國
立
交
通
大
學
應用化學所
博
士
論
文
合成不同插層劑對高分子/黏土奈米複合材料
物理性質之影響
Effect of Various Synthesized Intercalation Agents on
Physical Properties of Polymer/Clay Nanocomposites
研 究 生:葉定儒
指導老師:張豐志 教授
合成不同插層劑對高分子/黏土奈米複合材料
物理性質之影響
Effect of Various Synthesized Intercalation Agents on
Physical Properties of Polymer/Clay Nanocomposites
研 究 生:葉定儒 Student:Ting-Ju Yeh
指導教授:張豐志 Advisor:Feng-Chih Chang
國 立 交 通 大 學
應用化學所
博 士 論 文
A Dissertation
Presented to Institute of Applied Chemistry
College of Science
National Chiao Tung University
In Partial Fulfillment of the Requirements
For the Degree of
Doctor of Philosophy
In
Applied Chemistry
September 2004
Hsinchu, Taiwan, Republic of China
誌 謝
從未中斷的求學之路暫時要告一段落了,對我而言這一路走來真的很辛 苦,幸運地我遇到許多貴人的幫助,才使得我能順利拿到博士學位。我要感謝我 的指導教授張豐志博士,這些年勞心勞力修改學生的論文,以及提供良好的研究 環境使我能專心在研究上,老師培養我具備獨立思考的能力與自動自發的精神, 老師的待人處世與領導統御皆值得我學習的地方。感謝口試委員:段葉芳教授、 芮祥鵬教授、邱顯堂教授、葉正濤教授、廖建勛教授、吳震裕教授、韋光華教授 與黃華宗教授提供寶貴的意見,使學生的論文更臻完善。 感謝實驗室的學長郭紹偉博士、黃智峰博士、陳文億博士與蘇一哲博士在 實驗上的幫忙與親身經驗的傳承使我獲益良多。我要感謝與我共同奮鬥的同學老 隆、muscle、阿錫、凱方,有你們真好,讓我研究所生活多采多姿。謝謝阿明、 寶寶、小呆、阿吉與加菲貓,因為你們的搞笑使得實驗室生氣勃勃。感謝我帶的 學弟ㄚ廣實驗上的協助,使我順利完成論文。謝謝婉君、小杜、漢清、芷伶爲實 驗室辛苦的付出,管帳、大陸學者來台、實驗室計畫有勞你們。感謝學弟妹英傑、 DiDi、怡婷、佩儀、春雄維持實驗儀器正常的運轉、藥品目錄清楚、採買實驗室 必需品,我才能順利畢業,感謝實驗室所有成員。 感謝大學的專題老師段葉芳教授,在大學什麼都不懂的情況下訓練我具備 基礎的研究能力。謝謝大學好友誌民、誌銘、福特豐富我大學生活,且時常關心 我、幫助我,真的非常謝謝你們。我還要感謝在大學期間非常照顧我的叔叔和嬸 嬸,因為有他們的幫忙,我才能快速適應台北的生活。 我要感謝一直陪在我身邊默默支持我的女朋友瑜芝,因為你我對唸書不在 排斥,因為你我繼續求上進,很高興能與你在一起,認識你是我這輩子最快樂的 事。 我最感謝我的家人,沒有他們的栽培,我無法完成博士學位,我的榮耀獻 給最偉大的父母親。Outline of Contents
Outline of Contents I
List of Tables VI
List of Schemes VII
List of Figures VIII
Abstract (in Chinese) XIV
Abstract (in English) XVI
Chapter 1 Introduction 1
1.1 Introduction 1
1.2 Organically Modified Clay 6
1.3 Polymer/Clay Nanocomposite Processing 10
1.4 Surface-Initiated Polymerization (SIP) 14
1.5 Types of Polymer Matrix 20
1.5.1 Polyamide Matrices 20
1.5.2 Polyimide Matrices 20
1.5.3 Polypropylene and Polyethylene Matrices 22
1.5.4 Polymethylmethacrylate/Polystyrene Matrices 22
1.5.5 Epoxy and Polyurethane Matrices 23
1.5.6 Polyelectrolyte Matrices 24
1.5.7 Rubber Matrices 25
1.5.8 Others 25
1.6 Properties of Nanocomposites 26
1.6.1 Dimensional Stability 26
1.6.2 Thermal Stability and Flammability 28
1.6.4 Gas Barrier Properties 34
1.6.5 Electrical and Optical Properties 35
1.7 Summary 37 References 38
Chapter 2 Enhanced Thermal Properties of PS Nanocomposites formed from Inorganic POSS-Treated Montmorillonite
43
Abstract 43
2.1 Introduction 44
2.2 Experimental 46
2.2.1 Materials 46
2.2.2 Preparation of CPC-Modified Clays 46
2.2.3 Preparation of POSS-Modified Clays 46
2.2.4 Preparation of Polystyrene/Clay Nanocomposites 47
2.2.5 Instrumentations 47
2.3 Results and Discussion 49
2.3.1 X-Ray Diffractions 49
2.3.2 TEM Measurements on the Nanocomposites 51
2.3.3 Infrared Spectroscopy 52
2.3.4 Analyzing Glass Transition Temperatures 52
2.3.5 Molecular Weights of the Nanocomposites 52
2.3.6 Characterization by TGA 53
2.4 Conclusions 55
Chapter 3 Thermal Properties of Exfoliated Polystyrene Nanocomposites formed from Rigid Intercalation Agents-Treated Montmorillonite
66
Abstract 66
3.1 Introduction 67
3.2 Experimental 69
3.2.1 Materials 69
3.2.2 Synthesis of Intercalation Agent of 4-(4-adamantyl
phenoxy)-1-butanamine (APB) 69
3.2.3 Preparation of Modified Clays 70
3.2.4 Preparation of Polystyrene/Clay Nanocomposites 70
3.2.5 Instrumentations 71
3.3 Results and Discussion 73
3.3.1 Preparation of 4-(4-adamantylphenoxy)-1-butanamine (APB) 73
3.3.2 X-Ray Diffractions 73
3.3.3 TEM Measurements on the Nanocomposites 75
3.3.4 Analyzing Glass Transition Temperatures 75
3.3.5 Molecular Weights of the Nanocomposites 75
3.3.6 Coefficient of Thermal Expansion 76
3.3.7 Characterization by TGA 76
3.4 Conclusions 78
References 79
Chapter 4 Enhanced Thermal Properties of PS Nanocomposites formed from Montmorillonite Treated with a Surfactant/Cyclodextrin Inclusion Complex
Abstract 89
4.1 Introduction 90
4.2 Experimental 92
4.2.1 Materials 92
4.2.2 Preparation of Inclusion Complex 92
4.2.3 Preparation of Surfactant-Modified Clays 93
4.2.4 Preparation Polystyrene/Clay Nanocomposites 93
4.2.5 Instrumentations 94
4.3 Results and Discussion 95
4.3.1 X-Ray Diffraction 95
4.3.2 TEM Characterization 96
4.3.3 Stoichiometry of the Complex 97
4.3.4 Solid State 13C NMR Spectroscopic Analysis 97
4.3.5 Glass Transition Temperatures 98
4.3.6 Characterization by TGA 99
4.4 Conclusions 100
References 101
Chapter 5 Synthesis of a Novel Benzoxazine Monomer-Intercalated Montmorillonite and the Curing Kinetics of Polybenzoxazine/Clay Hybrid Nanocomposites 111 Abstract 111 5.1 Introduction 112 5.2 Experimental 114 5.2.1 Materials 114
5.2.2 Synthesis of Monofunctional benzoxazine Monomer (MBM) 114
5.2.3 Preparation of MBM-Modified Clays 115
5.2.4 Preparation of Polybenzoxazine/Clay Nanocomposites 115
5.2.5 Isothermal Curing 115
5.2.6 Dynamic Curing 116
5.2.7 Instrumentation 116
5.3 Results and Discussion 118
5.3.1 Preparation of Monofunctional Benzoxazine Monomer (MBM) 118
5.3.2 X-Ray Diffractions 118
5.3.3 TEM Measurements of the Nanocomposites 119
5.3.4 Investigating the Curing Behavior of PBZ/Clay Nanocomposites
Using DSC 119
5.3.5 Kinetic Analysis 121
5.3.5.1 Dynamic Kinetic Method 122
5.3.5.2 Isothermal Kinetic Analysis (Autocatalytic Model) 123
5.3.6 Analyzing Glass Transition Temperatures 124
5.3.7 Thermal Stability of PBZ/Clay Nanocomposites 125
5.4 Conclusions 126
References 127
Chapter 6 Conclusions and Future Outlook 143
List of Publications 146
List of Tables
Table 2-1 Results of TGA and DSC for Polystyrene Nanocomposites 58 Table 2-2 Molecular Weights of Polystyrene Nanocomposites 58 Table 3-1 Results of TGA and DSC for Polystyrene Nanocomposites 81 Table 3-2 Molecular Weights of Polystyrene Nanocomposites 81 Table 4-1 Results of TGA and DSC Data for the Polystyrene Nanocomposites 103 Table 5-1 Activation energies obtained using the Kissinger and Ozawa methods
for PBZ/clay nanocomposites.
