Original contribution
Low-cost telepathology system for intraoperative
frozen-section consultation: our experience
and review of the literature
Wen-Yih Liang MD
a, Chih-Yi Hsu MD
a, Chiung-Ru Lai MD
a,
Donald Ming-Tak Ho MD, PhD
a,b, I-Jen Chiang PhD
c,⁎
a
Department of Pathology, Taipei Veteran General Hospital, Taipei, Taiwan, ROC
b
National Yang-Ming University School of Medicine, Taipei City 112, Taiwan, ROC
c
Department of Medical Informatics, Taipei Medical University, Taipei City 110, Taiwan, ROC
Received 25 January 2007; revised 9 April 2007; accepted 9 April 2007
Summary We have established a low-cost noncommercial system of dynamic real-time telepathology for light microscopic diagnosis that was used to aid intradepartmental consultation for frozen-section diagnosis. Fifty cases were performed. For each case, multiple diagnoses were made and compared, namely, those made by the pathologist on duty (D1), by a subspecialist or senior using telepathology (D2), by the same pathologist using a light microscope (D3), and the final diagnosis (D4). A comparison of D1 and D2 revealed that 37 cases (74%) were diagnosed more precisely by D2. In 9 (18%) of 50 cases, there was a positive major impact on the operation as a result of teleconsultation. The results of D2 and D3 showed good agreement (κ = 0.97). The average time span required for telepathology is short compared with routine intradepartmental consultation. Our experience showed that telepathology is a good tool for frozen-section consultation and imposes little additional cost.
© 2008 Elsevier Inc. All rights reserved.
Keywords: Telepathology; Internet; Frozen section; Consultation
1. Introduction
Telepathology is a branch of telemedicine that uses telecommunication linkages to electronically capture, store, retrieve, and transmit images to distant sites for a variety of purposes. Telepathology can be performed in 2 distinct modes: (1) dynamic, in which live images are transmitted from a server site and viewed electronically in real time at the other site [1-3], and (2) static, in which still images are transmitted to a remote site in a near real-time manner[4-6].
The application of telepathology to intraoperative con-sultation (frozen-section diagnosis) is thought to be useful. In 1989, investigators in Norway were the first to provide intraoperative frozen-section services to several rural hospitals via telepathology [7]. Telepathology makes it possible for small hospitals without a pathologist on site to provide operations requiring an intraoperative histopatholo-gic diagnosis. Telepathology also can be used to support an isolated pathologist for second or expert consultation or even to transfer the diagnostic work completely to another facility, as described by Dunn et al[8].
In our department, the office and main facilities are 500 m away from the main building of the hospital and the operating room—a walk of about 15 minutes. One of our 11
www.elsevier.com/locate/humpath
* Corresponding author.
E-mail address: tech1@obsgyn.net (I. -J. Chiang).
0046-8177/$– see front matter © 2008 Elsevier Inc. All rights reserved. doi:10.1016/j.humpath.2007.04.023
staff pathologists is on duty for the frozen-section service, determined by a monthly schedule, and remains at the frozen-section room in the main hospital. When facing an uncertain or difficult case, the on-duty pathologist may refer the case or call a subspecialist or senior pathologist for help. We had an experience with real-time telepathology for remote diagnoses, described in our previous study, and got excellent results [9,10]. Inspired by the merging of telepathology and its applications, we also developed a low-cost telepathology system for intraoperative frozen-section consultation in 2003. In this study, we examined the diagnosis made by the pathologist on duty (D1), a subspecialist or senior using telepathology (D2), the same pathologist as in D2 using a light microscope (D3), and the final diagnosis of the case (D4) and compared them. We were interested in the following questions: (1) whether telecon-sultation (D2) could improve the original frozen-section diagnosis (D1) and (2) the differences between the diagnoses rendered after teleconsultation (D2) and direct review (D3).
