ELSEVIER Materials Chemistry and Physics 49 (1997) 70-77
The effect of pyrochlore phase on formation mechanism and electrical
properties of perovskite PZMN relaxors
San-Yuan Chen a, Chien-Min Wang b, Syh-Yyuh Cheng b
a Institute of Materials Science and Engineering, National Chiao-Tung University, 1001 Ta-Hsueh, Hsinchu, Taiwan, Republic of China b Materials Research Laboratories, Industrial Technology Research Institute, Chutung, Hsinchu 31015 Taiwan, Republic of China
Received 17 April 1996; revised 18 November 1996; accepted 18 November 1996
Abstract
The reaction mechanism and electrical properties of phase formation of the perovskite Pb [ (Mg,,Zn, -,) 1&lb2,3] O&TiO, (PZMN-ST) were studied. Three major pyrochlore phase types, Pb2Nbz07, Pb,Nb,Os and PbaNb& were characterized and analyzed to understand their roles in the formation of perovskite PMN, PZN and PZMN phases. The formation qf a perovskite phase within PMN relaxors requires the presence of either Pb,Nb,O, or Pb3Nb20s pyrochlore phases in the mixture and is only slightly dependent on the presence of the stabilizer. On the other hand, the formation of a perovskite phase within PZN or PZMN relaxors requires the presence: of both PbsNbaOs pyrochlore phase and stabilizer. The maximum dielectric constant for PMN, PZN and PZMN systems can be obtained with the PbsNbsOs phase present in the starting raw materials because of the benefit of Pb,NbzOs to perovskite phase formation.
Keywords: Pb-based relaxers; Pb[ (Mg,,Zn, -,) ,JGJ~,~] 03-SrTi03; Pyrochlore phase; Perovskite phase; Reaction mechanism
1. Introduction
The Pb-based relaxors, because of their low temperature sinterability, high permittivity and low temperature coeffi- cient of capacitance (TCC), find widespread industrial applications such as multilayer ceramic capacitors and actu- ators. Currently, most R&D efforts have been concentrated on Pb(Mg,,,Nb,,,)O, (PMN), in spite of the fact that Pb(Zn1,3Nb2,3)03 (PZN) ceramic is evaluated as having higher market potential than the PMN owing to its excellent optical and electrostrictive properties f 11. One reason which hampers PZN application is the difficulty to prepare pure PZN or PZN-based ceramics with perovskite structure by conventional processing technique owing to the formation of pyrochlore phases [ 21, but the problem of pyrochlore for- mation has been studied extensively in PMN system and can be avoided by pre-reacting MgO with Nb205 to form MgNb206 columbite structure [ 31. Thus stabilizers such as PbTiO, [4], BaTiO, [5,6] are usually added to increase the tolerance factor and electronegativity difference in the PZN or PZMN systems [ 71.
As both PMN and PZN ceramics are lead-based A(B’B”)Oa type ferroelectric compounds with perovskite structure and are formed from an XPbO-YNb205 mixture [ 71, it is our conviction that the pyrochlore phase sequence present within the XPbO-YNb205 mixture during their for- 0254-0584/97/$17.00 0 1997 Elsevier Science S.A. AR rights reserved PUSO254-0584(96)01915-3
mation can influence the final perovskite content of the I?MN and PZN. The pyrochlore phases [ 8-101 present in the PbO- NbzOs mixture are of three m;ain compositions, namely, rhombohedral pyrochlore 2PbO I Nb205 (herein designated P,N,) , tetragonal pyrochlore 3PbO * Nbz05 (herein desig- nated PsN,) and cubic pyrochlore 3PbO * 2Nb20s (herein designated P3N4). P2N2 is a stoichiometric pyrochlore phase of AZB207,.P3N4 is an A-deficient pyrochlore phase, and PsNs is a B-deficient pyrochlore phase [ 10,l l] . Although the for- mation sequences of perovskite phase in PMN have been reported, the role of the formed pyrochlore compound in the phase formation of PMN or PZN perovskite has not been described [ 121.
In this paper we shall investigate how these pyrochlore phases within PbO-NbzOs mixture influence the perovskite content and their effects on dielectric properties of PMN, PZN and PZMN ceramics. To study the electrical properties in this ST-PZMN system, strontium titanate (ST) [ 131 was utilized as a stabilizer for PZMN.