130 Table 5-2 Results obtained from isothermal experiments on the PBZ/clay
nanocomposites.
130 Table 5-3 TGA and DSC data for PBZ/clay nanocomposites. 130
List of Schemes
Scheme 2-1 Chemical structures of the surfactants used to prepare the modified
clays. 59
Scheme 2-2 Schematic drawing of the clay intercalated by the POSS and
polystyrene. 60
Scheme 3-1 Structures of Intercalation Agent (a) APP (b) APB. 82
Scheme 3-2 Preparation of Intercalation Agent (APB). 83
Scheme 4-1 Schematic representation of clay intercalated by the CPC/α-CD
inclusion complexes. 104
Scheme 5-1 Preparation of polybenzoxazine (PBZ). 133
Scheme 5-2 Preparation of the monofunctional benzoxaine monomer (MBM). 134 Scheme 5-3 Schematic illustration of clay intercalated by the MBM
andpolybenzoxaine. 135
List of Figures
Figure 1-1 Schematic of nanoscale fillers 2
Figure 1-2 Schematic representation of various methods used to prepare polymer-layered-silicate nanocomposites.
4
Figure 1-3 Schematically illustration of three different types of thermodynamically achievable polymer/layered silicate nanocomposites.
5
Figure 1-4 Structures of the salts used to prepare the organically modified clays. 6 Figure 1-5 Structure of triphenylhexadecylstibonium trifluoromethylsulfonate. 7 Figure 1-6 Structure of VDAC used to prepare the organically modified clay. 7 Figure 1-7 Chemical structures of the surfactants used to prepare the modified
clays.
8
Figure 1-8 The structures of intercalation agent (a) CPC and (b) CPC/α-CD inclusion complex.
9
Figure 1-9 Schematic of the basic steps in processing clay-filled polymers 11 Figure 1-10 X-ray diffraction data showing the diffraction patterns that result from
(a), (b) exfoliated clays, (c) intercalated clays, (d) organically modified clays.
12
Figure 1-11 Schematic of the microstructures that can develop in clay-filled polymer composites.
13
Figure 1-12 (a) Synthetic scheme and structure of the bicationic free radical initiator. (b) Synthetic Scheme and structure of the monocationic free radical initiator.
15
Figure 1-13 X-ray powder diffraction patterns of pure clay and two intercalated clay samples.
Figure 1-14 (a-c) Schematic diagrams of the intercalation: (a) original clay, (b) clay intercalated with bicationic initiator, and (c) clay intercalated with monocationic initiator.
17
Figure 1-15 XRD spectra of the two SIP nanocomposites showing degree of exfoliation.
18
Figure 1-16 Schematic of the synthesis of polyimide-clay hybrid film. 21 Figure 1-17 PS and PS/clay nanocomposites after dimension stability test. Clay
loading is 5 wt % for all nanocomposites.
27
Figure 1-18 TGA cures for polystyrene, PS, and the nanocomposites. 28 Figure 1-19 Peak heat release rates for polystyrene and the three nanocomposites. 29 Figure 1-20 Storage modulus of (a) pure PS, (b) PS/MMT-1, (c) PS/MMT-2 and
(d) PS/MMT-3.
31
Figure 1-21 Tanδ values of (a) pure PS, (b) PS/MMT-1, (c) PS/MMT-2 and (d) PS/MMT-3.
31
Figure 1-22 (a) Tensile strengths, (b) Young’s modulus and (c) elongations at break of PS/MMT nanocomposites.
33
Figure 1-23 Formation of tortuous path in PLS nanocomposites. 34 Figure 1-24 Dependence of diffusion coefficient of water on clay content for
montmorillonite with a layer width of 100 nm, and saponite with a layer width of 50 nm.
35
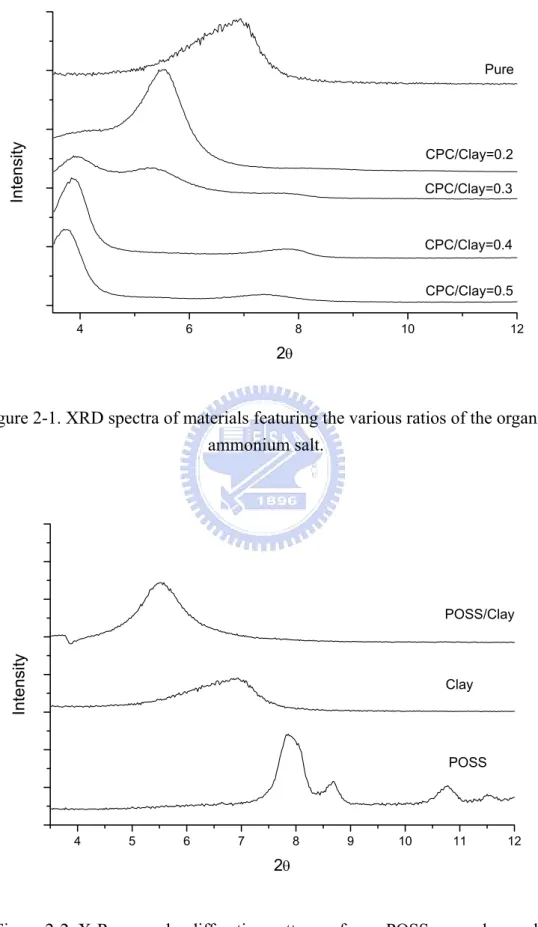
Figure 2-1 XRD spectra of materials featuring various ratios of the organic ammonium salt.
61
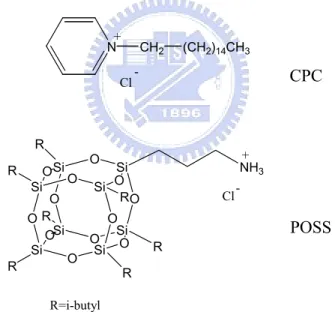
Figure 2-2 X-Ray powder diffraction patterns of pure POSS, pure clay, and intercalated clay.
Figure 2-3 XRD spectra of the two surfactant-containing nanocomposites, indicating the degree of exfoliation.
62
Figure 2-4 TEM micrographs of (a, top) the CPC-treated nanocomposite and (b, bottom) the POSS-treated nanocomposite.
63
Figure 2-5 IR spectra of pure clay, pure POSS, and intercalated clay. 64 Figure 2-6 DSC curves for determining the glass transition temperature of the
nanocomposites.
64
Figure 2-7 TGA traces of (a) pure clay, (b) clay intercalated with the POSS, and (c) clay intercalated with CPC.
65
Figure 2-8 TGA curves for the nanocomposites under a nitrogen atmosphere: (a) pure PS, (b) the nanocomposite formed with CPC, and (c) the
nanocomposite formed with POSS.
65
Figure 3-1 Proton NMR spectra of intercalation agent (APB) 84 Figure 3-2 X-ray diffraction patterns of (a) pure clay, (b) the APP-intercalated
clay, (c) the APB-intercalated clay.
84
Figure 3-3 WAXD analysis of PS nanocomposites prepared by emulsion polymerization: (a) APB treatment; (b) APP treatment.
85
Figure 3-4 TEM images of the APP-treated nanocomposite at low (left) and high (right) magnifications.
86
Figure 3-5 TEM images of APB-treated nanocomposite at low (left) and high (right) magnifications.
86
Figure 3-6 DSC curves for determining the glass transition temperature of (a) PS, (b) the nanocomposite formed using APP, and (c) the nanocomposite formed using APB
Figure 3-7 Coefficient of thermal expansion of nanocomposites. 87
Figure 3-8 TGA curves of (a) pure APP (b) pure APB. 88
Figure 3-9 TGA curves of the nanocomposites recorded under nitrogen
atmospheres: (a) pure PS, (b)the nanocomposite formed using APB, and (c) the nanocomposite formed using APP.