2. Materials and methods
2.1. Equipment
The Web-enabled charge coupled device camera used (AXIS 2420 Network Camera; Axis Communications, Lund, Sweden;www.axis.com/products/cam_2420/) is a digital net-work camera running TCP/IP. It includes all of the netnet-working connectivity required for distributing monitored images over a secure intranet or the Internet. With its own built-in server, the camera provides high-quality imaging and full Web-based control of the product management and configuration functions via a browser over a network. The camera, attached to a light microscope (Nikon Labophot-2 equipped with 4×, 10×, 40× and 100× E-PLAN objectives and trinocular microscope head and C-mount adapter (Nikon, Kanagawa, Japan)), is used in the room where frozen sections are prepared (Fig. 1). It contains an embedded Web server system and can connect directly to local networks or the Internet, transmitting high-quality streaming video at 30 (352 × 240) or 15 (704 × 480) frames per second. The pathologist also can use an attached monitor to get a larger and clearer view of the slide. The consultant requires only a computer connected to the intranet or Internet. By opening any Internet browser (such as Internet Explorer 5.0 or higher) with a given IP, the consultant at the client side is able to view the real-time images from the server site (Fig. 2). The resolution of the computer displays is 1024 × 768 dpi. The consultant can communicate with and instruct the pathologist at the server side by telephone to operate the microscope as necessary. No additional equipment is needed.
To get the best quality of image and video, we typically required a bandwidth of approximately 1.5 Mbit/s, which is minimal, as we perform these operations using the intranet of our hospital (100 Mbit/s).
The extra cost for using this system is about US$1500 to buy the Web-enabled CCD camera.
2.2. Selection and reading of cases
When the on-duty pathologist (D1) needed a subspecialist or senior pathologist (D2) for consultation on a frozen section, either traditional real-slide consultation or telecon-sultation could be chosen. There were no special require-ments for using the telepathology system other than agreement by both pathologists. Unlike other studies using robotic microscope systems, D1 opened the system, and pathologist D2 instructed D1 how to manipulate the microscope over the telephone. After full examination of the specimen via telepathology, D2 gave his diagnosis to D1, who made the diagnosis for the clinician and was responsible for it. The basic information on the patient and specimen; the total time span; and the diagnoses made by D1, D2 using telepathology, and the same subspecialist or senior pathol-ogist reviewing the frozen-section slide using a light microscope (D3) the same day; and the final diagnosis of the case (D4) were recorded. When the process was completed, the pathologist on duty was responsible for making a record of the consultation on a data sheet. From November 2003 to October 2006, nearly 9000 frozen-section diagnoses were rendered in our department, of which 50
cases were recorded completely and were included in this study. (Cases without a complete record were excluded.)
2.3. Data analysis
