REVIEW ARTICLE
New Insights into the Pathogenesis and Treatment of Patients with
Immunoglobulin A Nephropathy
Yasuhiko Tomino
1,2 *1Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine, Tokyo, Japan 2Juntendo University International Center (JUIC), Tokyo, Japan
a r t i c l e i n f o
Article history: Received: Aug 3, 2011 Revised: Sep 23, 2011 Accepted: Sep 23, 2011 KEY WORDS: ddY mouse; IgA nephropathy; respiratory mucosa; steroid pulse therapy; tonsillectomy;under-galactosylated IgA1
Immunoglobulin A (IgA) nephropathy (also called Berger’s disease) is the most common primary chronic glomerulonephritis worldwide, and wasfirst described by J. Berger et al in 1968. Histopathologically, IgA nephropathy is characterized by expansion of glomerular mesangial matrix with mesangial cell prolif-eration and/or mononuclear cell infiltration. Glomeruli typically contain generalized-diffuse granular mesangial deposits of IgA (mainly IgA1), IgG and C3. Electron-dense deposits are observed in the glomerular mesangial areas and partially in the glomerular basement membrane. Thus, this disease is considered to be an immune-complex-mediated glomerulonephritis. Clinically, patients with IgA nephropathy show microscopic and macroscopic hematuria and/or proteinuria. Advanced patients (almost 40% of patients) progress to end-stage kidney disease during 20 years of observation. However, the pathogenesis and radical treatment of IgA nephropathy have still not been established.
CopyrightÓ 2011, Taipei Medical University. Published by Elsevier Taiwan LLC. All rights reserved.
1. Introduction
Immunoglobulin A (IgA) nephropathy is considered to be an immune-complex-mediated glomerulonephritis, although the antigenic substances are still obscure.1Clinically, patients with IgA nephropathy show microscopic and macroscopic hematuria and/or proteinuria. Advanced patients progress to renal hypertension, renal anemia and end-stage kidney disease (ESKD). Progression to ESKD in patients with this disease is not as rare as originally thought. Thus, it is important to determine the mechanism of onset and progression in patients with IgA nephropathy. The objectives of this review are to introduce clinicopathological features of IgA nephropathy, and to summarize new insights into the pathogenesis and treatment of patients with IgA nephropathy.
2. Clinicopathological manifestations 2.1. Clinicalfindings
Patients with IgA nephropathy show microscopic and macroscopic hematuria and/or proteinuria. Macroscopic hematuria is occa-sionally observed after upper respiratory infections including acute
tonsillitis and/or pharyngitis. IgA nephropathy is frequently preceded by episodes of upper respiratory or gastrointestinal infections, which are presumed to have a viral or bacterial etiology. Many dysmorphic red blood cells, various cellular casts and acti-vated platelets in the urinary sediments are frequently observed in the advanced stage of this disease.2Serum IgA>350 mg/dL has frequently been observed in adult patients with IgA nephropathy. We have already reported the importance of four clinical markers in the diagnosis of patients with IgA nephropathy, or in the differ-ential diagnosis from other types of chronic glomerulonephritis as follows: (1) more thanfive red blood cells in urinary sediments; (2) persistent proteinuria (>0.3 g/day); (3) serum IgA level >315 mg/ dL; and (4) serum IgA/C3 ratio>3.01. Patients with three or four clinical markers have been diagnosed with IgA nephropathy in previous studies.2,3
A Joint Committee of the Special Study Group on Progressive Glomerular Disease, Ministry of Health, Labor and Welfare of Japan found in 1995 that it required>3 years on average from estimated onset to the first consultation and subsequent diagnosis of IgA nephropathy by renal biopsy.4 About 30% and 5e10% of IgA nephropathy patients develop ESKD within 15e20 years and 5 years, respectively. Conversely, about 60% can avoid ESKD. Notably, some of them spontaneously achieve natural remission or maintain a clinically stable condition without any treatment.
Clinical markers for poor prognosis of this disease are as follows: (1) renal (glomerular and tubulointerstitial) histopathological changes; (2) heavy proteinuria; (3) renal dysfunction at the time of
* Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine, 2-1-1 Hongo, Bunkyo-Ku, Tokyo 813-8421, Japan.