88
Figure 4-1 X-Ray diffraction patterns of (a) α-CD, (b) the CPC/α-CD inclusion complex, and (c) clay intercalated by the CPC/α-CD inclusion complex.
105
Figure 4-2 X-Ray diffraction patterns of (a) pure clay, (b) the CPC-intercalated clay, and (c) the CPC/α-CD intercalated clay.
105
Figure 4-3 WAXD analysis of PS nanocomposites prepared by emulsion polymerization: (a) CPC treatment; (b) CPC/α-CD treatment.
106
Figure 4-4 TEM images of the CPC-treated nanocomposite at low (left) and high (right) magnifications.
107
Figure 4-5 TEM images of CPC/α-CD-treated nanocomposite at low (left) and high (right) magnifications.
107
Figure 4-6 1H NMR spectrum (500 MHz) of the CPC/α-CD complex in DMSO-d6.
108
Figure 4-7 13C CP/MAS NMR spectra of (a) α-CD, (b) CPC/α-CD, and (c) CPC/α-CD intercalated clay.
108
Figure 4-8 DSC curves for determining the glass transition temperature of (a) PS, (b) the nanocomposite formed using CPC, and (c) the nanocomposite formed using CPC/α-CD.
Figure 4-9 TGA curves of (a) pure CPC and (b) the CPC/α-CD inclusion complex.
109
Figure 4-10 TGA curves of the nanocomposites recorded under nitrogen
atmospheres: (a) pure PS, (b) the nanocomposite formed using CPC, and (c) the nanocomposite formed using CPC/α-CD.
110
Figure 5-1 1H NMR spectrum of MBM. 136
Figure 5-2 XRD patterns of (a) pure clay and (b) the clay intercalated by MBM. 136 Figure 5-3 XRD patterns of the (a) 3, (b) 6, (c) 10, and (d) 15% clay
nanocomposites.
137
Figure 5-4 TEM micrographs of the (a) 3 and (b) 6% clay nanocomposites. 138 Figure 5-5 Dynamic exothermal curves of the PBZ/clay nanocomposites
recorded at a heating rate of 20℃/min.
139
Figure 5-6 Dynamic DSC exothermic curves of pure PBZ recorded at different scan rates.
139
Figure 5-7 Plots of reaction rate versus curing time for pure PBZ at different curing temperatures.
140
Figure 5-8 Plots of conversion as a function of cure time for the PBZ/clay nanocomposites cured at isothermal curing temperature of 200 oC.
140
Figure 5-9 Plots of reaction rate as a function of conversion for the nanocomposites cured at different temperatures.
141
Figure 5-10 Representations of Ozawa’s and Kissinger’s methods of calculating the activation energy from non-isothermal data for pure PBZ (Tp,
temperature at maximum reaction rate; β, heating rate).
Figure 5-11 DSC curves for determining the glass transition temperatures of the nanocomposites.
142
Figure 5-12 TGA curves of the PBZ/clay nanocomposites recorded under a nitrogen atmosphere.
摘 要
蒙托土應用於高分子材料上,改善了原有高分子的物理性質,例如:熱穩 定性、機械性質、阻氣性、尺寸安定性等。這些物理性質改善的程度取決於蒙托 土在高分子基材的分散程度,分散程度越好性質提升越顯著,反之則不然。本篇 論文分成四部份,主要探討不同插層劑對奈米複合材料物理性質的影響。 1. 本實驗利用乳化聚合的方法製備聚苯乙烯/蒙托土奈米複材,在蒙托土含量 3%時為脫層結構,在實驗當中使用 POSS 與 CPC 兩種插層劑改質蒙托土,由X-ray 鑑定出 POSS 與 CPC 都有成功插層進入蒙托土,在 TGA 實驗中,
POSS 有效地增加奈米複材的熱穩定性,主要因素是 POSS 較 CPC 具有剛性 結構,有別於市售的插層劑,其具有長的碳烷鏈較不受熱。GPC 分子量分 析得知蒙托土會妨礙高分子聚合反應的進行,分子量分布也較廣。 2. 我們合成插層劑 APB,其具有金剛烷的官能基,去改質蒙托土製備聚苯乙 烯/蒙托土奈米複材,先前文獻利用含磷的官能基作插層劑,目的提升奈米 複材的熱穩定性,本實驗研究金剛烷可否取代磷的官能基,作為插層劑另一 種選擇。TGA 結果顯示金剛烷確實較含磷的插層劑穩定,XRD 說明我們所 製備的複合材料具有奈米尺寸的分散程度,DSC 得知有添加蒙托土的奈米 複材玻璃轉移溫度較高,熱膨脹係數也下降44~55%,證實蒙托土有助奈米 複材的尺寸安定性。
3. 研究一般商業用插層劑CPC經過改質,套入環糊精對奈米複材的影響。研究 結果顯示CPC套環弧精有助將長的碳烷鏈拉直,避免它糾結在一起影響插層 效果,在1H NMR得知一個CPC的分子鏈能夠套入兩個環糊精,由XRD與13C NMR可以確定CPC確實有套入環弧精,TGA實驗證實有套環糊精的CPC熱 裂解溫度提升 50℃左右,主要原因是環糊精保護了CPC的碳烷鏈提升了裂 解溫度。 4. 我們首先合成插層劑 MBM 其具有 Benzoxazine 官能基,可以進行開環交聯 反 應 , 促 使 蒙 托 土 達 到 奈 米 分 散 程 度 。 我 們 使 用 溶 劑 的 摻 混 方 法 將 Benzoxazine 單體與改質後的蒙托土均勻混合,並進行恆溫與非恆溫的交聯 動力學實驗,結果得知有添加蒙托土會促進交聯反應提早發生,原因是因為 蒙托土表面有催化交聯反應,反應的級數在2.4~2.8 之間,活化能隨著蒙托 土的含量增加而降低。在 3%的蒙托土含量時為脫層結構,大於 3%時為插 層結構。
Abstract
Nanoclay-filled polymeric systems offer the prospect of greatly improving many of the properties of their mother polymers. In the recent literature, there have been reports of nanoclay-filled polymeric systems that display significant improvements in tensile and thermal properties, heat distortion temperatures, and resistance to flammability and reduced permeability to small molecules and reduced solvent uptake. A common observation emerging from these studies is that the magnitude of improvement depends strongly on the state of dispersion of the clay layers in the polymer matrix. The experiment work in this dissertation was divided into four areas: 1. We have prepared polystyrene/clay nanocomposites using an emulsion
polymerization technique. The nanocomposites were exfoliated at up to a 3 wt % content of pristine clay relative to the amount of polystyrene (PS). We used two different surfactants for the montmorillonite: the aminopropylisobutyl polyhedral oligomeric silsesquioxane (POSS) and the ammonium salt of cetylpyridinium chloride (CPC). The nanocomposite prepared from the clay treated with the POSS containing surfactant is exfoliated, while an intercalated clay was obtained from the CPC-treated surfactant. The value of Tg of the PS component in the
nanocomposite is 8 °C higher than the virgin PS and its thermal decomposition temperature (21 °C) is also higher significantly. The presence of the POSS unit in the MMT enhances the thermal stability of the polystyrene.
2. We synthesized intercalation agent APB and prepared polystyrene/clay nanocomposites using an emulsion polymerization technique. We used two different intercalation agents to treat clay: the phosphonium salt (APP) and the ammonium salt (APB). We expected that the intercalation agent APB containing rigid adamantine group also has high thermal stability besides phosphonium
group. The molecular weights of polystyrene (PS) obtained from the nanocomposite is slightly lower than the virgin PS formed under similar polymerization conditions. The coefficient of thermal expansion (CTE) was obtained from thermomechanical analysis. A 44~55 % decrease of CTE is observed for APB- and APP-intercalated clay nanocomposites relative to the pure PS.
3. We employed two surfactants for the montmorillonite: cetylpyridinium chloride (CPC) and the CPC/α-CD inclusion complex. The inclusion complex was characterized by X-ray diffraction, 13C CP/MAS NMR spectra, and 1H NMR spectroscopy, and TGA. The 1H NMR spectra of the complexes indicate that the stoichiometry of the complexes is 1:2 (i.e.,one CPC molecule and two α-CD units). The CPC/α-CD-treated clay is more effective than is virgin CPC-treated clay at enhancing the thermal stability of polystyrene.