The 4 diagnoses for each case were put into 4 categories: benign, borderline or uncertain, malignant, and deferred. The final diagnosis of the case (D4) was regarded as the standard reference diagnosis. If the consultative diagnosis corrected a wrong diagnosis by D1, and as a result, the patient received the correct operative procedure, it was regarded as a positive major impact on the operation. If the consultative diagnosis was incorrect and altered patient management inappropri-ately, it was classified as a negative major impact. Diagnostic agreement of the categories is described as percentage of the corresponding D1, D2, D3, and D4. The level of agreement between diagnoses made by the same pathologist using telepathology and light microscope (D2 and D3) was quantitated usingκ statistics. Comparisons of the agreement between D1 and D2 and between D2 and D4 also were performed. The value of κ ranged from 0 to 1, with higher values reflecting stronger agreement.
3. Results
All 50 cases were reviewed by 4 authors (Liang, Hsu, Lai, and Ho). The results are listed inTable 1, and the origins of the tissues are listed inTable 2. Most of the tissues (n = 49) were obtained for primary diagnosis, including nonneoplas-tic diseases (5), benign neoplasms (13), and malignant neoplasms (32). One sample of intra-abdominal tissue from a patient with a renal neoplasm was processed for staging and margin evaluation. Two cases received a more precise diagnosis in real-slide review (D3) than teleconsultation (D2), including a breast carcinoma that had an undetected small invasive focus (case 1) and a deferred diagnosis of reactive atypia related to inflammation (case 9). Three cases
received inferior results from consultations, including an ovarian serous carcinoma that had been diagnosed as a granulose cell tumor (case 21), a glioblastoma in which endothelial proliferation was not detected (case 38), and a poorly differentiated malignant epithelioid tumor. The diagnosis in this last case (case 44) was obtained by international consultation. The lesion involving the sellar region had been considered Langerhans cell histiocytosis. There was no D1, and the final sign-out pathologist (D4) had been the same pathologist, whereas in 38 (76%) of 50 cases, D2 and D4 had been the same. A comparison of the diagnoses made by D1 and D2 revealed that 37 cases (74%) received a more precise diagnosis, 10 (20%) showed no difference, and 3 (6%) received a less adequate diagnosis. In 9 (18%) of the 50 cases, there was a positive major impact on the operation as a result of teleconsultation, and no negative major impact occurred. Comparison of the agreement of diagnostic categories between D1 and D2 showed poor agreement (κ = 0.20). Teleconsultation showed excellent agreement of diagnostic categories with real-slide consulta-tion (κ = 0.97). Teleconsultaconsulta-tion showed good agreement of diagnostic categories with the final diagnosis (κ = 0.79). Real-slide consultation was slightly superior to teleconsulta-tion, because 2 cases (4%) received a better diagnosis. One case was a breast carcinoma with a small focus of stromal invasion that was not well recognized during teleconsulta-tion. Another case was an inflamed