E-mail: <yasu@juntendo.ac.jp>
Contents lists available atSciVerse ScienceDirect
Journal of Experimental and Clinical Medicine
j o u r n a l h o m e p a g e : h t t p : // w w w . j e c m - o n l i n e . c o m
1878-3317/$e see front matter Copyright Ó 2011, Taipei Medical University. Published by Elsevier Taiwan LLC. All rights reserved. doi:10.1016/j.jecm.2011.11.003
biopsy; (4) hypertension; (5) male sex; and (6) age<30 years.5The significance of gene polymorphisms of the renin angiotensin system in prognosis is still controversial in patients with IgA nephropathy.
2.2. Histopathologicalfindings
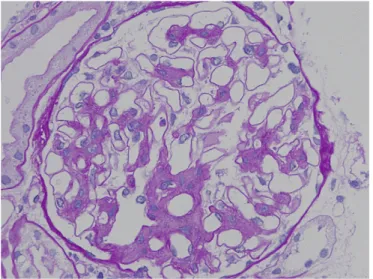
IgA nephropathy is characterized by expansion of glomerular mesangial matrix with mesangial cell proliferation and/or mono-nuclear cell infiltration. In light microscopy, typical periodic acid-eSchiff-positive hemispherical bodies with mesangial expansion and mesangial cell proliferation are observed in the glomerular paramesangial areas (Figure 1). Tubulointerstitialfibrosis, tubular atrophy and mononuclear cell infiltration are observed in patients with advanced IgA nephropathy (Figure 2). In Japan, the Special IgA Nephropathy Study Group of the Progressive Renal Diseases Study Committee organized by the Ministry of Health, Labor and Welfare conducted a multicenter retrospective caseecontrol study of IgA nephropathy in 2004 to develop an evidence- and lumped-system-based clinicopathological classification of IgA nephropathy for predicting long-term risk of progression to ESKD (Kawamura et al,
submitted for publication). The recently published Oxford classi fi-cation of this disease has identified prognostic pathological features, providing substantial evidence that histological grading systems can be used to predict renal outcome of IgA nephropathy.6 3. Initiation factors
3.1. Spontaneous animal model for IgA nephropathy
In the 40 years since IgA nephropathy wasfirst reported, the cause of this disease has never been clarified. The main reason for this appears to be that there has not been an appropriate animal model of this disease. A spontaneous animal model, the ddY mouse, is now used for investigating pathogenesis and treatment of IgA nephropathy.7In 1985, Imai et al8(Akita, Japan)first reported that the ddY strain of mouse can serve as a spontaneous animal model for human IgA nephropathy. ddY mice were imported from Germany before 1920 and have been maintained in Japan since that time. These ddY mice show mild proteinuria without hematuria and mesangioproliferative glomerulonephritis with glomerular IgA deposits. Marked deposition of IgA and C3 in the glomerular mesangial areas in association with an increase in the levels of macromolecular IgA appears in sera of these mice with aging (Figure 3). Electron-dense deposits are marked in the glomerular mesangial areas when observed by electron microscopy. These immunopathological findings appear at >40 weeks of age. Although the incidence of IgA nephropathy in ddY mice is highly variable, it appears that clinicopathological aberrations other than hematuria in ddY mice resemble those in human IgA nephropathy. Sequential renal biopsies were performed on 361 ddY mice. IgA nephropathy occurred in about 30% of the mice by 20 weeks (early onset group) and in about 30% of the mice by 40 weeks (late onset group). It did not occur in the remaining mice (quiescent group).9 When an“association study” on onset was performed in the early onset and quiescent groups, multiple disease receptor gene loci were observed.9One of the loci was found to be homologous with the gene locus reported for familial IgA nephropathy, therefore, at least some of these mice appear to be subject to the same genetic regulation as human IgA nephropathy, and ddY mice were judged to be useful as an animal model as described previously.9 A genome-wide scan with 270 microsatellite markers has identified three chromosomal regions on chromosomes 1, 9 and 10, which are significantly associated with glomerular injuries. The peak marker D10MIT86 on chromosome 10 is located in the region syntenic to human 6q22e23with IgAN1, which is the candidate gene respon-sible for familial IgA nephropathy.9,10 In addition, D1MIT16 on chromosome 1 is very close to the locus of the selectin gene, which is a known candidate for human IgA nephropathy. It appears that the three-group ddY mouse model can be a useful tool for identi-fying the susceptibility genes and also for examining their roles in the pathogenesis of IgA nephropathy.7
Figure 1 Paramesangial deposits in a glomerulus of an IgA nephropathy patient viewed by light microscopy (periodic acideSchiff staining).