4. We have used the solvent blending method to prepare polybenzoxazine/clay nanocomposites possessing various clay contents. We synthesized a monofunctional benzoxazine monomer (MBM) and then treated the clay with this intercalation agent. To better understand the curing kinetics of the polybenzoxazine/clay nanocomposites, we performed dynamic and isothermal differential scanning calorimetry (DSC) measurements. The Kissinger and Ozawa methods gave fairly close results for the calculated activation energies, which decreased upon increasing the clay content. The Kamal method-based on an autocatalytic model-suggested a total reaction order of between 2.4 and 2.8.
Chapter 1
Introduction
1.1 Introduction
Polymer composites are important commercial materials with applications that include filled elastomers for damping, electrical insulators, thermal conductors, and high-performance composites for use in aircraft. Materials with synergistic properties are chosen to create composites with tailored properties; for example, high-modulus but brittle carbon fibers are added to low-modulus polymers to create a stiff, lightweight composite with some degree of toughness. In recent years, however, we have reached the limits of optimizing composite properties of traditional micrometer- scale composite fillers, because the properties achieved usually involve compromises. Stiffness is traded for toughness, or toughness is obtained at the cost of optical clarity. In addition, macroscopic defects due to regions of high or low volume fraction of filler often lead to breakdown or failure.
Nanoscale fillers come in many shapes and sizes. For ease of discussion, we have grouped nanofillers into three categories (Figure 1-1). Fiber or tube fillers have a diameter <100 nm and an aspect ratio of at least 100. The aspect ratios can be as high as 106 (carbon nanotubes). Plate-like nanofillers (Figure 1-1) are layered materials typically with a thickness on the order of 1 nm, but with an aspect ratio in the other two dimensions of at least 25. Three dimensional (3D) nanofillers are relatively equi-axed particles <100 nm in their largest dimension. This is a convenient way to discuss polymer nanocomposites, because the processing methods used and the properties achieved depend strongly on the geometry of the fillers.
Figure 1-1 Schematic of nanoscale fillers.
Recently, a large window of opportunity has opened to overcome the limitations of traditional micrometer-scale polymer composites – nanoscale filled polymer composites – in which the filler is <100 nm in at least one dimension (Figure 1-1). Although some nanofilled composites have been used for more than a century, research and development of nanofilled polymers has greatly increased in recent years, for several reasons. First, unprecedented combinations of properties have been observed in some polymer nanocomposites. For example, the inclusion of equi-axed nanoparticles in thermoplastics, and particularly in semicrystalline thermoplastics, increases the yield stress, the tensile strength, and Young’s modulus compared to pure polymer. A volume fraction of only 0.04 mica-type silicates (MTS) in epoxy increases the modulus below the glass transition temperature by 58% and the modulus in the rubbery region by 450%. In addition, the permeability of water in poly(ε-caprolactone) decreases by an order of magnitude with the addition of 4.8% silicate by volume. Yano et al. showed a 50% decrease in the permeability of polyimides at a 2% loading of MTS. Many of these nanocomposites are optically transparent and/or optically active.
A second reason for the large increase in research and development efforts was the ‘discovery’ of carbon nanotubes in the early 1990s. Although more careful review has shown that nanotubes have been observed since the 1960s, it was only in the mid-1990s that they were made in the quantities required for property evaluation of composites. The properties of these carbon nanotubes, particularly strength and electrical properties, are significantly different from those of graphite and offer exciting possibilities for new composite materials.
Third, significant development in the chemical processing of nanoparticles and in the in situ processing of nanocomposites has led to unprecedented control over the morphology of such composites. It has also created an almost unlimited ability to control the interface between the matrix and the filler.
Thus, this is an exciting time to study nanocomposites, because of the unique combinations of properties that are achievable and because of the high potential for successful commercial development. Although the technical community has made advances in the processing of nanocomposites, we are just beginning to assemble the interdisciplinary teams required to understand, tailor, and optimize properties. We have at our fingertips, however, the ability to change the size, shape, volume fraction, interface, and degree of dispersion or aggregation. Thus, the opportunities may well become limitless when theory and experiment have assembled enough information to guide further development.
Polymerization of vinyl monomers intercalating into the montmorillonite (MMT) clay [1] were first reported in literature as early as 1961. The most recent methods to prepare polymer-layered-silicate nanocomposites have primarily been developed by several other groups. In general these methods (shown in Figure 1-2) are able to achieve molecular-level incorporation of the layered silicate (e.g. montmorillonite
silicate either to a polymerization reaction (in situ method), [2-4] to a solvent-swollen polymer (solution blending), [5] or to a polymer melt (melt blending). [6,7] Recently, a method has been developed to prepare the layered silicate by polymerizing silicate precursors in the presence of a polymer. [8]
Figure 1-2. Schematic representation of various methods used to prepare polymer-layered-silicate nanocomposites. [41]
In general, layered silicates have layer thickness on the order of 1 nm and very high aspect ratio (e.g. 10~1000). A few weight percent of layered silicates that are properly dispersed throughout the polymer matrix thus create much higher surface area for polymer/filler interaction as compared to conventional composites. Depending on the strength of interfacial interactions between the polymer matrix and layered silicate (modified or not), three different types of polymer/layered silicate (PLS) nanocomposites are thermodynamically achievable (see Figure 1-3):
a. Intercalated nanocomposites: in intercalated nanocomposites, the insertion of a polymer matrix into the layered silicate structure occurs in a crystallographically regular fashion, regardless of the clay to polymer ratio.
Intercalated nanocomposites are normally interlayer by a few molecular layers of polymer. Properties of the composites typically resemble those of ceramic materials.
b. Flocculated nanocomposites: conceptually this is same as intercalated nanocomposites. However, silicate layers are some times flocculated due to hydroxylated edge-edge interaction of the silicate layers.
c. Exfoliated nanocomposites: in an exfoliated nanocomposite, the individual clay layers are separated in a continuous polymer matrix by an average distances that depends on clay loading. Usually, the clay content of an exfoliated nanocomposite is much lower than that of an intercalated nanocomposite.
Figure 1-3 Schematically illustration of three different types of thermodynamically achievable polymer/layered silicate nanocomposites.
1.2 Organically modified clay
Zhu and co-workers [21] reported the preparation of three new organically modified clays and their corresponding preparation of PS/clay nanocomposites from these clays by bulk polymerization. Two are functionalized ammonium salts while the third is a phosphonium salt and structures of these salts are shown in Figure 1-4. TGA/FTIR showed that the phosphonium treatment results in the most thermally stable treatment when compared to the two ammonium salts.
N+ N+
OH
P+
VB16 OH16
P16
Figure 1-4. Structures of the salts used to prepare the organically modified clays. [21] Wang [22] used two different organic modifications of the montmorillonite, one contains a styryl monomer on the ammonium ion while the other contains no double bond. A double bond that may be involved in the polymerization reaction is present in the cation of the clay. Polystyrene-clay nanocomposite has been prepared by bulk, solution, suspension, and emulsion polymerization as well as by melt blending. The organic modification as well as the mode of preparation may determine whether the composite is either exfoliated or intercalated. Exfoliation is more likely to occur if the ammonium ion contains a double bond which can participate in the polymerization reaction. However, the mere presence of this double bond is not sufficient to always
produce an exfoliated system.
In addition, Wang and Wilkie [23] also reported the preparation and characterization of an antimony-containing clay and the preparation of polystyrene nanocomposites from this clay. The structure of antimony is shown in Figure 1-5. The objective of this study is to determine if this clay is more thermally stable than the common ammonium clays and thus could be used at higher temperatures for the processing of polymers, such as polycarbonate, that require processing at higher temperature.
Sb+ (SO
3CF3)
-Figure 1-5. Structure of triphenylhexadecylstibonium trifluoromethylsulfonate. [23] Fu and Qutubuddin [24] reported the synthesis of exfoliated polystyrene-clay nanocomposite. A reactive cation surfactant vinylbenzyldimethyldodecylammonium chloride (VDAC) was synthesized and used for ion exchange with sodium ions in MMT. The structure of VDAC is shown in Figure 1-6. The exfoliated polystyrene-clay nanocomposite was prepared by direct dispersion of organophilic MMT in styrene monomer followed by free radical polymerization.