soft-tissue mass with a few reactive atypical cells, the nature of which was unclear, and the diagnosis was deferred on frozen section. However, no negative major impact occurred in these 2 cases as a result of teleconsultation. The time span of telepathology (mean ± SD [range], 4.5 ± 2.8 minutes [1-17]) is rather short compared with routine intradepartmental consultation (get-ting from the main building of the hospital to the opera(get-ting room and making the diagnosis, 18.6 ± 4.1 minutes [14-28]). Cases that took longer were mainly the result of diagnostic problems. There was no record of or complaint related to problems with the computer system.
4. Discussion
Frozen-section diagnosis has been an important factor in intraoperative decision making since the end of the 19th century but is technically limited and more difficult than examination of formalin-fixed paraffin-embedded sections. Nevertheless, frozen section is regarded as an accurate means of diagnosis during surgery and often has a significant influence on the operation. The accuracy of frozen-section diagnosis reported in the literature ranges from 94% to 97.4%[11-13]. The principal indications for frozen section are to obtain a diagnosis of a pathologic process that could influence the remainder of the surgery, determine the presence or absence of tumor metastases in lymph nodes, and assess the adequacy of the resection margins of a malignant lesion.
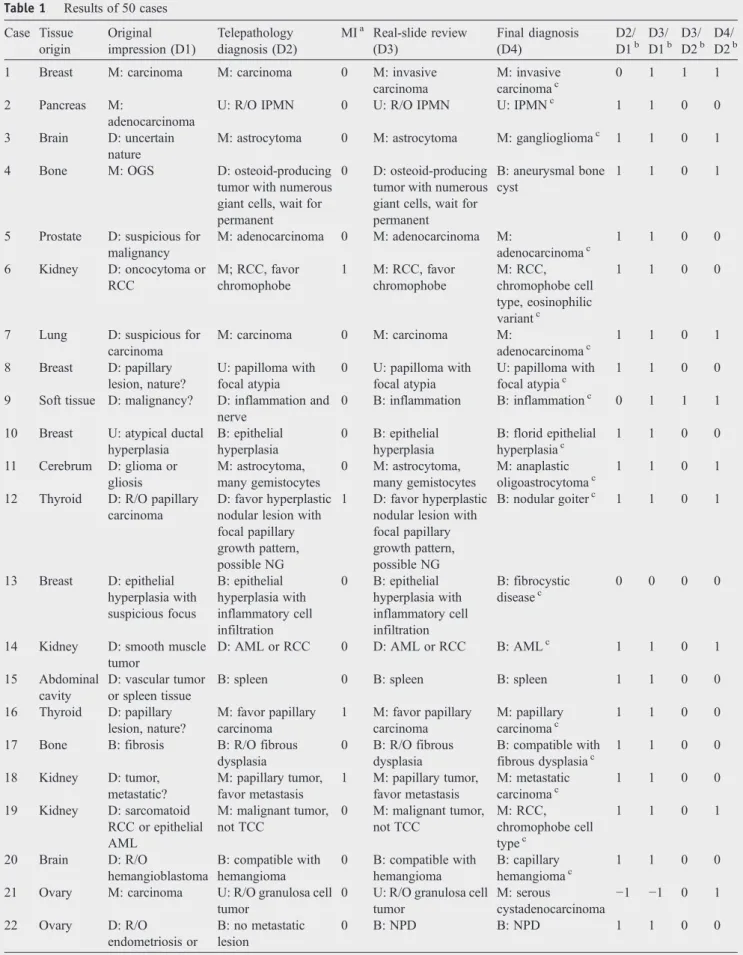
Table 1 Results of 50 cases Case Tissue origin Original impression (D1) Telepathology diagnosis (D2) MIa Real-slide review (D3) Final diagnosis (D4) D2/ D1b D3/ D1b D3/ D2b D4/ D2b 1 Breast M: carcinoma M: carcinoma 0 M: invasive
carcinoma M: invasive carcinomac 0 1 1 1 2 Pancreas M: adenocarcinoma
U: R/O IPMN 0 U: R/O IPMN U: IPMNc 1 1 0 0 3 Brain D: uncertain
nature
M: astrocytoma 0 M: astrocytoma M: gangliogliomac 1 1 0 1 4 Bone M: OGS D: osteoid-producing
tumor with numerous giant cells, wait for permanent
0 D: osteoid-producing tumor with numerous giant cells, wait for permanent
B: aneurysmal bone cyst
1 1 0 1
5 Prostate D: suspicious for malignancy M: adenocarcinoma 0 M: adenocarcinoma M: adenocarcinomac 1 1 0 0 6 Kidney D: oncocytoma or RCC M; RCC, favor chromophobe 1 M: RCC, favor chromophobe M: RCC, chromophobe cell type, eosinophilic variantc 1 1 0 0
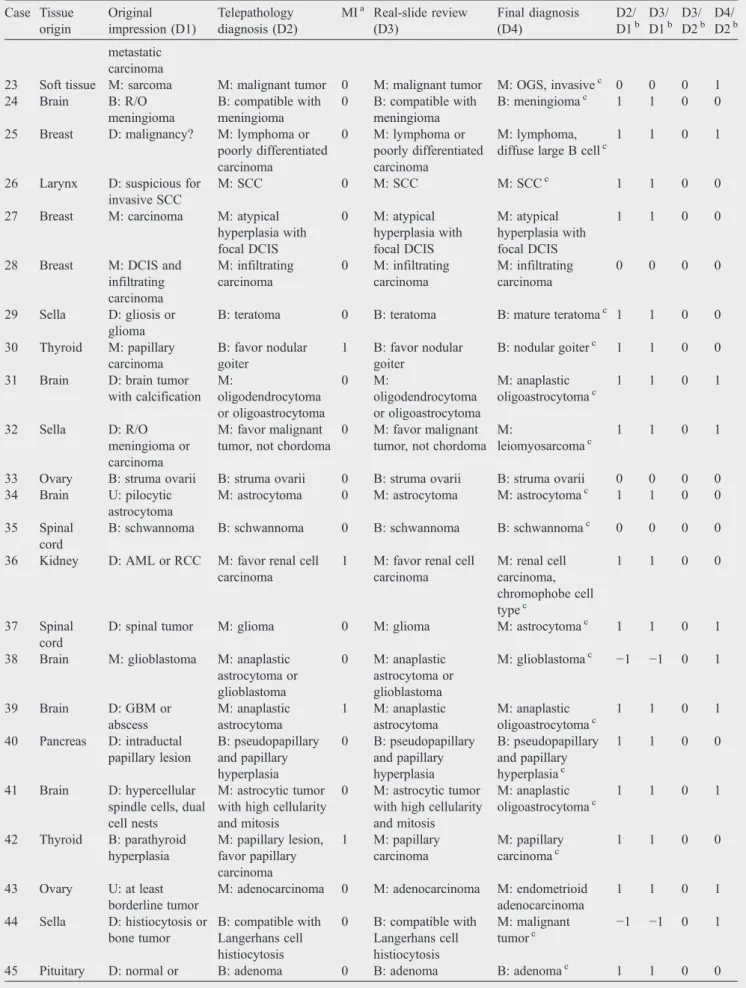
7 Lung D: suspicious for carcinoma M: carcinoma 0 M: carcinoma M: adenocarcinomac 1 1 0 1 8 Breast D: papillary lesion, nature? U: papilloma with focal atypia 0 U: papilloma with focal atypia U: papilloma with focal atypiac 1 1 0 0 9 Soft tissue D: malignancy? D: inflammation and
nerve
0 B: inflammation B: inflammationc 0 1 1 1 10 Breast U: atypical ductal
hyperplasia B: epithelial hyperplasia 0 B: epithelial hyperplasia B: florid epithelial hyperplasiac 1 1 0 0 11 Cerebrum D: glioma or gliosis M: astrocytoma, many gemistocytes 0 M: astrocytoma, many gemistocytes M: anaplastic oligoastrocytomac 1 1 0 1 12 Thyroid D: R/O papillary
carcinoma
D: favor hyperplastic nodular lesion with focal papillary growth pattern, possible NG