3.2. Abnormalities in the mucosal immunity and IgA molecule 3.2.1. Mucosal immunity
Many IgA nephropathy patients experience episodic macroscopic hematuria, which may be spontaneous or coincide with mucosal infection, usually of the respiratory or gastrointestinal tracts. The main production site of IgA is the mucosa of the respiratory tract and intestines, and the first symptoms in the onset of IgA nephropathy often suggest infections of the upper respiratory and gastrointestinal tracts. Almost all IgA secreted from the mucosa is dimeric or polymeric and the IgA deposited in the glomeruli of IgA nephropathy patients has the same properties,11 showing that mucosal immunity is the most important factor in this disease. IgA is secreted in the mucosa to prevent infiltration of pathogens or toxins by aggregation or neutralization. This IgA is polymeric and binds with secretory components or J-chains. Secretory IgA (SIgA) is also present in small amounts in the blood. IgA nephropathy patients often show macroscopic hematuria after infections of the upper respiratory and gastrointestinal tracts, therefore, the onset of mucosal infections acts as a trigger. Recently, a correlation between hematuria and the serum level of SIgA1 has been reported.12 J chains with IgA have been identified in the glomeruli of IgA nephropathy patients, and IgA eluted from the glomeruli of resec-ted kidneys of patients with relapsed IgA nephropathy after transplantation is polymeric SIgA1. This suggests the possibility that SIgA1 of mucosal origin plays an important role in glomerular deposition of IgA.
Toll-like receptors (TLRs) are a family of pathogen pattern recognition receptors that have several different classes of pathogen-related structures and active defense mechanisms, particularly in innate immunity. Myeloid differentiation factor 88 (MyD88) is a common adaptor molecule required for signaling mediated by TLR. Suzuki et al13 have reported the relationship between TLR9 and severity of renal injury in IgA nephropathy of ddY mice. MyD88 has been identified as a candidate gene for progression of renal injury in ddY mice. In a previous study, ddY mice were housed under either conventional or specific pathogen-free conditions. Expression of genes encoding TLR and signaling molecule MyD88 were quantified by real-time reverse transcrip-tase polymerase chain reaction in spleen cells. The severity of renal injuries was higher in the conventionally housed group, although the housing conditions did not affect the prevalence of IgA nephropathy. ddY mice that had a history of IgA nephropathy and were housed under conventional conditions had higher levels of TLR9 and MyD88 transcripts than the mice that had IgA nephrop-athy and were housed under specific pathogen-free conditions. Moreover, nasal challenge with CpG-oligodeoxynucleotides (DNA), which are ligands for TLR9, aggravated renal injury, led to strong Th1 polarization, and increased serum and mesangial IgA. It appears that activation of the TLR9/MyD88 pathway by common exogenous bacterial and/or viral antigens may affect the severity of IgA nephropathy.13 Thus, it is postulated that overproduction of under-galactosylated IgA1 in sera occurs due to common exoge-nous bacterial and/or viral infections in patients with IgA nephropathy.