N+ CH3
C H3
CH3
VDAC
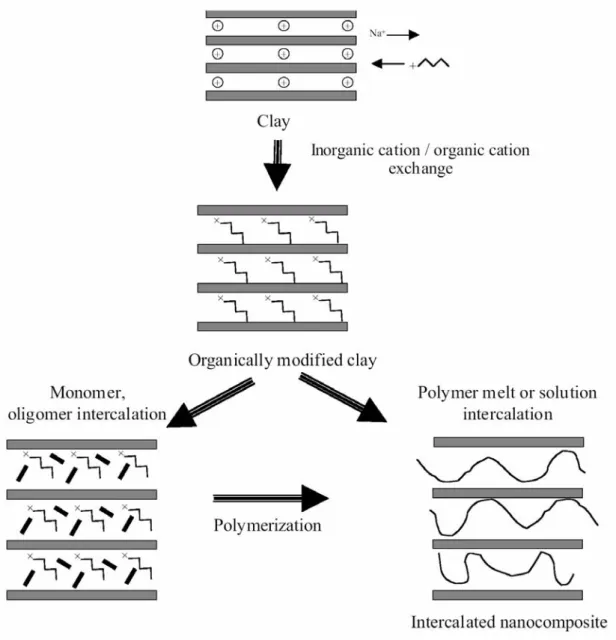
Chang and co-workers [27] reported the preparation of two types of nanocomposites formed from cetylpyridinium chloride (CPC)- and aminopropylisobutyl polyhedral oligomeric silsesquioxane (POSS)-treated clays (Figure 1-7). The PS/clay nanocomposite formed using the CPC-treated clay exhibited no significant improvement in thermal properties. [28-32] The major advantage of choosing POSS molecules is its thermal stability up to 300 oC, higher than the thermal degradation temperatures of most organic molecules. POSS consists of a rigid cubic silica core with 0.53 nm side length, to which organic functional groups can be attached at the vertices for further reactions. POSS derivatives containing amine functional groups can play the role of surfactants for the treatment of clay and the thermal stability of the resulting nanocomposite is enhanced.
Si O Si O Si O Si O Si O Si O Si O Si O O O O O R R R R R R R NH3 R=i-butyl N CH2 (CH2)14CH3 + Cl- CPC POSS + Cl
-Figure 1-7. Chemical structures of the surfactants used to prepare the modified clays. [27]
In addition, chang and co-workers also reported the preparation of two types of PS/clay nanocomposites formed from clays treated with either cetylpyridinium chloride (CPC) or the CPC/α-CD inclusion complex. [26] The structures of two intercalation agents were shown in Figure 1-8. We found that CPC, a linear aliphatic surfactant, is able to form a crystalline complex with cyclodextrin. Including CPC
within CD channels improves the thermal stability of the virgin CPC. The linear aliphatic chain within the CPC/α-CD cannot bend within the galleries of the clay and the d spacing of clay intercalated by the CPC/α-CD inclusion complex is significantly higher than that formed using pure CPC. The CPC/α-CD inclusion complex can promote exfoliated structure of clay.
N+ CH3 Cl -N+ CH3 Cl
-(a)
(b)
Figure 1-8. The structures of intercalation agent (a) CPC and (b) CPC/α-CD inclusion complex.
1.3 Polymer/clay nanocomposite processing
Scientists have known for about 40 years that polymers interact strongly with montmorillonite and that the clay surface can act as an initiator for polymerization. Patents for clay/Nylon 6 composites were not issued until the 1980s, at which point the clay/polymer nanocomposites were commercialized. [83] The improvement that led to commercialization was the appropriate dispersion of the clays at the nanometer scale.
The first step in achieving nanoscale dispersion of clays in polymers is to open the galleries and to match the polarity of the polymer or monomer so that it will intercalate between the layers. This is done by exchanging an organic cation for an inorganic cation (Figure 1-9). The larger organic cations swell the layers and increase the hydrophobic properties of the clay (Figure 1-9), resulting in an organically modified clay. The organically modified clay can then be intercalated with polymer by several routes. Solution processing involves dispersion of both the organically modified clay and the polymer in a common solution. Variations on this process include emulsion or suspension polymerization. [84] Highly polar polymers such as Nylon and polyimides are more easily intercalated than nonpolar polymers such as polypropylene, because polar polymers have a higher affinity for the polar clay galleries. In situ polymerization intercalates monomer directly into the organically modified clay galleries, and the monomer can either adsorb onto the layer surface or be anchored by free radical techniques. Melt intercalation involves mixing the clay and a polymer melt, with or without shear. The success of melt intercalation is surprising, given that the gallery spacing is only about 2 nm and the radius of gyration of the polymer is significantly larger than this. Even more surprising is that the speed of melt intercalation is faster than that of self diffusion of polymers and scales with
the inverse of the molecular weight.
Figure 1-9 Schematic of the basic steps in processing clay-filled polymers.
As the layer spacing increases, the process can be monitored by x-ray diffraction (XRD). Intense peaks between 3˚ and 9˚ indicate an intercalated composite, but if the peaks are extremely broad or disappear completely, this indicates complete exfoliation. Figure 1-10 shows the XRD pattern of organically modified montmorillonite (d), an intercalated montmorillonite (c), and two exfoliated montmorillonite nanocomposites (a, b).
Figure 1-10 X-ray diffraction data showing the diffraction patterns that result from (a), (b) exfoliated clays, (c) intercalated clays, (d) organically modified clays.
The resulting nanocomposites can have several structures (Figure 1-11). The structure of an intercalated nanocomposite is a tactoid with expanded interlayer spacing, but the clay galleries have a fixed interlayer spacing. Exfoliated nanocomposites are formed when the individual clay layers break off the tactoid and are either randomly dispersed in the polymer (a disordered nanocomposite) or left in an ordered array.
Figure 1-11 Schematic of the microstructures that can develop in clay-filled polymer composites.
1.4 Surface-initiated polymerization (SIP)
Rather than modifying the clay with organic quaternized ammonium salts, cationically modified polymerization initiators can also be used to prepare organophilic clays. In this method, in the situ polymerization is initiated by the activation of these initiators which are ionically bound to the clay particle surfaces, that is, through a surface-initiated polymerization (SIP) process. The advantage of SIP is based on the assumption that as the polymer chain grows through surface initiation, the ordered silicate layers can be gradually pushed apart, ultimately exfoliating to discrete laths, resulting in a well-dispersed structure of the final product. Also, theoretically, if all initiators are tethered to clay surfaces, a higher efficiency of intergallery polymerization is expected compared to that of free, or unattached initiators. Exfoliated polystyrene-clay nanocomposites with controllable MW have been prepared by intercalating a charged living free radical polymerization (LFRP) initiator into montmorillonite. [33] A (1,1-diphenylethylene) DPE derivative initiator was used to synthesize polystyrene-clay nanocomposite materials through living anionic surface-initiated polymerization (LASIP). [34,35] However, only intercalated structures were obtained.
In efforts to conduct SIP from clay surfaces, Xiaowu and co-workers [36] recently synthesized two initiators for free radical SIP, both contain quaternized amine endgroups for cation exchange with montmorillonite particles. The initiator molecule design is as follow: (1) symmetric, with two cationic groups at both chain ends (named bicationic free radical initiator hereafter) and (2) asymmetric, with one cationic group at one end (named monocationic free radical initiator hereafter). The synthetic schemes and structures of these initiators are shown in Figures 1-12a and 12b. They are both AIBN-analogue initiators for free radical polymerization. The use
of another symmetric bicationic azo compound, 2,2’-azobis(isobutyramidine hydrochloride) (AIBN), has also been proven to be feasible for styrene SIP on high surface area mica powder. [37] However, no structural information for these SIP products has been reported. Asymmetric azo initiators in the form of silanes have also been successfully employed to free radically polymerize styrene from spherical silica gel surfaces. [38,39] To the best of our knowledge, there have been no reports on a direct free radical SIP approach from surface-bound monocationic azo initiators on individual clay nanoparticles.
