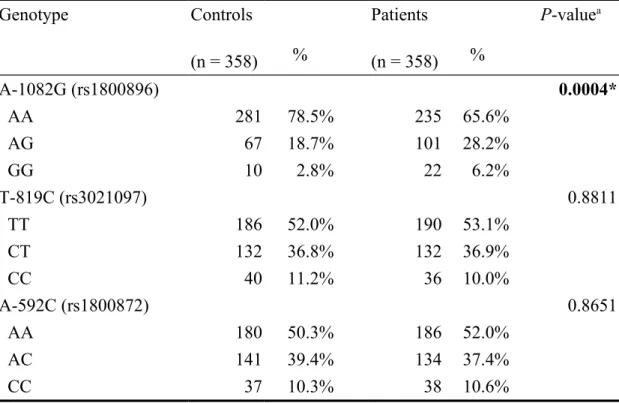
The frequencies for IL-10 A-1082G, T-819C and A-592C promoter genotypes among the controls and gastric cancer patients are summarized and analyzed of their differential distributions in Table III. Among the three polymorphic sites analyzed, the distribution of genotypic frequencies at IL-10 A-1082G was significantly different among the gastric cancer patients and non-cancer controls, and The P-value was significant (P = 0.0004). In detail, the percentages of AA, AG and GG genotypes at IL-10 A-1082G were 78.5%, 18.7% and 2.8% among the controls and 65.6%, 28.2% and 6.2% among the gastric cancer patients, respectively (Table III). Obviously, the AG and AA genotypes were of higher percentages for the gastric cancer patients than in the controls
(Table III). As for the other two polymorphic sites, IL-10 T-819C and A-592C, there was no difference among gastric cancer patients and controls in the distribution of their genotypic frequencies (P = 0.8811 and 0.8651, respectively) (Table III).
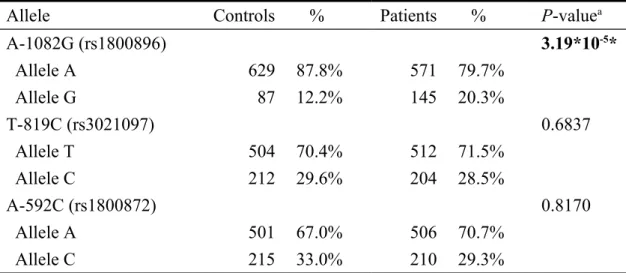
The analysis of allelic distributions at IL-10 1082G, T-819C and A-592C among the non-cancer controls and gastric cancer patients are summarized and presented in Table IV. For the three promoter sites genotyped, only the distribution of IL-10 A-1082G was significantly different among controls and gastric cancer patients (P = 3.19 x 10-5). In detail, the percentage of variant G allele in the gastric cancer patients (20.3%) was much higher than that in the controls (12.2%) (Table IV). As for IL-10 T-819C and A-592C, there was no difference between gastric cancer patient and control groups in the distribution of their allelic frequencies (P = 0.6837 and 0.8170, respectively) (Table III). From the results of Tables III and IV we can concluded that individuals carrying G allele on IL-10 A-1082G were at higher risk of gastric cancer in Taiwan. The genetic-lifestyle interaction was of interest and hereby the interaction between IL-10 A-1082G and personal smoking status on gastric cancer risk was analyzed. The results showed that the genotypic distribution of
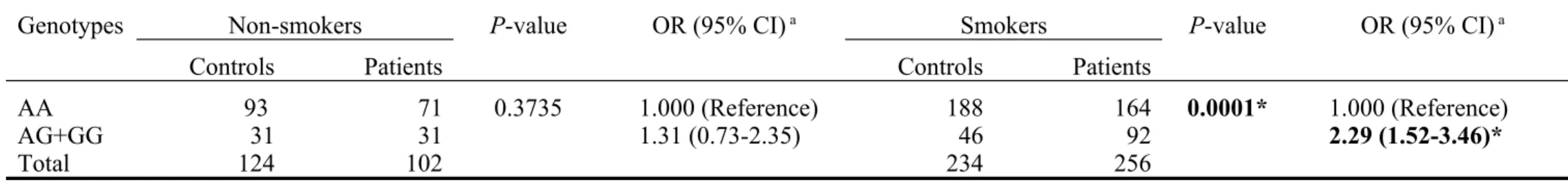
IL-10 A-1082G was significantly different between cancer and control
groups only among those who have smoking habits (OR=2.29, 95% CI=1.52-3.46, P=0.0001), but not among those who are non-smokers (OR=1.31, 95% CI=0.73-2.35, P=0.3735) (Table V). Consistent with the findings in Table III and IV, the percentage of AG and GG carriers (35.9%) were significantly higher in gastric cancer patients who smoked than the controls (19.7%). There was no such difference observed in the non-smoker groups (30.4% vs 25.0%).