Conversely, when bone marrow transplantation (BMT) is per-formed in ddY mice using marrow of ddY and normal mice, BMT from the quiescent group ameliorates not only glomerular injury, but also mesangial deposition of IgA and IgG in the early onset group of ddY mice. BMT from the early onset group causes glomerular injury with IgA and IgG deposition in the quiescent group of ddY mice. Moreover, BMT from the early onset group of ddY mice causes acute progression of glomerular injury in the same group. Therefore, it appears that bone marrow cells may contribute to the glomerular IgA and IgG depositions and glomerular injury. It
is necessary to determine the roles of bone marrow cells, including immune responses.14
3.2.2. IgA molecules
Serum IgA in IgA nephropathy patients has many truncated O-linked glycans with little galactose in the hinge region. The expression of
b
1,3-galatosyltransferase and its molecular chap-erone Cosmc that promotes galactosylation of IgA1 in peripheral B cells of IgA nephropathy patients is decreased.15,16 Therefore, attention has been focused on aberrant galactosylation in the IgA1 molecule as one of the causes of IgA nephropathy. IgA1 with decreased galactosylation of the O-linked glycans suggests that self-aggregation of the hinge region structure and formation of a compound between the hinge region epitope and the IgA anti-body are apt to occur under unstable conditions.17e19This aber-rantly galactosylated IgA1 is deposited in the glomerular mesangial cell region by various mechanisms including binding with recep-tors, phagocytosis and electrical charge, and IgA nephropathy occurs.20Experimentally, this aberrantly galactosylated IgA1 is also known to promote mesangial cell proliferation.In IgA nephropathy patients, circulatory IgA1 and IgA1 in mesangial deposits contain elevated amounts of galactose-deficient IgA1 (Gd-IgA1). Suzuki et al have hypothesized that a fraction of Gd-IgA1 from the glomerular deposits may be excreted in the urine and thus may represent a disease-specific marker (submitted). Recently, they have evaluated the utility of sodium dodecyl sulfate-polyacrylamide gel electrophoresis/western blot-ting and enzyme-linked immunosorbent assay (ELISA) for detec-tion of urinary polypeptide biomarkers for diagnosis of IgA nephropathy. Levels of IgA and Gd-IgA1 were determined in urine samples from cohorts recruited in the USA and Japan. Urine samples were collected from 62 patients with IgA nephropathy, 25 with other renal diseases (disease controls), and 53 healthy controls. Urinalysis indicated proteinuria in 87% of IgA nephropathy patients, 88% of disease controls, and 0% of healthy controls. Gd-IgA1 was determined by ELISA using Helix aspersa lectin specific for terminal N-acetylgalactosamine. Urinary IgA was elevated in patients with IgA nephropathy as well as in disease controls. However, elevation of urinary Gd-IgA1 levels was more frequent in patients with IgA nephropathy. These exploratoryfindings should be evaluated in a prospective study with concurrent renal biopsy and longitudinal sampling. If validated, it may be feasible to develop these assays into a novel noninvasive test to detect renal injury at earlier stages of IgA nephropathy and to assess clinical manifestations and response to therapy (Suzuki et al, submitted for publication).
4. Progressive factors
There are many progressive factors including genetic background in patients with IgA nephropathy (Table 1).21Complement activation, podocyte injury and mast cell infiltration in the interstitium are summarized in this chapter.
4.1. Complement activation
Earlier studies have indicated that IgA per se activates the alter-native pathway of complement, whereas more recent data have also shown activation of the lectin pathway in patients with IgA nephropathy. Roos et al22 have reported that both mannose-binding lectin andL-ficolin contribute to the progression of IgA
nephropathy. They have hypothesized that serum levels of complement components and regulatory proteins in patients with IgA nephropathy are correlated with its pathogenesis. Onda et al23 have clarified the variations in serum levels of
complement components and regulatory proteins (CRPs) in patients with IgA nephropathy. It appears that hyper-complementemia occurs in the progression of IgA nephropathy and is controlled by an increase of CRPs. Higher levels of serum C4 binding protein in particular may indicate the severity of histological injury in this disease.23
4.2. Podocyte injury
Development of glomerulosclerosis in several human and experi-mental diseases is associated with podocyte injury. The number of podocytes per glomerulus might be a podocyte injury parameter and provide prognostic information in patients with IgA nephropathy. Podocyte injury may be caused by apoptosis, necrosis, detachment from the glomerular basement membrane (GBM), or autophagy of such cells. It is speculated that depositions of IgA, IgG and C3 in the glomerular capillary walls and/or some cytokines produced by mesangial cells might induce podocyte injury.
Lemley et al24have reported that podocyte loss, that is, podo-cytopenia, is concurrent with increasing disease severity in patients with IgA nephropathy. They have found no corresponding corre-lations between the clinical indices of injury and the number of mesangial and endothelial cells in this disease. Endothelial and mesangial cell damage is followed by regeneration, but this is not the case with podocytes. Podocytes in adults do not undergo mitosis, and normally, the only way to respond to injury is by cell hypertrophy. Thus, increased podocyte surface area reflects the extent of podocyte hypertrophy. In severe patients, segments of the GBM become denuded. Direct contact of the naked portion of the GBM with the parietal epithelium of Bowman’s capsule might lead to adhesion to Bowman’s capsule, and potentially, segmental sclerosis.25Hishiki et al26have reported that podocyte injuries, that is, reduction of absolute number per glomerulus and increase of glomerular surface area covered by one podocyte, are related to disease progression in patients with IgA nephropathy. Thus, podocyte injury might provide additional prognostic information in such patients.