N N CN CN OH O O H O N N CN CN O O O O Br Br DCC/DMAP Br OH N(CH3)3 N N CN CN O O O O N+ N+ C H3 CH3 C H3 CH3 CH3 CH3 Br- Br -N N CN CN OH O O H O DCC/DMAP O H CH3 N N CN CN OH O O O C H3 DCC/DMAP Br OH N N CN CN O O O O C H3 Br N(CH3)3 N N CN CN O O O O C H3 N +CH3 CH C H3 3 Br
-Figure 1-12 (a) Synthetic scheme and structure of the bicationic free radical initiator. (b) Synthetic Scheme and structure of the monocationic free radical initiator. [36]
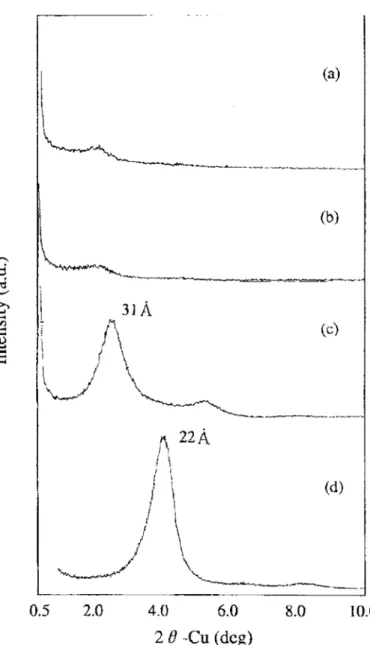
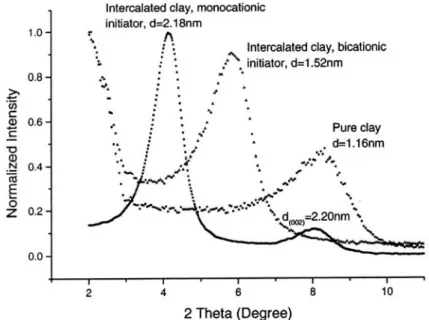
X-ray powder diffraction patterns of the pristine clay and two initiator-intercalated clay samples are shown in Figure 13. Lamellar periodicity was maintained on the organophilic clay despite the rigorous sonication-centrifugation procedure to intercalate the initiators. By using the Bragg equation, nλ=2dsinθ, the
accordance with data from other sources. [40] The XRD patterns of the intercalated clays indicate the successful insertion of the initiator molecules into the galleries of the silicate platelets since both intercalated clay samples gave increased d spacing values. In addition, the sharper shape and the higher diffraction intensities of these peaks after intercalation provide the evidence of a better-ordered swollen structure than that of the original clay. This result demonstrates that the layered framework of inorganic clay can accommodate the AIBN derivative molecules of various functionalities with better long-range periodicity.
Figure 1-13. X-ray powder diffraction patterns of pure clay and two intercalated clay samples. [36]
On further analysis, the d spacing values seemed to be inconsistent with the steric sizes of the two initiators. The d spacing of bicationic intercalated clay (1.52 nm) is substantially smaller than that of the monocationic intercalated clay although their molecular dimensions are comparable (both chain length values are 2.20 nm, as estimated by Chem 3D software). The bicationic initiator molecule possesses charged groups on both ends that can have two intercalation possibilities: (1) these two cationic endgroups interact electrostatically with two different but neighboring
platelets’ surfaces, or (2) they interact on the same side surface of a single clay particle. The combination of these two possibilities makes the intercalated structure less spatially ordered which accounts for the broadened reflection for this sample as compared with the peak of the clay intercalated by the monocationic initiator. Furthermore, since XRD collects the average information from a large area of a powder sample, a synergic effect of these two possibilities accounts for an intermediate d spacing value. This interpretation is schematically shown in Figure 1-14. The interlamellar height shown in the figure is calculated by Δd = d spacing – thickness of one platelet (~1.0 nm).
Figure 1-14. (a-c) Schematic diagrams of the intercalation: (a) original clay, (b) clay intercalated with bicationic initiator, and (c) clay intercalated with monocationic
initiator. [36]
to the long-range order of the polystyrene matrix. Similar broad peaks were also observed in the diffractogram of the PS-0 reference sample (not shown). Sample bi-PS-M-2 shows a small peak at 2θ=5.9o, which is about the same as the peak position of the corresponding intercalated clay (Figure 1-8), implying that this product still contains fraction of the intercalated clay structure. On the contrary, there is no peak on the XRD trace of the mono-PS-M-2 sample, indicating that a completely exfoliated structure was achieved in this sample.
Figure 1-15. XRD spectra of the two SIP nanocomposites showing degree of exfoliation. [36]
This observed result is quite unexpected. We would anticipated that these adjacent clay layers in the clay/bicationic initiator system will be gradually pushed apart during SIP, if these two immobilized free radicals are simultaneously generated. As a result, the intercalated clay stacks would be totally delaminated, forming a fully exfoliated nanocomposite product. However, the polymer can only grow within the clay gallery when monomer molecules are able to diffuse and make contact with effectively with the tethered radicals within the interlayer spacing. The time scale of diffusion is such that access to the monomer from within the layers is limited.
Considering the rapid kinetics for free radical polymerization in solution, this intercalative monomer diffusion is significantly slower toward monomer addition. Thus, free initiators exhaust the monomers while SIP inside clay lamellar is delayed by diffusion. Furthermore, there is also competition from the surface-perimeter-attached initiators of the clay particles. Even if some of the bication initiators were activated and grew to become oligomers, the growing chains will likely be terminated by recombination or disproportionation by nearby immobilized growing chains/initiators in the same gallery. Hence the low molecular weight and high polydispersity obtained by bi-PS-M-2 can be explained.
By comparison, an intercalated monocationic initiator is easier to be delaminated than a bicationic initiator. The monocationic initiator molecule is also more organophilic. The weaker van der Waals interaction between the alkyl headgroups of the monocationic initiator and clay surfaces makes the intercalated clay easier to be swelled by the solvent and monomer. Once the clay intercalated with monocationic initiator is exfoliated by sonicating and stirring, the attached initiators have more accessibility to monomer and thus results in better monomer intercalative diffusion.
1.5 Types of Polymer matrix
1.5.1 Polyamide Matrices
Nylon-6/Nylon-12/clay hybrid composites were the first exfoliated smectic clay composites made. [40] Montmorillonite, with a CEC of 119 mEq/100 g, was intercalated with 12-aminolauric acid, which increased the intergallery spacing from 1.0 to 1.7 nm. This ‘12-montmorillonite’ was then mixed with ε-caprolactam, which increased the intergallery spacing even further, to 4.0 nm, indicating that the ε-caprolactam had intercalated into the galleries. Heating to 250 oC led to polymerization, forming a clay/Nylon-6 nanocomposite. Further research [41] determined that ε-caprolactam could intercalate directly into the galleries of montmorillonite in a hydrochloric acid solution and, upon intercalation, becomes oriented vertically in the galleries. The modified montmorillonite then mixed easily with additional molten ε-caprolactam and 6-aminocaproic acid, yielding a Nylon-6 homopolymer/clay nanocomposite. The montmorillonite was completely exfoliated. Recently [42], montmorillonite/Nylon 6 nanocomposites were processed by melt intercalation. Although the degree of exfoliation was not as high as in nanocomposites produced by the above methods, at weight fractions less than 0.1 the composites were primarily exfoliated.
1.5.2 Polyimide Matrices
The preparation of polyimide matrix clay nanocomposites involves several steps [43] (Figure 1-16). By intercalating montmorillonite with the ammonium salt of dodecylamine, it becomes soluble in dimethylacetamide (DMAC). DMAC is also a solvent for 4,4’-diaminodiphenylether and pyrometllitic dianhydride, the precursors for polyamic acid and, as such, polyimides. After intercalation of the ammonium salt of dodecylamine, x-ray studies [44] showed that hectrite (CEC = 55 mEq/100 g) has
one monolayer of organic material between the layers, whereas saponite, montmorillonite, and synthetic mica (all with CEC > 100 mEq/100 g) have two. After composite formation, however, only the montmorillonite and the synthetic mica have exfoliated completely, but the hectrite and saponite remain in a somewhat aggregated state. Lan et al. [45] found aggregates of montmorillonite after using a similar procedure. More recently, P-phylenediamine in an HCl solution was also found to form organic-modified montmorillonite that dissolves in DMAC. [46] This same study showed that the presence of a small amount of nanoscale organoclay can decrease the imidization temperature by 50 oC (from 300 oC to 250 oC), and at 250 oC the imidization time decreased by 15 min. The activation energy decreased by 20 %. Clearly, the organoclay surface is acting as a catalyst.