Discussion
Previously, our team has found several potential genetic markers for gastric cancer early detection and prediction in Taiwan (23-26). In the current study, we aimed at investigating the association of IL-10 genotypes and gastric cancer risk in Taiwan. We have selected and genotyped three promoter polymorphic sites IL-10 A-1082G, T-819C and A-592C among the 358 gastric cancer cases and 358 non-cancer controls. It was found that individuals carrying the AG and GG genotypes were of higher risk of gastric cancer compared with those carrying GG genotype on IL-10 A-1082G (Table III). As for IL-10 T-819C and A-592C, there was no similar differentially genotypic distribution found (Table III). In addition, the results of allelic frequency distribution analysis supported the idea that those individuals carrying the variant G allele were of higher risk of gastric cancer compared with those carrying wild-type A allele (Table IV). Furthermore, there is synergistic genetic-lifestyle interaction for IL-10 A-1082G and personal smoking habit (Table V), however, whether IL-10 A-1082G genotype has interaction with other environmental factors, such as H. pylori infection and/or fruit and vegetable intakes, needs further investigations.
In literature, the contribution of IL-10 promoter genotypes to gastric cancer risk has been investigated by several groups but the findings remain conflicting and inconclusive (12-18). Recently, Asia meta-analyses reported that IL-10 promoter genotypes may be associated with increased gastric cancer risk (18, 27, 28), and at the meanwhile, Western population studies have showed a reverse association (14, 29). It is reasonable that ethnic differences in the distribution of the genotypes may affect the findings and conclusions of genotyping work. In addition, any difference criteria in the inclusion and exclusion of sampling, study designing, recording of patient age at diagnosis, genotyping methodologies, and lifestyle background may also influence the overall findings.
IL10 promoter genotypes were reported to control the production of
IL10 (30, 31), and it was reported that IL10 levels were elevated in gastric mucosa after H. pylori infection, and were higher in patients that have severe chronic inflammation (32). In advanced stages in gastric carcinogenesis, IL10 mRNA expression and serum levels were also elevated (33, 34). As mentioned in the introduction, IL10 plays a role in carcinogenesis acting not only as an anti-inflammatory cytokine but also
as an immunosuppressant (35). Thus, H. pylori-induced IL10 production may have beneficial effects via limiting the inflammation-induced tissue damage, but adding the risk via rendering the mucosal immune cells unable to adequately defense against malignant cells at the same time (32). The current study unrevealed the association of IL-10 genotypes and their interaction with smoking status, but did not provide answers for interactions of other environmental factors such as H. pylori infection or obesity with IL-10 genotypes to gastric carcinogenesis. The roles of IL10 and its contribution to the pathogenesis of gastric cancer require further investigations from DNA, mRNA, protein and functional angles.
In conclusion, our study found that IL10 A-1082G genotypes may play a role in gastric carcinogenesis in Taiwan via an interaction with personal cigarette smoking status. The results provided evidence supporting that gastric carcinogenesis is a multiple steps that involve both genetic and environmental factors. The G allele of IL10 A-1082G may be a useful marker in gastric oncology for early cancer detection and prediction.
Acknowledgement
This study was supported by research grants from Terry Fox Cancer Research Foundation and Taichung Armed-Forces General Hospital (103A04). The assistance from Tsai-Ping Ho in data collection, and genotyping work from Hong-Xue Ji, Chieh-Lun Hsiao, Lin-Lin Hou and Chia-En Miao were highly appreciated by the authors.
References
1 Jemal A, Siegel R, Xu J and Ward E: Cancer statistics, 2010. CA Cancer J Clin 60: 277-300, 2010.
2 Jemal A, Bray F, Center MM, Ferlay J, Ward E and Forman D: Global cancer statistics. CA Cancer J Clin 61: 69-90, 2011.
3 Nagini S: Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 4: 156-169, 2012.
4 Parkin DM: The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118: 3030-3044, 2006.
5 Correa P and Piazuelo MB: Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol Hepatol Rev 7: 59-64, 2011.
6 Mocellin S, Marincola FM and Young HA: Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 78: 1043-1051, 2005.
7 Kingo K, Ratsep R, Koks S, Karelson M, Silm H and Vasar E: Influence of genetic polymorphisms on interleukin-10 mRNA expression and psoriasis susceptibility. J Dermatol Sci 37:
111-113, 2005.