1 D: favor hyperplastic nodular lesion with focal papillary growth pattern, possible NG B: nodular goiterc 1 1 0 1 13 Breast D: epithelial hyperplasia with suspicious focus B: epithelial hyperplasia with inflammatory cell infiltration 0 B: epithelial hyperplasia with inflammatory cell infiltration B: fibrocystic diseasec 0 0 0 0
14 Kidney D: smooth muscle tumor
D: AML or RCC 0 D: AML or RCC B: AMLc 1 1 0 1 15 Abdominal
cavity
D: vascular tumor or spleen tissue
B: spleen 0 B: spleen B: spleen 1 1 0 0 16 Thyroid D: papillary lesion, nature? M: favor papillary carcinoma 1 M: favor papillary carcinoma M: papillary carcinomac 1 1 0 0 17 Bone B: fibrosis B: R/O fibrous
dysplasia 0 B: R/O fibrous dysplasia B: compatible with fibrous dysplasiac 1 1 0 0 18 Kidney D: tumor, metastatic? M: papillary tumor, favor metastasis 1 M: papillary tumor, favor metastasis M: metastatic carcinomac 1 1 0 0 19 Kidney D: sarcomatoid RCC or epithelial AML M: malignant tumor, not TCC 0 M: malignant tumor, not TCC M: RCC, chromophobe cell typec 1 1 0 1 20 Brain D: R/O hemangioblastoma B: compatible with hemangioma 0 B: compatible with hemangioma B: capillary hemangiomac 1 1 0 0 21 Ovary M: carcinoma U: R/O granulosa cell
tumor
0 U: R/O granulosa cell tumor M: serous cystadenocarcinoma −1 −1 0 1 22 Ovary D: R/O endometriosis or B: no metastatic lesion 0 B: NPD B: NPD 1 1 0 0 (continued on next page)
Table 1 (continued) Case Tissue origin Original impression (D1) Telepathology diagnosis (D2) MIa Real-slide review (D3) Final diagnosis (D4) D2/ D1b D3/ D1b D3/ D2b D4/ D2b metastatic carcinoma
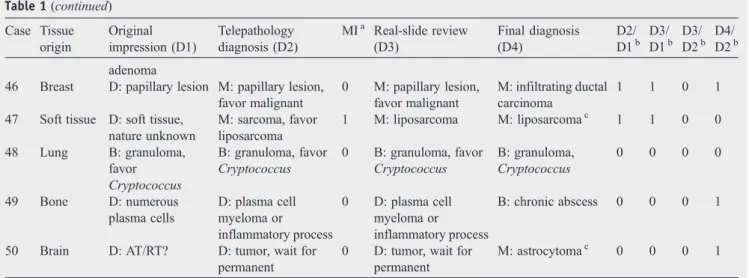
23 Soft tissue M: sarcoma M: malignant tumor 0 M: malignant tumor M: OGS, invasivec 0 0 0 1 24 Brain B: R/O meningioma B: compatible with meningioma 0 B: compatible with meningioma B: meningiomac 1 1 0 0 25 Breast D: malignancy? M: lymphoma or
poorly differentiated carcinoma 0 M: lymphoma or poorly differentiated carcinoma M: lymphoma, diffuse large B cellc
1 1 0 1
26 Larynx D: suspicious for invasive SCC
M: SCC 0 M: SCC M: SCCc 1 1 0 0 27 Breast M: carcinoma M: atypical
hyperplasia with focal DCIS 0 M: atypical hyperplasia with focal DCIS M: atypical hyperplasia with focal DCIS 1 1 0 0
28 Breast M: DCIS and infiltrating carcinoma M: infiltrating carcinoma 0 M: infiltrating carcinoma M: infiltrating carcinoma 0 0 0 0 29 Sella D: gliosis or glioma
B: teratoma 0 B: teratoma B: mature teratomac 1 1 0 0 30 Thyroid M: papillary carcinoma B: favor nodular goiter 1 B: favor nodular goiter B: nodular goiterc 1 1 0 0 31 Brain D: brain tumor
with calcification M: oligodendrocytoma or oligoastrocytoma 0 M: oligodendrocytoma or oligoastrocytoma M: anaplastic oligoastrocytomac 1 1 0 1 32 Sella D: R/O meningioma or carcinoma M: favor malignant tumor, not chordoma
0 M: favor malignant tumor, not chordoma
M:
leiomyosarcomac
1 1 0 1
33 Ovary B: struma ovarii B: struma ovarii 0 B: struma ovarii B: struma ovarii 0 0 0 0 34 Brain U: pilocytic
astrocytoma
M: astrocytoma 0 M: astrocytoma M: astrocytomac 1 1 0 0 35 Spinal
cord
B: schwannoma B: schwannoma 0 B: schwannoma B: schwannomac 0 0 0 0 36 Kidney D: AML or RCC M: favor renal cell
carcinoma
1 M: favor renal cell carcinoma M: renal cell carcinoma, chromophobe cell typec 1 1 0 0 37 Spinal cord
D: spinal tumor M: glioma 0 M: glioma M: astrocytomac 1 1 0 1 38 Brain M: glioblastoma M: anaplastic