2. Experimental procedure
ST-PZMN ceramics with composition O.l2SrTiO,- 0.88Pb[ (Mgsn, -,) 1 ,sNb2,s] 0, (ST-PZMN) were chosen for these studies. Raw materials including SrTiOs, PbO, ZnO,
MgO and Nbz05 were weighed in appropriate proportions and mixed together in a polyethylene jar with ZrOz as the grinding medium and alcohol solution as the mixing agent. After drying, the mixture was subjected to calcination at temperatures in the range 500-950°C for 4 h. The pre-cal- cined powder with the formula of PbmYO, pyrochlore phases was first synthesized by reacting PbO with Nb,Os powders at 480-800°C. PZMN-ST ceramics were then fab- ricated by reacting the constituents with the pyrochlore phases.
The relative amount of pyrochlore phase to perovskite phase was determined by measuring the major integrated intensities of XRD peaks for perovskite ( 110) and for pyro- chlore (222) phases (P2N2, P3N2, P3N4) as described by Klug and Alexander [ 141. The percentage of formed perov- skite phase was calculated by the following equation: %perovskite = 100 X Zp,,,,/ (Z,,,, + ZpyrO)
where I,,,, and IpyrO represent the intensities of XRD peaks for perovskite and pyrochlore phases, respectively. To deter- mine the reaction sequences of perovskite formation, differ- ential thermal analysis (DTA) was used to examine phase reactions during the calcination. Dielectric measurements were performed on an automated system wherein a temper- ature box (Delta 9023) and Hp 4192A Impedance analyzer at a frequency of 1 kHz were controlled by a desktop computer.
3. Results
3.1. Pyrochlore phase formation within XPbO-YNb205 mixtures
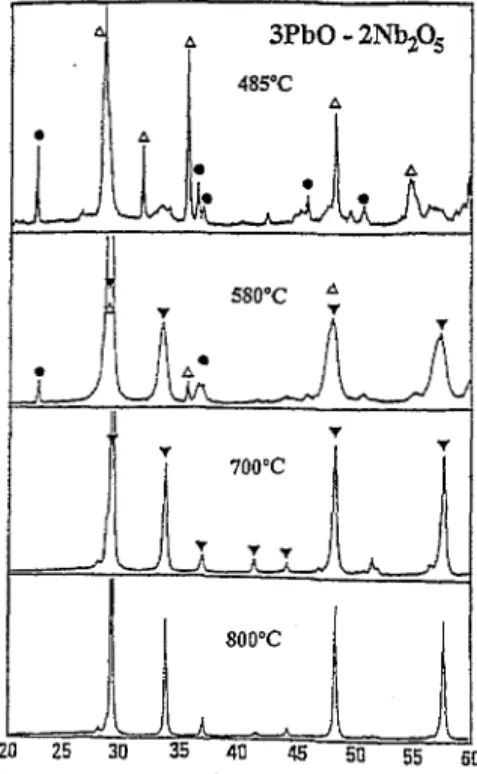
The formation reactions of pyrochlore phases for XPbO- YNb205 mixtures at different calcination temperatures were studied and analyzed with XRD. For the 3PbO-2Nb205 mix- ture (Fig. l), cubic P3N4 pyrochlore phase is first formed around 580°C and remains stable throughout the whole range of calcination temperatures. For the 2PbO-Nb205 mixture (Fig. 2)) P3N4 is still first observed pyrochlore phase, but it was further transformed into P,N, pyrochlore phase by reac- tion with PbO at calcination temperature of = 700°C. Similar results were also observed in the 3PbO-Nb,05 mixture (Fig. 3): P3N4 was the first formed pyrochlore phase and P,N, phase was subsequently observed with increasing cal- cination temperature. P3Nz phase began to appear at 700°C and become dominant at temperature of 800°C. This change from P3N4 to P2Nz and finally to P3N2 is strongly dependent on PbO amount and phase stability.
The phase formations of the XPbO-YNb20S mixtures were further confirmed by DTA results as shown’in Fig. 4. Only one exothermic peak was observed at around 560°C in the 3PbO-2Nbz05 mixture. The peak corresponds to the forma- tion of P3N4 pyrochlore phase according to the XRD analysis. The starting formation temperature was approximately
530°C. In the 2PbO-NbzO, mixture, corresponding to Fig. 2 of XRD, there is one more exothermic peak around 630°C other than the peak of P3N4 formation. The peak at 630°C was believed to correspond to the formation of P,N, phase. Furthermore, as shown in Fig. 4, three exothermic peaks,
I!
58O’C “,
Fig. 1. XRD patterns of calcined 3PbO-2 Nbz05 mixture: A, PbO; 7, PbxNb$&; *, NbZOx.
Fig. 2. XRD patterns of calcined 2PbO-Nb205 mixture: A, PbO; 7, Pb3Nb40,3; l , Nbz05; + , Pb,Nb,O,.