1.5.3 Polypropylene and Polyethylene Matrices
Nonpolar polymers are very difficult to intercalate into smectic clays, because the clays are strongly polar. This challenge has been met [47, 48] by first intercalating stearylamine into montmorillonite and synthetic mica. Melt-mixing the organoclays with maleic anhydride-modified polypropylene oligomers results in PP-MA intercalation. The modified organoclay is then melt-mixed with a polypropylene matrix. There is a balance between creating a polar oligomer with enough maleic anhydride to intercalate well, but nonpolar enough to mix with the polypropylene. Unfortunately, the oligomer limits the extent of property improvement achieved to date. Polyethylene has also been successfully melt-mixed with modified montmorillonite and saponite after ion exchange with dioctadecyldimethylammonium bromide. The degree of dispersion is not excellent, and the layers are certainly not exfoliated; yet, significant modification of both the crystal structure and properties has been observed. [49]
1.5.4 Polymethylmethacrylate/Polystyrene Matrices
The processing of clay/PMMA or clay/PS composites was first done by directly intercalating the monomer into the clay, followed by polymerization. [50] This method was not successful in exfoliating the clays. At issue again is the compatibility between the clay and the monomer. One solution for PMMA has been to use appropriate ammonium salts, [51, 52] which may be reactive. Another solution is to use a comonomer as a compatibilizer. [53] A similar solution was found for polystyrene by using the reactive cationic surfactant vinylbenzyldimethyldodecylammonium as the intercalant. Exfoliated graphite/polystyrene composites have been made by similar processing methods. Recently, a commercially viable process was developed [54] for polystyrene in which
montmorillonite intercalated with octadecyl trimethyl ammonium chloride was melt-mixed with a styrene methylvinyloxazoline copolymer. This process resulted in complete exfoliation, which could not be achieved with pure polystyrene. The hypothesis is that the hybridization is due to strong hydrogen bonding between the oxazoline groups and oxygen groups in the silicate clays.
1.5.5 Epoxy and Polyurethane Matrices
Epoxy is a widely used thermoset, with applications ranging from household
glues to high-performance composites. To improve performance, increasing the Tg of
epoxy and improving its properties above the Tg are desirable. Adding clays and
layered silicic acids to epoxy [55-57] can greatly improve its mechanical performance,
particularly at temperatures above Tg. The processing has been studied in detail. In the
smectic clay/epoxy composites, the length of the intercalated organic amine determines the ease of exfoliation, and only clays with primary and secondary onium ions form exfoliated nanocomposites. After intercalation of the organic amines, the epoxy resin or a combination of resin and curing agent can be intercalated into the smectic clays or layered silicic acids. If enough resin and curing agent are intercalated and the curing process is controlled, exfoliated nanocomposites result. The acidic onium ions catalyze the intragallery polymerization or curing of the resin. If this reaction occurs more rapidly than extragallery curing, then the clay exfoliates. Otherwise, an intercalated nanocomposite results. Therefore, careful control of temperature and time is required, or the ratio of resin to curing agent must be significantly less than the stoichiometric ratio in order to achieve exfoliation. An approach similar to that used for epoxy composites was used to make intercalated montmorillonite/polyurethane composites.
1.5.6 Polyelectrolyte Matrices
Polyelectrolytes can be used in electrochemical devices such as solid-state batteries, electrochromic devices, and sensors. [69] The addition of layered silicates to polyelectrolytes increases the conductivity, improves the mechanical stability, and improves the interfacial stability with electrode materials. Polyelectrolytes are characterized by a large number of ionizable groups and thus are highly polar. This makes them excellent candidates for intercalation into smectic clays. Polyvinylpyridines are of particular interest because of the variety of processing methods available. Intercalated nanocomposites can be formed easily from the water-soluble hydrobromide salt of the 1,2 or 1,6 polyelectrolyte (1,2 or 2,6 polyvinylpyridinium cations). However, only a single layer of polymer intercalates, and exfoliation does not occur. A slower, but ultimately more effective process, uses neutral poly-4-vinylpyridine and results in an exfoliated composite. A second method involves intercalation of 4-vinylpyridinium salts, followed by polymerization.
Poly(ethylene oxide) (PEO) matrix composites have also been processed both by intercalating PEO in solution into organically modified smectic clays [69] and by melt- mixing clay with PEO and PEO/PMMA mixtures. [70, 71] In neither case does an exfoliated composite result. Aranda and Ruiz-Hitzky [69] dissolved PEO in acrylonitrile and found that the structure of the PEO changed when the interlayer
cation was changed. Use of Na+ montmorillonite or NH4+ montmorillonite resulted in
either a helical PEO or a bilayer zigzag PEO structure in the galleries. The PEO arrangement was reversible with exchange of the interlayer cations.
1.5.7 Rubber Matrices
Several applications of rubbers might benefit from inclusion of exfoliated clays. Their greatly reduced permeability [72] would be useful for the inner liners of tires and inner tubes. [73] In addition, modification of the glass transition temperature and/or the loss modulus might be useful in a variety of damping applications. Montmorillonite has been ion-exchanged with a protonated form of butadiene and acrylonitrile copolymer. This was subsequently mixed with nitrile butadiene rubber in the presence of crosslinking agents and resulted in highly dispersed nanocomposites. Nanocomposites have also been prepared from dioctadecyldimethyl ammonium- exchanged montmorillonite in poly(styrene-b-butadiene) matrices. [74]
1.5.8 Others
Clay/polymer nanocomposites that include poly(e-caprolactone) have been made via in-situ polymerization. Composites that include poly(p-pheylenevinylene) have been made via intercalation of poly(xylylenedimethylsulfonium bromide) and subsequent elimination of the dimethylsulfide and HBR. [75] Those including cyclic polycarbonate [76] or polyethyleneterephthalate have been made via monomer intercalation and subsequent polymerization; and those including polyaniline via in-situ polymerization of aniline monomer. [77]
1.6 Properties of Nanocomposites
1.6.1 Dimensional Stability
Dimensional stability is critical in many applications. For example, if the layers of a microelectronic chip have different thermal or environmental dimensional stabilities, then residual stresses can develop and cause premature failure. Poor dimensional stability can also cause warping or other changes in shape that affect the function of a material. Nanocomposites provide methods for improving both thermal and environmental dimensional stability. The possible mechanism by which nanofillers can affect the coefficient of thermal expansion (CTE) of a polymer has also been observed in traditional fillers.
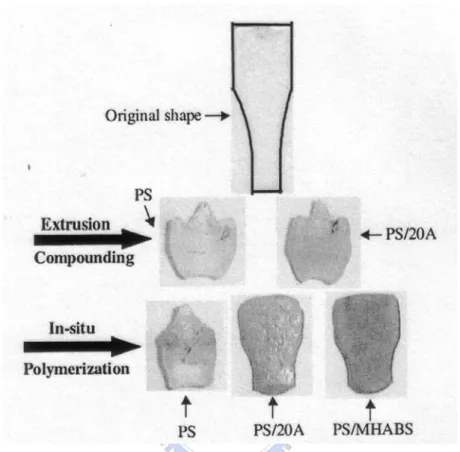
The dimension stability of nanocomposites was studied by Zeng and Lee. [58] Figure 1-17 shows the shape changes of injection molded PS and PS/clay nanocomposites under the aforementioned thermal cycle (50 oC, 1 h; 75 oC, 1 h; 105
oC, 1 h; and 135 oC, 1h). The original sample shape is shown in the first row. Pure PS
and the extruded PS/20A (dimethyl dehydrogenated tallow ammonium montmorillonite, 20A) nanocomposite are shown in the second row for comparison. The third row shows the in-situ polymerized pure PS, PS/20A, and PS/MHABS (2-methacryloyloxyethylhexadecyldimethylammonium bromide, MHABS) nanocomposites. All the nanocomposites contain 5 wt % clay. In the absence of clay, the sample shrank greatly, and the shape became highly irregular. Dimension stability at elevated temperature was improved significantly when 5 wt % of clay was present in the in-situ polymerized nanocomposites, as shown in the third row. The exfoliate PS/MHABS exhibited the best dimensional stability. After the heating cycle, although the sample shrank to a certain extent, the original shape and surface smoothness remained. It is noteworthy that the PS/20A nanocomposite prepared by extrusion
compounding did not show much improvement in dimension stability at elevated temperature, as compared to the in-situ polymerized PS/20A nanocomposite with the same clay content.
Figure 1-17. PS and PS/clay nanocomposites after dimension stability test. Clay loading is 5 wt % for all nanocomposites. [58]
1.6.2 Thermal Stability and Flammability
Delaminated composites have significantly higher degradation temperatures than intercalated nanocomposites or traditional clay composites [61]. Some speculate that this increase in stability is due to the improved barrier properties of the composites. If oxygen cannot penetrate, then it cannot cause oxidation of the resin [62]. In addition, the inorganic phase can act as a radical sink to prevent polymer chains from decomposing. The improved thermal stability of some composites may be limited by the lower thermal stability of alkylammonium ions. For example, in intercalated clay/polystyrene composites, the intercalating agent decomposes at about 250 oC. Bonding the intercalating ion to the polystyrene matrix noticeably improved the thermal stability.
Jin and co-worker investigated thermal property of polymer-clay nanocomposites by TGA and cone calorimetry. [21] The thermal stability of the nanocomposite is enhanced relative to that of virgin polystyrene and this is shown in Figure 1-18. Typically, the onset temperature of the degradation is about 50 oC higher for the nanocomposites than for virgin polystyrene.