astrocytoma or glioblastoma 0 M: anaplastic astrocytoma or glioblastoma M: glioblastomac −1 −1 0 1 39 Brain D: GBM or abscess M: anaplastic astrocytoma 1 M: anaplastic astrocytoma M: anaplastic oligoastrocytomac 1 1 0 1 40 Pancreas D: intraductal papillary lesion B: pseudopapillary and papillary hyperplasia 0 B: pseudopapillary and papillary hyperplasia B: pseudopapillary and papillary hyperplasiac 1 1 0 0 41 Brain D: hypercellular spindle cells, dual cell nests
M: astrocytic tumor with high cellularity and mitosis
0 M: astrocytic tumor with high cellularity and mitosis M: anaplastic oligoastrocytomac 1 1 0 1 42 Thyroid B: parathyroid hyperplasia M: papillary lesion, favor papillary carcinoma 1 M: papillary carcinoma M: papillary carcinomac 1 1 0 0 43 Ovary U: at least borderline tumor
M: adenocarcinoma 0 M: adenocarcinoma M: endometrioid adenocarcinoma 1 1 0 1 44 Sella D: histiocytosis or bone tumor B: compatible with Langerhans cell histiocytosis 0 B: compatible with Langerhans cell histiocytosis M: malignant tumorc −1 −1 0 1
Most published studies of telepathology for frozen section are retrospective and an evaluation of the validity of the system[4,6,14-16]. In contrast, we applied the system to our daily frozen-section service as a choice of consultative method. All of our 11 staff members were included, so the study was not limited to certain pathologists. Previous studies mainly compared the diagnoses made by the same pathologist using a conventional device or telepathology and showed excellent correlation. We recorded the original impression before teleconsultation and evaluated the func-tion of the telepathology system. Forty-six (92%) of 50 cases received correct consultative diagnoses by telepathology, and 37 cases (74%) received a more precise diagnosis than the original impression (D1). Nine of the 50 telepathology reviews (18%) had a positive major impact on the operation, and no negative major impact occurred. In addition, teleconsultation showed good agreement with real-slide findings (κ = 0.97).
As we discussed in our previous article, our goal is to establish a cost-effective model that other pathology laboratories can follow or emulate. Our self-assembled system is similar to that of Petersen et al[17]and Marchevsky et al[18]. For the server site, aside from the conventional microscope and personal computer, our system requires only a high-resolution CCD Webcam and adapter. There are essentially no extra requirements for the remote site. Anyone who has a personal computer that can connect to the Internet is able to access the images using a common Web browser such as Netscape Communicator or Internet Explorer.
Another characteristic of our experimental design is the use of the ordinary telephone system to facilitate commu-nication between the 2 pathologists at the remote and local
sites. In this study, the telephone was used for communica-tion, such as notification of new cases being transmitted, instructions for changing the field or magnification, giving the patient's clinical data (such as site and size of the lesion, age, sex, history, etc), and giving the diagnosis. The pathologist at the local site cannot give his/her opinion about the diagnosis on the telephone.