S.-Y. Chen et al. /Materials Chemistry and Physics 49 (1997) 70-77
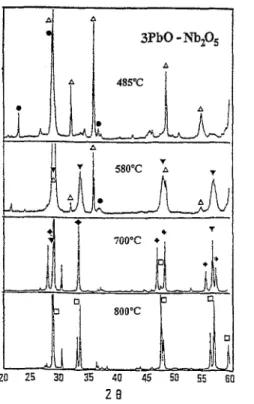
Fig. 3. XRD patterns of calcined 3PbO-Nb,05 mixture: A, PbO; V, Pb,Nb~O,,; a, Nb205; q , Pb3NbTb20s; +, Pb2Nb207.
I
I , I I I I
400 500 600 700 800 900 1000
Temperature (“C)
Fig. 4. DTA curves in XPbO-YNb205 with/SrTiO, mixture,
representing the formation of P3N4, P,N, and P,N, phases, were found in the 3PbO-Nb,05 mixture.
3.2. Phase evolution of SrTiO,-Pb[(Mg,Zn, -x)INNbu3]03 system
The phase formation and evolution at different calcination temperatures in ST-Pb [ (Mg,,Zn* -,) 1J4b2,3] O3 system with various compositions (x) were studied and analyzed by X-ray diffraction patterns. The results were summarized in Table 1. For the composition of ST-PMN (x = 1) , both P3N4 and P,N, were considered to be main intermediate pyrochlore phases below 700°C prior to the perovskite PMN phase for- mation. With increasing calcination temperature (especially above SOO”C), the relative amount of PzNz formation decreases sharply with the formation of perovskite PMN
Table 1
Effect of calcination temperature on phase formation in 0.88 Pb[ (MG-
Zn, -,) r,aNb2,s] O+W2SrTi03 compdion
Composition: PZMN-ST Calcination temperature P-3 Phases X=1 500-600 P, N, M, &r ST 600-700 p2N2> P&L P, M, ST ’ 700-800 pMN, WL PsN.,. M, ST 800-900 PMN, P&, ST x=0.5 500-600 P, &f&r W Z, ST 600-700 p,Iv2, P&J’, Z M, ST 700-800 pMN, P,N,, PaNd, Z, ST 800-900 pzMN, P3N4 x=0 5004500 P, p3N4, N, Z ST 600-700 p2N2, P&t, P. Z ST 700-800 p3N2, f’& Z, ST 800-900 pzN, P&
P=PbO, N=Nb205, M=MgO, Z=ZnO, ST=SrTiOa, PZN=Pb(Zn,,a- Nb&Oa, PMN = Pb( Mg,,,Nb,,,) 03, P2N2 = Pb2Nb20,, PsNz = Pbs- NbzOs, P3N4= Pb3Nb40r3, PZMN-ST = 0.88 Pb[ (Mg,,.Znr -x)1,3Nb2,3] - 03-0.12SrTiOa
while P,N4 seems relatively unchanged. On the other hand, in the case of PZN perovskite formation (x=0), the pyro- chlore phase formation reactions are very similar to those of ST-PMN when the calcination temperature is below 700°C. However, when the calcination tl:mperature increases, e.g. to 750°C the decrease of PzNz is accompanied by an the increase of P,N,. Later, both P3N2 and SrTi03 phases gradually dis- appear and perovskite PZN fo.rms at temperatures above 800°C. The formation reaction sequences of ST-PZN per- ovskite indicates that P,N, is the main pyrochlore precursor for PZN pcrovskite formation in ST-PZN system. For x= 0.5 of ST-PZMN system, the P3N4 is the first observed pyr- ochlore phase (at 500-6OO”C), followed by PzN2 (at 600- 7OO’C). XRD patterns revealed that a small amount of P3Nz phase can be observed at 750°C. P,N, appears together with PMN at the expense of PzNz and the disappearance of MgO. PZMN appears at 800-900°C with the disappearance of P3N2, ZnO and stabilizer SrTi03.
3.3. The role of pyrochlore phase type in perovskite phase formation of PMN and PZN
Two main different mixture systems were chosen to study the phase evolution and perovskite content for both compo- sitions of PMN (x= 1) and PZN (x= 0). In one mixture system, the raw materials used were PbO, Nb205, ZnO, MgO and SrTiO,, and in the other system, pyrochlore phases were used instead of PbO and Nb205 while the other constituents remained the same. For the PMN-ST system (Table 2)) per- ovskite phase still can be produced in the 3PbO-MgO-Nbz05 mixture system without the stabilizer SrTiO,. That means the stabilizer has little effect on the formation of PMN perovskite.