8 Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ and Hutchinson IV: An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 24: 1-8, 1997. 9 Tseng LH, Lin MT, Shau WY, Lin WC, Chang FY, Chien KL,
Hansen JA, Chen DS and Chen PJ: Correlation of interleukin-10 gene haplotype with hepatocellular carcinoma in Taiwan. Tissue Antigens 67: 127-133, 2006.
10 Langsenlehner U, Krippl P, Renner W, Yazdani-Biuki B, Eder T, Koppel H, Wascher TC, Paulweber B and Samonigg H: Interleukin-10 promoter polymorphism is associated with decreased breast cancer risk. Breast Cancer Res Treat 90: 113-115, 2005.
11 Havranek E, Howell WM, Fussell HM, Whelan JA, Whelan MA and Pandha HS: An interleukin-10 promoter polymorphism may influence tumor development in renal cell carcinoma. J Urol 173: 709-712, 2005.
12 Xue H, Wang YC, Lin B, An J, Chen L, Chen J and Fang JY: A meta-analysis of interleukin-10 -592 promoter polymorphism
associated with gastric cancer risk. PLoS One 7: e39868, 2012. 13 Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT and Lin JT:
Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer 104: 617-623, 2003. 14 El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA,
Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni JF, Jr. and Chow WH: Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124: 1193-1201, 2003.
15 Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Sugimura H and Hishida A: Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol 22: 1443-1449, 2007.
16 Garcia-Gonzalez MA, Lanas A, Quintero E, Nicolas D, Parra-Blanco A, Strunk M, Benito R, Angel Simon M, Santolaria S, Sopena F, Piazuelo E, Jimenez P, Pascual C, Mas E, Irun P, Espinel J, Campo R, Manzano M, Geijo F, Pellise M, Gonzalez-Huix F, Nieto M, Espinos J, Tito L, Bujanda L and Zaballa M:
Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol 102: 1878-1892, 2007.
17 Lee JY, Kim HY, Kim KH, Kim SM, Jang MK, Park JY, Lee JH, Kim JH and Yoo JY: Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett 225: 207-214, 2005.
18 Kim J, Cho YA, Choi IJ, Lee YS, Kim SY, Shin A, Cho SJ, Kook MC, Nam JH, Ryu KW, Lee JH and Kim YW: Effects of interleukin-10 polymorphisms, Helicobacter pylori infection, and smoking on the risk of noncardia gastric cancer. PLoS One 7: e29643, 2012.
19 Fortis C, Foppoli M, Gianotti L, Galli L, Citterio G, Consogno G, Gentilini O and Braga M: Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett 104: 1-5, 1996.
20 Tsai CW, Tsai MH, Shih LC, Chang WS, Lin CC and Bau DT: Association of interleukin-10 (IL10) promoter genotypes with nasopharyngeal carcinoma risk in Taiwan. Anticancer Res 33:
3391-3396, 2013.
21 Wang HC, Liu CS, Chiu CF, Chiang SY, Wang CH, Wang RF, Lin CC, Tsai RY and Bau DT: Significant association of DNA repair gene Ku80 genotypes with breast cancer susceptibility in Taiwan. Anticancer Res 29: 5251-5254, 2009.
22 Liu CJ, Hsia TC, Tsai RY, Sun SS, Wang CH, Lin CC, Tsai CW, Huang CY, Hsu CM and Bau DT: The joint effect of hOGG1 single nucleotide polymorphism and smoking habit on lung cancer in Taiwan. Anticancer Res 30: 4141-4145, 2010.
23 Chiu CF, Wang CH, Wang CL, Lin CC, Hsu NY, Weng JR and Bau DT: A novel single nucleotide polymorphism in XRCC4 gene is associated with gastric cancer susceptibility in Taiwan. Ann Surg Oncol 15: 514-518, 2008.
24 Bau DT, Wang HC, Liu CS, Chang CL, Chiang SY, Wang RF, Tsai CW, Lo YL, Hsiung CA, Lin CC and Huang CY: Single-nucleotide polymorphism of the Exo1 gene: association with gastric cancer susceptibility and interaction with smoking in Taiwan. Chin J Physiol 52: 411-418, 2009.
polymorphisms of DNA double strand break gene Ku70 and gastric cancer in Taiwan. BMC Cancer 11: 174, 2011.
26 Lin CH, Lin CC, Tsai CW, Chang WS, Yang CW and Bau DT: Association of Caveolin-1 Genotypes with Gastric Cancer in Taiwan. Anticancer Res 34: 2263-2267, 2014.
27 Zhuang W, Wu XT, Zhou Y, Liu L, Liu GJ, Wu TX, Yao X, Du L and Wei ML: Interleukin10 -592 promoter polymorphism associated with gastric cancer among Asians: a meta-analysis of epidemiologic studies. Dig Dis Sci 55: 1525-1532, 2010.