Some possible drawbacks may be present in our design as well as in other dynamic systems. The Internet connection speed between the home base and the client determines the speed of frame transmission, and Internet congestion might cause stagnation or interruption of the image flow. Never-theless, the quality of single frames is not affected. Consequently, slowing down the motion and taking more time to reach a diagnosis could overcome this problem. In
Table 2 Distribution of organ systems involved in 50 telepathology frozen-section consultations
Organ/tissue No. of cases No. (%) of correct consultative diagnosis Brain/spinal cord 13 12 (92)
Bone/soft tissue 6 6 (100) Breast 7 6 (86) Head and neck 2 2 (100) Kidney/prostate 6 6 (100) Lung 2 2 (100) Ovary 4 3 (75) Pancreas 2 2 (100) Pituitary/sella 4 3 (75) Thyroid 4 4 (100) Total 50 46 (92) Table 1 (continued) Case Tissue origin Original impression (D1) Telepathology diagnosis (D2) MIa Real-slide review (D3) Final diagnosis (D4) D2/ D1b D3/ D1b D3/ D2b D4/ D2b adenoma
46 Breast D: papillary lesion M: papillary lesion, favor malignant 0 M: papillary lesion, favor malignant M: infiltrating ductal carcinoma 1 1 0 1 47 Soft tissue D: soft tissue,
nature unknown M: sarcoma, favor liposarcoma 1 M: liposarcoma M: liposarcomac 1 1 0 0 48 Lung B: granuloma, favor Cryptococcus B: granuloma, favor Cryptococcus 0 B: granuloma, favor Cryptococcus B: granuloma, Cryptococcus 0 0 0 0 49 Bone D: numerous plasma cells D: plasma cell myeloma or inflammatory process 0 D: plasma cell myeloma or inflammatory process B: chronic abscess 0 0 0 1
50 Brain D: AT/RT? D: tumor, wait for permanent
0 D: tumor, wait for permanent
M: astrocytomac 0 0 0 1
Abbreviations: MI indicates major impact; diagnostic categories: B, benign; U, borderline or uncertain behavior; M, malignant; D, deferred; R/O, rule out; IPMN, intraductal papillary-mucinous neoplasm; OGS, osteogenic sarcoma; AML, angiomyolipoma; NG, nodular goiter; RCC, renal cell carcinoma; TCC, transitional cell carcinoma; NPD, no pathologic diagnosis; DCIS, ductal carcinoma in situ; SCC, squamous cell carcinoma; GBM, glioblastoma multiforme; AT/RT, atypical teratoid/rhabdoid tumor.
our experience, the time required for diagnosis through the intranet and the Internet did not differ substantially. We had also tried different connection bandwidths, including 128, 384, 568 kb/s, and T1. Diagnosis could be obtained at all available speeds.
These results proved the validity of our system. However, just as previous literature showed, because of limited information, user unfamiliarity with the new equipment and method of telepathology (electric image, resolution, wideness of field, inability to manipulate the slide directly, and time lag) diagnosis made by telepathology cannot be better than that made by conventional methods. We also had similar results and found that real-side consultation was slightly superior in 2 cases (4%). This was an acceptable difference for a new convenient system that does not require much time for training and wastes less time waiting for the consultant. Maybe someone will contend that accuracy is the essential issue for pathologic diagnosis. However, the time span required to make a diagnosis is another important thing that should be considered in an urgent task, the intraoperative frozen section. The mean reported time span ranged from 3 to 14.2 minutes [16,19-21]. The average time span of our system was only 4.5 minutes. These results are hard to compare, because they depend on the hardware and software of the telecommunication system and the method of case selection in the study. However, all of these are acceptable in real-life practice. Our goal was to use telepathology in place of direct consultation requiring travel to minimize turn-around time and in the process negate, manage, and minimize the risk of having a solo pathologist during all important frozen-section analyses where treatment and outcome could be directly impacted by frozen-section diagnosis. Furthermore, this system was not limited to intradepartmental consultation. It could be applied to interdepartmental consultation; even consultants a hundred miles away could give their opinion via the Internet. In our opinion, the core value of telepathology is to let the specialist save a solo pathologist from a dangerous situation, in which he or she has to make a prompt decision rather than employ a specialist, and to replace the pathologist in the distant clinic with a technician.