Table 2
Pyrochlore content and reactions between pyrochlore phases in PbO-Nbz05 system and PbO, MgO, SrTi03
Mixture system *
Role of PbO and stabilizer ST 3PbO-Nb20rMg0
ZPbO-Nb20FMg0
3PbO-NbzOsMgO-SrTi03
3PbO-2Nb&-MgO
Role ofpyrochlore phase
Pb2Nb207-MgO
PbJVb&-MgO Pb$ib.,&,-MgO
Enhancing perouskite formation by excess PbO
Pb,Nb40,,-MgOSrTiO,PbO Pb$Ib,O,,-Mg03PbO Phase (content) P3N4 (21%), Pero. PMN (79%) P3N4 (47%), Pero. PMN (53%) P3N4 (16%), Pero. PMN (84%) P3N4 (96%) P3N4 (62%), Pero. PMN (38%) P3N4 (24%). Pero. PMN (76%) P,N, (97%) P,N, and P,NZ (22%)) Pero. PMN (78%) P3N4 (29%), Pero. PMN (71%) a Calcined at 900°C for 4 h.
MgO mixture system has the higher relative perovskite con- tent than other mixture systems. Similar observation was also found in the mixture system using pyrochlore as raw material. Pb3Nbz08-MgO gives a higher perovskite content than other pyrochlore phase systems. This further confirms our results that P3N2 is more important than PzNz for perovskite forma- tion of PMN phase. Therefore, when the pre-synthesized P3Nz phase was used as the starting material, the P3Nz phase acts as a nucleation center to form perovskite PMN. Table 2 also indicates that the presence of Pb3Nb4013 ( P3N4) inhib- ited perovskite phase formation as found in the Pb3Nb4013- MgO-(SrTi03) system. However, the addition of a 3 mol excess of PbO to the Pb3Nb4013-Mg0 mixture with or with- out SrTiQ can promote the formation of perovskite phase.
ZnO-SrTiO, mixture systems, showing the possibilities of forming PZN perovskite phase. In other words, no perovskite phase can be observed in Pb$?b4013-Zn0-SrTi0, and PbJ$O,-ZnO-SrTiO, mixture systems. Similarly, the addition of a 3 mol excess of PbO to the Pb3Nb40,,-ZnO- SrTi03 system promotes the formation of perovskite PZN, but without SrTiO, stabilizer, the excessive PbO is still not effective for perovskite formation as observed in the Pb3Nb4013-Zn0-3Pb0 system.
3.4. The effect of preformed pyrochlore phase on PMN, PZN and PZMN system
For the PbO-ZnO-Nbz05 system, the resulting PZN phases are always pyrochlore, independent of mixture com- positions and phases (Table 3). These phenomena are com- pletely different from those of the PbO-MgO-Nbz05 system. However, with the addition of stabilizer SrTi03, the situatiod changes for both 3PbO-Nb205-ZnO-SrTi03 andPb,Nb,Os-
Experimental results indicate that the preformed pyro- chlore phases can exert similar effects to those of pyrochlore phases present in the mixture, with P3N2 pyrochlore phase still leading the other two phases in the production of the perovskite content within the final product (see Pb3Nbz08- MgO mixture system in Table 2 and Pb3Nb208-ZnO-SrTi03 mixture system in Table 3). Pyrochlorephases areconsidered
Table 3
Pyrochlore content and reactions between pyrochlore phases in PbO-Nbz05 system and PbO, ZnO, SrTi03
Mixture system a Phase (content)
Stabilizer ST is importantfor Pero. PZN
3PbO-Nb,O&nO ZPbO-Nb,O,ZnO 3PbO-Nb20~ZnOSrTi0;I SPbO-2Nb105-ZnO
Pyrochlore Pa2 and ST are required for Pero. PZN Pb,Nb20BZn0
PbZNbzO,-ZnO-SrTi03
Pb$&OgZnOSrTiO,
Pb$&O,,-ZnO-SrTi03
Enhancing Pero. PZN in excess PbO Pbfib,O,,ZnO-SrTiO,-3Pb0 PbSNb40,3-Zn0-3Pb0 P3N4, Pyro. PZN P3N4. Pyro. PZN P3N4 (19%), Pero. PZN (81%) P3N4, Pyro. PZN PjNq, P3N2, Pyro. PZN P,N, (93%), Pero. PZN (7%) P3N4 (13%), Pero. PZN (87%) P3N4, Pyro. PZN P3N4 (19%), Pero. PZN (81%) P3N4, Pyro. PZN a Calcined at 900°C for 4 h.