28 Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, Du L, Wei ML and Wu XT: Interleukin-10 -1082 promoter polymorphism associated with gastric cancer among Asians. Eur J Cancer 44: 2648-2654, 2008.
29 Zambon CF, Basso D, Navaglia F, Belluco C, Falda A, Fogar P, Greco E, Gallo N, Rugge M, Di Mario F and Plebani M: Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 29: 141-152, 2005.
genotypes in SLE. J Biomed Biotechnol 2010: 838390, 2010.
31 Suarez A, Castro P, Alonso R, Mozo L and Gutierrez C: Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation 75: 711-717, 2003.
32 Bodger K, Wyatt JI and Heatley RV: Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut 40: 739-744, 1997.
33 De Vita F, Orditura M, Galizia G, Romano C, Infusino S, Auriemma A, Lieto E and Catalano G: Serum interleukin-10 levels in patients with advanced gastrointestinal malignancies. Cancer 86: 1936-1943, 1999.
34 Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, Gerhard M and Prinz C: Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut 53: 1082-1089, 2004.
faces of interleukin 10 in human infectious diseases. Lancet Infect Dis 6: 557-569, 2006.
Table I. Distributions of selected characteristics among gastric cancer patients and controls.
Characteristics Controls (n = 358) Patients (n = 358) P-value a
n % Mean (SD) n % Mean (SD) Age (y) 62.1 (9.5) 63.8 (11.4) 0.5811 Gender 0.2219 Male 242 67.6% 258 72.1% Female 116 32.4% 100 27.9%
Personal smoking status
Cigarette smokers 234 65.4% 256 71.5% 0.0912
Non-smokers 124 34.6% 102 28.5%
Table II. The primer sequences, polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) conditions for Interleukin-10 A-1082G, T-819C and A-592C genotyping work.
Polymorphisms (locations)
Primer sequences Restriction
enzyme
SNP sequence
DNA fragment size (bp) A-1082G (rs1800896) F: 5’-CTCGCTGCAACCCAACTGGC-3’ R: 5’-TCTTACCTATCCCTACTTCC-3’ Mnl I A G 139 bp 106 + 33 bp T-819C (rs3021097) F: 5’-TCATTCTATGTGCTGGAGAT-3’ R: 5’-TGGGGGAAGTGGGTAAGAGT-3’ Mae III T C 209 bp 125 + 84 bp A-592C (rs1800872) F: 5’-GGTGAGCACTACCTGACTAG-3’ R: 5’-CCTAGGTCACAGTGACGTGG-3’ Rsa I C A 412 bp 236 + 176 bp *F and R indicate forward and reverse primers, respectively
Table III. Distribution of Interleukin-10 A-1082G, T-819C and A-592C
genotypes among gastric cancer patients and controls.
Genotype Controls (n = 358) % Patients (n = 358) % P-valuea A-1082G (rs1800896) 0.0004* AA 281 78.5% 235 65.6% AG 67 18.7% 101 28.2% GG 10 2.8% 22 6.2% T-819C (rs3021097) 0.8811 TT 186 52.0% 190 53.1% CT 132 36.8% 132 36.9% CC 40 11.2% 36 10.0% A-592C (rs1800872) 0.8651 AA 180 50.3% 186 52.0% AC 141 39.4% 134 37.4% CC 37 10.3% 38 10.6%
Table IV. Distribution of allele frequencies at 1082G, T-819C and
A-592C of Interleukin-10 gene among gastric cancer patients and controls.
Allele Controls % Patients % P-valuea
A-1082G (rs1800896) 3.19*10-5* Allele A 629 87.8% 571 79.7% Allele G 87 12.2% 145 20.3% T-819C (rs3021097) 0.6837 Allele T 504 70.4% 512 71.5% Allele C 212 29.6% 204 28.5% A-592C (rs1800872) 0.8170 Allele A 501 67.0% 506 70.7% Allele C 215 33.0% 210 29.3%
Table V. Distribution of Interleukin-10 A-1082G genotypes in gastric cancer patients after stratification by personal smoking status.
Genotypes Non-smokers P-value OR (95% CI) a Smokers P-value OR (95% CI) a
Controls Patients Controls Patients
AA 93 71 0.3735 1.000 (Reference) 188 164 0.0001* 1.000 (Reference)
AG+GG 31 31 1.31 (0.73-2.35) 46 92 2.29 (1.52-3.46)*
Total 124 102 234 256
a OR: Odds ratio, CI: confidence interval; ORs were estimated with multivariate logistic regression analysis. *Statistically identified as significant.