In conclusion, a low-cost telepathology system provid-ing real-time imagprovid-ing, costprovid-ing only US$1500, has the potential to provide a remote frozen-section consultation to anywhere there is an Internet connection. No additional software is needed for the consultant except the Internet browser used in daily life. No special training is needed. The diagnostic accuracy of telepathology shows good agreement with that of real-slide consultation. The time needed to make a diagnosis by telepathology is acceptable. Furthermore, this system makes it possible to obtain valuable opinions from consultants long distances away who are unable to reach the frozen-section room immediately. It can improve the performance in frozen-section diagnoses.
References
[1] Weinstein MH, Epstein JI. Telepathology diagnosis of prostrate needle biopsies. HUMPATHOL1997;28:22-9.
[2] Weinstein LJ, Epstein JI, Edlow D, et al. Static image analysis of skin specimens: the application of telepathology to frozen section evaluation. HUMPATHOL1997;28:30-5.
[3] Dunn BE, Almagro UA, Choi H, et al. Dynamic-robotic telepathology: Department of Veterans Affairs feasibility study. HUMPATHOL1997; 28:8-12.
[4] Becker Jr RL, Specht CS, Jones R, et al. Use of remote video microscopy (telepathology) as an adjunct to neurosurgical frozen section consultation. HUMPATHOL1993;24:909-11.
[5] Briscoe D, Adair CF, Thompson LD, et al. Telecytologic diagnosis of breast fine needle aspiration biopsies. Intraobserver concordance. Acta Cytol 2000;44:175-80.
[6] Winokur TS, McClellan S, Siegal GP, et al. A prospective trial of telepathology for intraoperative consultation (frozen sections). HUM
PATHOL2000;31:781-5.
[7] Nordrum I, Eide TJ. Remote frozen section service in Norway. Arch Anat Cytol Pathol 1995;43:253-6.
[8] Dunn BE, Choi H, Almagro UA, et al. Routine surgical telepathology in the Department of Veterans Affairs: experience-related improve-ments in pathologist performance in 2200 cases. Telemed J 1999;5: 323-37.
[9] Pan CC, Liang WY, Huang CW, et al. Diagnosing minimal adenocarcinoma on prostate needle biopsy by real-time dynamic telepathology through the Internet: evaluation of an economic technology for remote consultation. HUMPATHOL2002;33:242-6.
[10] Liang WY, Pan CC, Chiang H. Real-time dynamic telepathology through the Internet: evaluation of a new and economic technology at Taipei Veterans General Hospital. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64:277-82.
[11] Kaufman Z, Lew S, Griffel B, et al. Frozen-section diagnosis in surgical pathology. A prospective analysis of 526 frozen sections. Cancer 1986;57:377-9.
[12] Lessells AM, Simpson JG. A retrospective analysis of the accuracy of immediate frozen section diagnosis in surgical pathology. Br J Surg 1976;63:327-9.
[13] Rogers C, Klatt EC, Chandrasoma P. Accuracy of frozen-section diag-nosis in a teaching hospital. Arch Pathol Lab Med 1987;111:514-7. [14] Adachi H, Inoue J, Nozu T, et al. Frozen-section services by
telepathology: experience of 100 cases in the San-in District, Japan. Pathol Int 1996;46:436-41.
[15] Mizushima H, Uchiyama E, Nagata H, et al. Japanese experience of telemedicine in oncology. Int J Med Inform 2001;61:207-15. [16] Kaplan KJ, Burgess JR, Sandberg GD, et al. Use of robotic
telepathology for frozen-section diagnosis: a retrospective trial of a telepathology system for intraoperative consultation. Mod Pathol 2002;15:1197-204.
[17] Petersen I, Wolf G, Roth K, et al. Telepathology by the Internet. J Pathol 2000;191:8-14.
[18] Marchevsky AM, Lau SK, Khanafshar E, et al. Internet teleconferen-cing method for telepathology consultations from lung and heart transplant patients. HUMPATHOL2002;33:410-4.
[19] Demichelis F, Barbareschi M, Boi S, et al. Robotic telepathology for intraoperative remote diagnosis using a still-imaging–based system. Am J Clin Pathol 2001;116:744-52.
[20] Chorneyko K, Giesler R, Sabatino D, et al. Telepathology for routine light microscopic and frozen section diagnosis. Am J Clin Pathol 2002; 117:783-90.
[21] Moser PL, Lorenz IH, Sogner P, et al. The accuracy of telediagnosis of frozen sections is inferior to that of conventional diagnosis of frozen sections and paraffin-embedded sections. J Telemed Telecare 2003;9: 130-4.