74 S.-Y. Chen et al. /Materials Chemistry and Physics 49 (1997) 70-77
to be the intermediate products in the formation of perovskite phase. The effect of P,N, pyrochlore phase raw material on PZMN-ST system is shown in Fig. 5. If the pyrochlore phase P,N, was used as the raw material, the formation behavior of PZMN-ST perovskite phase (labeled as P,N,-Z-M-ST sys- tem) is different from that formed by the conventional method (labeled as P-Z-M-N-ST system). As indicated in Fig. 5, the pyrochlore precursor to form perovskite phase is probably P2N2 in the P-Z-M-N-ST system but the dominant pyrochlore phase to form perovskite phase is P,N, in the
s: ST 7: Pew. T . q 1 z P 1 m z --1-~ 30 min ~ T P ” P 7 T I 20 h A 40 50 60 2 0 (degree 1 (b) l z:ZnO wP3N4 5 min S:ST 7: Pero.
l
:P3N2 . l . . z h,\ iz . 30 min : i 2 9 (degree)Fig. 5. XRD patterns of (a) P-Z-M-N-ST and (b) P3N2-Z-MST with
Mg(x) =0.35 systems calcined at 800°C.
,Beaction Kinetics of Perovskite Pormntioq (a) P-ZM-N-ST System: consiantnucmion mte
--I
(SW-WC)
1
PZMN+I%N4 ---____---_________
@) PNU-M-ST System: Fixed numb~ofnude~
T
+
(SOO-900%)
nuclA for perovsldte Pm+ hN4
Fig. 6. The overall reaction mechanism of perovskite phase formation in (a)
P-Z-M-N-ST and (b) P,N2-ZM-ST systems.
P3N2-Z-M-ST system, although P3N4 phase always remains in both precursor systems. An improvement in perovskite content is observed for the P&,-Z-M-ST system, and its reaction kinetics for perovskite formation is shown in Fig. 6. 3.5. Electrical properties
Two major compowtlons, . . 0.88 (PbJ%,O,-MgO)- 0.12SrTi03 and 0.8S(Pb$b,,0z-ZnO)-0.12SrTi03, were chosen to study the effect of pyrochlorephase on the electrical properties of SrTiO,-PMN and SrTi03-PZN. Figs. 7 and 8
5000
1
- &loo- iii $ 3000- v f 2000- 2 FI 1000- 01. I I -50 0 50 100 150 : Temperature (“c) 10Fig. 7. Temperature dependence of dielectric constant in Pb,Nb,O,-MgO- SrTiOB systems.
-I
-50 0 SO 100 150 7.00 -.
Temper&n-e (“C)
Fig. 8. Temperature dependence of dielectric constant in Pb,Nb,O,-ZnO- SrTi03 systems.
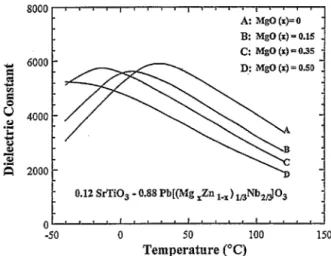
8000 8 n ’ I I ’ r b n 1 I * * * I ’ ’ 8 8 A: MgO(r)=O - B: MgO (I) = 0.15 _ c: Mgo (I) - 035 . 2 6000 - D,: MgO (I) = 0.50 5 0 ” 4000 - .z P 3 R 2000- _ 0.12 SrTiO, - 0.88 Pb[(Mg =Zn 1mx) ,,Nb,dO, 0 **ll’llq”lt’l”l*’ -50 0 50 100 150 Temperature (“C)
Fig. 9. Dielectric constant vs. temperature for PZMN-ST composition. show the temperature dependences of dielectric constant at 1 kHz for the three main pyrochlore phase systems containing MgO and ZnO, respectively. The maximum attainable die- lectric constant was found in P3N2 pyrochlore-containing sys- tem but a flat curve with low dielectric constant was observed in the P3N4 pyrochlore phase-containing system. The dielec- tric constant of either P,N,-MgO-ST or PaN,-ZnO-ST sys- tems still can be modified and enhanced by adding a 3 mol excess of PbO. These phenomena can possibly be explained via P3N2 formation as shown in the following reactions: P3N4 + P + 2( P,N,) and P2N2 + P --) P,N,. In order to com- pare with P,N,-ZnO-ST system, the temperature depend- ence of dielectric constant of P-Z-M-N-ST system was studied as shown in Fig. 9. It was found that the dielectric properties are comparable for the same composition such as 0.12ST-O.88PZN in these two systems 0.88( Pb$&,Os- Zn0)-0.12SrTi03 (curve A, Fig. 8) and 088Pb(Zn,,,- Nb,l,0,)-0.12ST (curve A in Fig. 9).