4.3. Mast cell infiltration in the interstitium
Mast cells are derived from hematopoietic progenitors and migrate into inflammatory lesions. Human mast cells can be classified into two types according to their protease composition: those con-taining only tryptase (MCTs) and those concon-taining both tryptase and chymase (MCTCs). MCTs are involved in immunological responses, whereas MCTCs seem instead to play a role in
angiogenesis and tissue remodeling. IgA nephropathy patients have more MCTCs than MCTs in the interstitial lesions. Mast cells are observed around but not in the conglomerate of angiotensin II (Ang-II)-positive cells. The number of Ang-II-positive cells is correlated with MCTCs and MCTs in patients with IgA nephropathy with the most severe pathological changes. It appears that chymase-dependent Ang-II synthesis induced by human mast cells may be involved in the inflammatory and fibrotic processes of IgA nephropathy.27
5. Treatment (Table 2)
5.1. Management of dietary salt and protein intake
Prognosis of IgA nephropathy depends not only on medication but also on dietary control. Low-salt diet is usually recommended to control blood pressure because high salt intake is a major cause of blood pressure increases. Long-term protein restriction is generally considered to reduce the levels of urinary protein and ameliorate glomerular injuries in patients with IgA nephropathy. Thus, assessment of salt and protein intakes is very important in the management of IgA nephropathy. A high level of knowledge con-cerning IgA nephropathy and diet should improve compliance. My colleagues and I have suggested that an educational program may prove to be effective for improving patients’ knowledge, that is, efficacy of dietary and protein intake, and blood pressure control by dietary salt restriction in patients with chronic kidney disease and IgA nephropathy.28,29
5.2. Drug therapy
5.2.1. Olmesartan: antihypertensive drug
Angiotensin-converting enzyme (ACE) inhibitors and/or angio-tensin II receptor blockers (ARBs) exert a marked renoprotective effect in patients with IgA nephropathy. Combination therapy with an ACE inhibitor and ARB has been reported to produce a more marked decrease in proteinuria progression in normotensive patients with IgA nephropathy. However, in Japan, ACE inhibitors and ARBs are not used in normotensive patients. My research group has evaluated the antiproteinuric effect of ARB olmesartan in normotensive patients with IgA nephropathy in a multicenter controlled trial.30 Olmesartan was given to 25 patients for 16 weeks. The initial dose was 5 mg and was increased stepwise to 10 mg, 20 mg and 40 mg. Final doses were 40 mg (n¼ 11), 20 mg (n¼ 5), 10 mg (n ¼ 7) and 5 mg (n ¼ 2). The change in urinary protein to creatinine ratio was e56.2%. Creatinine clearance showed no changes throughout the study period. The average blood pressure was 119 /77 mmHg in the lead-in period and
Table 2 Treatment in patients with IgA nephropathy 1. Basic therapy
Strict control of blood pressure
Low protein, low salt and high calorie diet 2. Drug therapy
a) Immunotherapy
Corticosteroids: ordinary and/or pulse therapy
Immunosuppressants: cyclosporin A, mizoribin, mycophenolate mofetil Tonsillectomy
Tonsillectomy with steroid pulse therapy Plasma exchange
b) Non-immunotherapy
Dilazep hydrochoride, dipyridamole Angiotensin-converting enzyme inhibitor Angiotensin II AT1 receptor blocker Fish oil (ethyl icosapentate)
Others: heparin sodium, warfarin potassium, urokinase, carbazochrome sodium sulfate
Table 1 Progressive factors in patients with IgA nephropathy Clinicalfindings
Heavy proteinuria
Renal dysfunction at the time of renal biopsy Hypertension Male sex Age<30 y Genetic background Others Pathologicalfindings
Severe renal (glomerular and tubulointerstitial) histopathological injury Podocyte loss
Increase of cytokine, chemokine and/or growth factor activity Increase of reactive oxygen species activity
Increase of complement activity
Increase of platelet aggregation and blood coagulation Increase of adhesion molecule and cell infiltration Others
decreased to 107/77 mmHg at week 16. On completion of treatment with olmesartan, no correlation was observed between changes in urinary protein to creatinine ratio and mean blood pressure based on an investigation of dispersion diagrams. It appears that olme-sartan monotherapy showed a robust reduction of urinary protein in normotensive IgA nephropathy patients, suggesting that this effect is independent of its blood-pressure-lowering properties.30 5.2.2. Tonsillectomy with steroid pulse therapy