4. Discussion
4.1. The effect of PbO and Nb205 contents on pyrochlore phase within XPbO-Wb205 mixture
Three main pyrochlore phases, namely, rhombohedral P2N2, tetragonal P3Nz and cubic P3N4 have been considered to play important roles in perovskite formation of Pb-based relaxors f S-lo]. Experimental results of this study [XRD
(Figs. l-3) and DTA (Fig. 4) ] indicate that P3N4 is the first formed pyrochlore phase within the XPbO-YNb205 mixture; the formation of P,N, and P3N2 pyrochlore phases will depend on the contents of PbO and Nb205 as well as the calcination temperature. PzNz starts to appear at 2PbO- Nb205 mixture and at 3PbO-Nbz05 mixture at=700”C. These are mixtures with lower Nb205 content than the 3PbO- 2Nbz05 mixture where only the P3N4 pyrochlore phase exists regardless of calcination temperature. P,N, starts to appear at 800°C for the 3PbO-Nbz05 mixture where the Nb205 con- tent still remains low but the PbO content is higher than that
for the 2PbO-Nb205 mixture. The phase formation sequences for these mixtures were further confirmed by DTA and the results are shown in Fig. 4. These results indicate that higher NbzOs content does not favor P,N2 and P3N2 formation; also the changes in pyrochlore phase sequence within the mixture from P,N, to P,N, and finally to P3N2 are strongly dependent on the PbO content. Excess PbO, besides favoring the for- mation of P3N2 within the mixture, also promotes the for- mation of perovskite phase within the PMN and PZN system. A similar observation was also reported and conlirmed by Lejeune et al. f 151. The latter was confirmed in this study by adding a 3 mol excess of PbO to Pb3Nb40i3-MgO (Table 2) and PbsNbQO1,-ZnO-SrTiO, mixtures (Table 3) exhibiting only P3N4, perovskite PMN and perovskite PZN were formed, respectively. These observations are in agreement with the report that perovskite phase content increases with excess PbO [ 161.
4.2. The effect of mixture pyrochlore phase on the perovskite phase of PMN, PZN and PZMN
The pyrochlore phases present in the XPbO-YNb205 mixtures can influence the final phases of the PMN, PZN and PZMN. Their effects on each of these systems are as shown below.
4.2.1. PMN system
Perovskite PMN phase is easily attained in XPbO- YNbzO,-MgO mixture provided no excess of Nbz05 is pres- ent as in 3PbO-2Nb205-MgO mixture which exhibits only P3N4 phase (Table 2). The latter’s situation can be altered with the addition of a 3 mol excess of PbO. The mixture composition which exhibits the most perovskite PMN is 3PbO-Nb,OFMgO. As this composition favors the forma- tion of P,N, phase, we conclude that P,N, phase is important for the formation of perovskite PMN. In fact a complete conversion to perovskite PMN from an equimolar mixture of Pb3Nb208 and MgO was also reported by Guhaand Anderson
[171.
The role of P3N2 in the perovskite phase formation was in good agreement with the opinion of Lejune and Boilot [ 81 and that of Villegas et al. [ 113 for the PMN-PZT system. It was reported that the decomposition of columbite MgNbzOE, to react with PbO was followed by the formation of P3N2 which, having a partially vacant B06 octahedral sublattice, would easily accommodate the MgO to form a B-deficient pyrochlore structure with the formula of Pb2(Nb1,33- Mg0.17) O5.5 whose subsequent reaction with MgO and PZT yielded PMN-PZT perovskite phase. The effect of raw pre- cursor type on the formation mechanism of perovskite phase was also examined by Jang et al. [ 181 using two different types of columbite precursor, (Mg,Zn) Nb206 and MgNb,06 + ZnNb206.
The fact that the X-ray peak of the stabilizer SrTi03 was still clearly observed at the higher calcination temperatures such as 900°C leads to the postulation that the stabilizer
76 S.-Y. Chen et al. /Materials Chemistv and Physics 49 (1997) 7&77
SrTi03 has no effect on the content of perovskite PMN, but it influences its intermediate pyrochlore phase. For in PMN- ST system (Table 1) , no P3N2 phase existed during its for- mation. PMN synthesized with 3PbO-Nb205 mixture having P3N2 phase exhibits higher perovskite PMN content than the 2PbO-Nb205 mixture which shows only P,N, phase. Since preferred phase formation of either P2N2 or P,N, pyrochlore type is strongly influenced by PbO content, we conclude that the formation of perovskite PMN requires the presence of either P2N2 or P,N, phase with P3Nz phase being the preferred phase for PMN. Furthermore, both P3N2 and PMN phase formation probably occur immediately and simultaneously.
4.2.2. PZN system
The formation of perovskite PZN requires the presence of stabilizer (see Table 3). Without any stabilizer, the final phase of PZN will be pyrochlore, regardless of the mixture’s composition and phase condition. Besides stabilizer, theexis- tence of P,N2 pyrochlore phase in the mixture is another pre- requisite for the formation of perovskite PZN. For 2PbO- NbzO,-ZnO-SrTiO, system, the mixture exhibits only lim- ited amount of perovskite PZN (7%) which may be negligible owing to experimental error. All these are further confirmed by the phase sequences exhibiting in the formation of PZN-ST (Table 1) . They are as follows:
PbO-Nb205-ZnO-SrTi03 + P3N4 + P3N4 + P2N2 + P3N4 + P3N2 + P3N4 + PZN Comparing ST-PMN (x = 1) with ST-PZN (x = 0) revealed that P3N2 phase plays a dominating role in the formation of PZN perovskite and a stabilizer such as SrTiO, is needed for such reactions, although the SrTi03 stabilizer is less impor- tant for perovskite PMN formation. In addition, P,N, phase seems to react with ZnO under the influence of SrTiO, sta- bilizer to form PZN perovskite at above 850°C.
4.3. Effect of raw material phase on formation mechanism ofPZMN-STperovskitephase
Although the usage of preformed P3N2 pyrochlore phase as starting raw material in the formation of PMN and PZN does not increase the perovskite content of the final product as compared with that of using raw material mixture, its usage in the formation of PZMN-ST shows a complete different mechanism as shown in Fig. 5.
XRD results indicate that the first major formedperovskite is PMN phase because the maximum content of perovskite phase obtainable corresponds to the disappearance of MgO content at the temperatures below 850°C. In other words, the relative XRD peak intensities of ZnO to perovskite phase are almost constant up to 800°C as shown in Fig. 5(a). The dissolution of ZnO into the perovskite phase seems to be initiated only beyond 800°C. Moreover, it was found that the effect of stabilizer SrTi03 on the formation of PMN or PMN-
rich perovskite phase is negligible. On the other hand,
(Fig. 5 (b) ) in P,N*-&M-ST system, the formation of per- ovskite phase was accompanied by the gradual disappearance of ZnO peak with increasing calcination time at 800°C (Fig. 5(b)). It is believed that ZnO may react with P,N, or the formed PMN phase to form Zn-rich PZMN perovskite even the temperatures below 850°C.
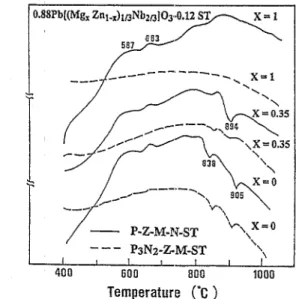
These phenomena are further confirmed by the DTA anal- ysis shown in Fig. 10. The first endothermal peak around 838°C was observed especially for Zn-rich ST-PZMNorpure PZN compositions in both P,N:,-CM-ST and P-Z-M-N- ST systenis. As no obvious endothermic peak was observed in the composition of x = 0, i.e. for the P3N2-M-ST system, this peak is probably caused by the ZnO dissolving into the lead-rich P3N2 phase, forming the liquid phase. In the litera- ture, several new but weak X-ray peaks have been reported for the Pb(ZnJ4bl -JO0,35 _ 1,5x pyrochlore phase when the powder mixture of PbO-ZnO-N&O5 was calcined at BOO”C, [9] and they are probably caused by the variation of Zn concentration in the pyrochlore phases. However, the other endothermal peak at 900°C which was observed in the ST- PZMN system, but not in ST-PMN and PbO-ZnO-Nb205 systems, was the result of the formation reaction of PZMN perovskite phase under the catalysis of stabilizer SrTiOj. From the above-mentioned results, we may conclude that the P,N2 phase is required for perovskite PZMN phase formation. The overall reaction mechanism of perovskite phase forma- tion can be summarized as in Fig. 6.
In P-Z-M-N4T system, pyrochlore phases (P,N, and P,N,) which are believed to be the nucleation centers for perovskite phase formation are constantly produced through the reaction of preformed P3N,* with unreacted PbO. The structure of the perovskite phasl: is mainly PMN at temper- atures below 850°C and is attained through diffusion and reaction between P3N2 or P,N, phases and MgO [ 1’7,191. The stabilizer SrTi03 plays its role only when the temperature is higher than 85O”C, and the perovskite phase is almost completely formed at a temperature above 900°C.
O.BPb[(Mg,
1 (/--;-$;;5
>?I
400 600 1000
Temperature (‘C )
On the other hand, in the P,N,-Z-M-ST system, P3N2 dominates the formation of PMN or Zn-containing PMN by reacting with MgO and ZnO phases at calcination tempera- tures below 850°C. Formation of perovskite PZMN is nearly complete when the temperature is higher than 900°C. How- ever, P3N4 once formed remained unchanged throughout these reactions.