The tonsils are mucosa in very close contact with extrinsic antigens, especially infectious antigens, and are the site of initiation of immune responses. In recent years, the focus has been centered on the tonsils as part of the immune system and a site of infection. TLRs are a family of pathogen pattern recognition receptors that have several different classes of pathogen-related structures and active defense mechanisms, particularly in innate immunity as described above. Macroscopic hematuria is occasionally observed after acute tonsillitis in patients with IgA nephropathy. Clinical effects of tonsillectomy with steroid pulse therapy on the treatment of proteinuria and hematuria in patients with IgA nephropathy have been reported mainly from Japan.31,32It has been reported that tonsillar TLR9 expression was correlated with the therapeutic efficacy of tonsillectomy followed by steroid pulse therapy. About 23% of patients showed high tonsillar expression levels of TLR9 and high remission rates of hematuria and proteinuria after tonsillec-tomy with steroid pulse therapy. Recently, Suzuki et al have examined the relationship between expression profiles of tonsillar TLR9 or TLR9 single nucleotide polymorphism (SNP) and the clin-ical effects of tonsillectomy with steroid pulse therapy. In patients with IgA nephropathy, it is clear that the TT genotype of the TLR9 known rs35410 SNP and the histological severity show a significant correlation.13 In a group of IgA nephropathy patients with high TLR9 expression levels in resected tonsils and a TT genotype group, the therapeutic effects of tonsillectomy and steroid pulse therapy on hematuria and proteinuria were high, suggesting that exposure to extrinsic antigens in the mucosa, and especially the tonsils, is important in the progression of IgA nephropathy via abnormal TLR9 activation. It appears that the treatment response of tonsil-lectomy to steroid pulse therapy may be correlated with the tonsillar TLR 9 expression level and genotype of TLR9 SNP (rs352140) (Sato et al, in press). At present, a multicenter randomized controlled trial on concomitant tonsillectomy and steroid pulse therapy in IgA nephropathy patients is underway in Japan and the results are awaited.
6. Conclusion
I have reviewed new insights into the mechanisms of initiation and progression, and treatment in patients with IgA nephropathy. Controversial results have been obtained, therefore, the results of future research and clinical trials are eagerly awaited.
Acknowledgments
I sincerely thank my colleagues in the Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine, Tokyo, Japan. A part of this manuscript was reported at the International Symposium on New Advance in Nephrology in Taipei Medical University on June 11, 2011.
References
1. Berger J, Hinglais N. Les Depots intercapillaries d’IgA-IgG. J Urol Nephrol 1968;74:694e5.
2. Nakayama K, Ohsawa I, Maeda-Ohtani A, Murakoshi M, Horikoshi S, Tomino Y. Prediction of diagnosis of immunoglobulin A nephropathy prior to renal biopsy and correlation with urinary sedimentfindings and prognostic grading. J Clin Lab Anal 2008;22:114e8.
3. Maeda A, Gohda T, Funabiki K, Horikoshi S, Shirato I, Tomino Y. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J Clin Lab Anal 2003;17:73e6.
4. Koyama A, Igarashi M, Kobayshi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research group on progressive renal diseases. Am J Kidney Dis 1997;29:526e32.
5. Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospec-tive cohort study. Nephrol Dial Transplant 2009;24:3068e74.
6. Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, et al, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76:534e45.
7. Tomino Y. IgA nephropathy: lessons from an animal model, the ddY mouse. J Nephrol 2008;21:464e7.
8. Imai H, Nakamoto Y, Asakura K, Miki K, Yasuda T, Miura AB. Spontaneous glomerular IgA deposition in ddY mice: An animal model of IgA nephritis. Kidney Int 1985;27:756e61.
9. Suzuki H, Suzuki Y, Yamanaka T, Hirose S, Nishimura H, Toei J, Horikoshi S, et al. Genome-wide scan in a novel IgA nephropathy model identifies a susceptibility locus on murine chromosome 10 in a region syntenic to human IGAN1 on chromosome 6q22-23. J Am Soc Nephrol 2005;16:1289e99.
10. Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6Q22-23. Nat Genet 2000;26:354e7.
11. Tomino Y, Endoh M, Nomoto Y, Sakai H. IgA1 and IgA nephropathy. N Engl J Med 1981;305:1159e60.
12. Oortwijn BD, van der Boog PJ, Roos A, van der Greest RN, de Filter JW, Daha MR, van Kooten C. A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int 2006;69:1131e8.
13. Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, Kanou T, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol 2008;19:2384e95.
14. Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, Pang H, Horikoshi S, et al. Th1 polarization in murine IgA nephropathy directed by bone-marrow-derived cells. Kidney Int 2007;72:319e27.
15. Suzuki H, Moldveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 2008;118:629e39.
16. Qin W, Zhou Q, Yang LC, Li Z, Su BH, Luo H, Fan JM. Peripheral B lymphocyte beta-galactosyltransferase and chaperone expression in immunoglobulin A nephropathy. J Intern Med 2005;258:467e77.
17. Kokubo T, Hiki Y, Iwase H, Tanaka A, Toma K, Hotta K, Kobayashi Y. Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J Am Soc Nephrol 1998;9:2048e54.
18. Yan Y, Xu LX, Zhang JJ, Zhang Y, Zhao MH. Self-aggregated deglycosylated IgA1 with or without IgG were associated with the development of IgA nephrop-athy. Clin Exp Immunol 2006;144:17e24.
19. Bonner A, Furtado PB, Almogren A, Kerr MA, Perkins SJ. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J Immunol 2008;180:1008e18.
20. van der Boog PJ, van Kooten C, de Fijter JW, Daha MR. Role of macromolecular IgA in IgA nephropathy. Kidney Int 2005;67:813e21.
21. Tomino Y. Pathogenesis of IgA nephropathy. IgA nephropathy today. In: Tomino Y, editor. Contributions to nephrology, 157. Basel: Karger; 2007. p. 1e7.
22. Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 2006;17:1724e34.
23. Onda K, Ohi H, Tamano M, Ohsawa I, Wakabayashi M, Horikoshi S, Fujita T, et al. Hypercomplementemia in adult patients with IgA nephropathy. J Clin Lab Anal 2007;21:77e84.
24. Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int 2002;61:1475e85.
25. Kriz W, Kretzler M, Nagata M, Provoost AP, Shirato I, Uiker S, Sakai T, et al. A frequent pathway to glomerulosclerosis: detection of tuft architecture-podocyte damage-segmental sclerosis. Kidney Blood Press Res 1996;18: 245e53.
26. Hishiki T, Shirato I, Takahashi Y, Funabiki K, Horikoshi S, Tomino Y. Podocyte injury predicts prognosis in patients with IgA nephropathy using a small amount of renal biopsy tissue. Kidney Blood Press Res 2001;24:99e104. 27. Sakamoto-Ihara T, Suzuki Y, Kurusu M, Yamashita M, Horikoshi S, Tomino Y.
Possible involvement of mast cells in renal fibrosis in patients with IgA nephropathy. Inflamm Res 2007;56:421e7.
28. Yamaji K, Kurusu A, Okamoto M, Sekiguchi Y, Horikoshi S, Tomino Y. Effect of educational hospitalization on chronic kidney disease (CKD) patients. Clin Nephrol 2007;68:401e4.
29. Kobayasi T, Yamaji K, Takeda Y, Maiguma M, Omote K, Sekiguchi Y, Tsuge T, et al. Efficacy of educational short-term hospitalization in patients with chronic kidney disease (CKD): second report. Nephrology Frontier 2011;10: 94e8.
30. Tomino Y, Kawamura T, Kimura K, Endoh M, Hosoya T, Horikoshi S, Utsunomiya Y, et al. Antiproteinuric effect of olmesartan in patients with IgA nephropathy. J Nephrol 2009;22:224e31.
31. Hotta S, Miyazaki M, Furuta T, Tomioka S, Chiba S, Horigome I, Abe K, et al. Tonsillectomy and steroid pulse therapy significantly impact clinical remission in patients with IgA nephropathy. Am J Kidney Dis 2001;38:736e43. 32. Miura N, Imai H, Kikuchi S, Hayashi S, Endoh M, Kawamura T, Tomino Y, et al.
Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephrop-athy: a nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol 2009;13:460e6.