4.4. Pyrochlore phase versus electrical properties
As shown in Figs. 7 and 8, PMN and PZN compounds formed with P3N2 mixtures exhibited the highest dielectric constant while those from P3N4 mixtures showed the lowest dielectric constant. Although the addition of a 3 mol excess PbO to P3N4 mixtures can boost the dielectric constant values, still they cannot exceed those obtained from P,N, mixtures. These delineate once more the importance of having P3Nz pyrochlore phase in the system for obtaining a final product with a high dielectric constant.
The fact that there is not much difference in dielectric constant for PZN of composition 0.12ST-O.88PZN obtained either via PbO-Nbz05 mixture (curve A in Fig. 9) or via preformed P,N, pyrochlore phase (curve A in Fig. 8) con- f%ms once more that pyrochlore phase is the major factor in determining the electrical properties of the system regardless of the mean of obtaining it.
5.
1.
2.
3.
Conclusions
The formation of perovskite phase within PMN relaxers requires the presence of either P,N, or P,N, pyrochlore phases in the mixture and is only slightly dependent on the presence of the stabilizer.
The formation of perovskite phase within PZN or PZMN relaxors require the presence of both P,N, pyrochlore phase and stabilizer.
Excess PbO can be used to modify the pyrochlore phase present in the mixture and promote perovskite forma- tion through the pyrochlore reaction of P3N4 +P + 2 (P,N,) ; P2N2 + P + P3Nz
4. Maximum dielectric constant for PMN, PZN and PZMN systems can be obtained with the P,N, phase present in the starting raw materials.
Acknowledgements
The authors are thankful to Ms. Kathy Hu for helpful sug- gestions. This work was made possible by the financial sup- port from The Ministry of Economic Affairs of The Republic of China.
References
[I] J. Kuwata, K Uchino and S. Nomura, Ferroelectrics, 22 (1979) 863- 867.
[21 0. Furukawa, Y. Yamashita, M. Harata, T. Takahashi and K. Inagaki,
Jpn. J. Appl. Phys., 24 (Suppl. 24-3) (1985) 96-99.
131 S.L. Swartz and T.R. Shrout, Mater. Res. Bull., 17 (10) ( 1982) 1245- 1250.
[4] J. Kuwata, K Uchino and S. Nomura, Ferruelectrzcs, 37 (1981) 579- 587.
[5] S.Y. Chen, S.Y. Cheng and C.M. Wang, J. Am. Ceram. Sot., 74 (2)
(1991) 4owo5.
[6] A. Halliyal, U. Kumar, R.B. Newnham and L.E. Cross, Am. Gram.
Sot. Bull., 66 (4) (1987) 671-676.
[7] T.R. Shrout and A. Halliyal, Am. Gram. Sot. BuZl., 66 (4) (1987)
704-711.
[8] M. Lejeune and J.P. Boilot, Ceram. Int., 8 (3) (1982) 99-105. 191 H.C. Ling, M.F. Yan and W.W. Rhodes, J. Mater. Sci., 24 (1989)
541.
[ 101 F. Chaput, J.P. Boilot, M. Lejeune, R. Papiemik andL.H. Pfalzgraf, J.
Am. Ceram. Sot.. 72 (8) (1989) 1355-1357.
[ 111 M. Villegas, J.R. Jurado, C. Moure and P. bran, J. Mater. Sci., 30
(1995) 1391-1396.
[ 121 M. Inada, Jpn. Natl. Tech. Rep. (Matsushita Elect. ind. Co.), 27 (1)
(1977) 95.
El31 J.R. Belsick, A. Halliyal, U. Kumar and R.E. Newnham, Am. Gram. Sot. Bull., 66 (4) (1987) 66467.
[ 141 H.P. Klug and L.E. Alexander, X-may Diffraction Procedures for Polycrystalline and Amorphous Materials, Wiley, New York, 1974, pp. 411-41.5.
[ 151 M. Lejeune and J.P. Boilot, Ferruelectrics, 54 (1982) 191. [ 161 M. Lejeune and J.P. Boilot, Mater. Res. BulZ., 20 (1985) 493-499.
[ 171 J.P. Guha and U.U. Anderson, J. Am. Ceram. Sot., 69 (1986) C-287.
[ 181 H.M. Jang, S.R.ChoandK-M.Lee,J. Am. Gram. Sot., 78 (2) (1995) 297-304.
[ 191 S.Y. Chen, CM. Wang and S.Y. Cheng, J. Am. Ceram. Sot., 74 (10) (1991) 2506-2512.