One invariably finds that nanocomposites have a much lower peak heat release rate (PHRR) than the virgin polymer. The peak heat release rate for polystyrene and the three nanocomposites are also shown graphically in Figure 1-19. P16-3 means that the nanocompoite was formed using 3 % of P16 clay with polystyrene. The peak heat release rate falls as the amount of clay was increased. The suggested mechanism by which clay nanocomposites function involves the formation of a char that serves as a barrier to both mass and energy transport. [59] It is reasonable that as the fraction of clay increases, the amount of char that can be formed increases and the rate at which heat is released is decreased. There has been a suggestion that an intercalated material is more effective than is an exfoliated material in fire retardancy. [21]
Figure 1-19. Peak heat release rates for polystyrene and the three nanocomposites. [21]
The production of a char barrier must serve to retain some of the polymer and thus both the energy released and the mass loss rate decrease. The amount of smoke evolved, specific extinction area, also decreases with the formation of the
formation of the nanocomposite gives a reduction in smoke, however, the presence of additional clay does not decrease smoke.
1.6.3 Mechanical Properties
The cyclic deformation of PS/MMT nanocomposites as a function of temperature was measured by DMA. The temperature dependence of storage modulus and tanδ were shown in Figure 1-20 and 21, respectively. The storage modulus of PS/MMT nanocomposites were greater than that of pure PS and monotonically increased with the clay content in both the glassy and rubbery regions. However, the improvements in the rubbery region were much greater than those in the glassy region. This behavior indicates that the restricted segmental motions at the organic-inorganic interface are due to large aspect ratios of the clay platelets, and the polymer chains were also well confined inside the clay galleries at the nanoscale level. [63,64] The storage modulus of PS/MMT-3 was 1.2 times higher than that of pure PS, which is comparable to the earlier reported data (1.4 times improvement). [63] The Tgs of the
nanocomposites were estimated from the peak values of tanδ in Figure 1-18, which were shifted towards higher temperature with increasing the clay content. These results indicate that nanoscale clay platelets strongly restrict the polymer segmental motions, resulting in the significant increase in Tg. This improvement in Tg is higher
than those of other researchers even though the smaller clay content was used in this experiment. [65,66]
Figure 1-20. Storage modulus of (a) pure PS, (b) PS/MMT-1, (c) PS/MMT-2 and (d) PS/MMT-3.
Figure 1-21. Tanδ values of (a) pure PS, (b) PS/MMT-1, (c) PS/MMT-2 and (d) PS/MMT-3.
The effects of clay loadings on tensile properties of the PS/MMT nanocomposites are shown in Figure 1-22. The tensile strength and Young’s modulus were significantly enhanced in the presence of the small contents of clay, while the elongation at break was reduced with increasing the clay content. The increase in tensile strength was attributed to the stronger interfacial adhesion between PS and the clay platelets. However, the enhancement of modulus was reasonably ascribed to the high resistance exerted by the clay platelets against the plastic deformation and the stretching resistance of the oriented polymer backbones in the galleries. The improvement of tensile strength in PS/MMT-3 compared to pure PS was ~47%, which is greater than the earlier reported value in the literature (~21%) for PS/MMT nanocomposite with 3wt% MMT prepared by melt blending. [64] Similarly, the enhancement of Young’s modulus in PS/MMT-3 compared to pure PS was ~25%, which is much greater than the reported value (7.4% improvement for PS/MMT nanocomposite with 5wt% clay prepared by emulsion polymerization). [67] However, the elongations at break were reduced with increasing the clay content. Similar results were earlier reported. For example, the reduction of elongation at break in PS/MMT nanocomposite with 4.4wt% MMT prepared by melt blending was reported to ~26%. [63]
Figure 1-22. (a) Tensile strengths, (b) Young’s modulus and (c) elongations at break of PS/MMT nanocomposites. [68]
1.6.4 Gas barrier properties
Clays are believed to increase the barrier properties by creating a maze or “tortuous path” (Figure 1-23) that retards the progress of the gas molecules through the matrix resin. The direct benefit of the formation of such a path is clearly observed in polyimide/clay nanocomposites by dramatically improved barrier properties, with a simultaneous decrease in the thermal expansion coefficient. [78,79] The polyimide/layered silicate nanocomposites with a small fraction of OMLS exhibited reduction in the permeability of small gases, e.g. O2, H2O, He, CO2, and ethylacetate
vapors. [80] For example, at 2 wt % clay loading, the permeability coefficient of water vapor was decreased ten-fold with synthetic mica relative to pristine polyimide. By comparing nanocomposites made with layered silicates of various aspect ratios, the permeability was seen to decrease with increasing aspect ratio.
Figure 1-23 Formation of tortuous path in PLS nanocomposites.
Apparently, significant improvements in barrier properties are also achievable with nonplate-like nanoparticles. [81] Nano Material Inc. reports that a PVA/EVOH matrix composite with 7 nm silica and titania nanoparticles exhibits a gas permeability of 1 cc m-2 d-1 atm-1 and moisture permeability of less than 1 g m-2 d-1. Although this is achieved at very high loadings, the material is melt processable.
The absorption of water into composites is significant. For example, one of the limitations of Nylon is the reduction in mechanical properties that accompanies the
absorption of water. The addition of exfoliated montmorillonite increases the resistance to water permeation after 30 min from 2% to 1% at 5 wt. % of filler. [82] The mechanism of the reduction is attributed to the constrained region of the Nylon. If the constrained region is taken into account, the diffusion coefficient follows a rule of mixtures. Figure 1-24 shows the change in diffusion coefficient of water in Nylon in response to clay content.
Figure 1-24 Dependence of diffusion coefficient of water on clay content for montmorillonite with a layer width of 100 nm, and saponite with a layer width of 50
nm. [82]
1.6.5 Electrical and Optical Properties
The electrical and optical properties of nanofilled polymers are exciting areas of research. This is particularly true because of the possibility of creating composites with unique combinations of functionalities, such as electrically conducting composites with good wear properties that are optically clear. Such properties can result because nanoparticles, with diameters distinctly below the Rayleigh scattering limit, still display their solid-state physical properties when embedded in transparent matrices.
active nanoparticles embedded in a transparent host material, often a polymer. Optical composites take advantage of the optical properties of materials that are hard to grow in single-crystal form or that require protection from the environment and give them the ease of processing afforded many polymers. In addition, sometimes the material must be used at the nanoscale to achieve specific optical properties, and the matrix is used just to hold the particles together and provide processability. For example, high-grade optical composites, with properties otherwise obtainable only in optical glasses, become accessible through the use of polymer molding techniques.
1.7 Summary
The nanocomposite presented here is a composite material reinforced with silicate sheets. Silicate sheet is an ultrafine filler of nanometer size, which is almost equal to the size of the matrix polymer. Although the content of the filler is as little as several wt%, individual filler particles exist at a distance as close as tens of nanometers from each other because of their ultrafine size. One end of the polymer is strongly restrained to the silicate sheet by polar interaction. Thus, the nanocomposite has a microstructure that has never been seen in conventional composites. The characteristic properties of the nanocomposite are derived from this very structure. Considering the properties, the nanocomposite may be, in a sense, an embodiment of the ideal polymer composite, or a completely novel composite. Silicate sheet can be regarded as a rigid inorganic polymer. In this sense, the nanocomposite realized is a molecular composite in which a silicate sheet is used instead of an organic rod-like polymer.
References
[1] Blumstein, A. Bull. Chim. Soc. 1961, 899.
[2] Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. J. Mater. Res. 1993, 8, 1179.
[3] Lan, T.; Pinnavaia, T. J. Chem. Mater. 1994, 6, 2216.
[4] Usuki, A.; Kato, M.; Okada, A.; Kurauchi, T. J. App. Polym. Sci. 1997, 63, 137. [5] Jeon, H. G.; Jung, H. T.; Lee, S. D.; Hudson, S. Polymer Bulletin 1998, 41, 107. [6] Giannelis, E. Adv. Mater. 1996, 8, 29.
[7] Fisher, H.; Gielgens. L.; Koster, T. Nanocomposites from Polymers and Layered Minerals; TNO-TPD Report, 1998.
[8] Carrado, K. A.; Langui, X. Microporous Mesoporous Mater. 1999, 27, 87. [9] Friedlander, H. Z.; Grink, C. R. J. Polym. Sci., Polym. Lett. 1964, 2, 475. [10] Blumstein, A. J. Polym. Sci., Part A. 1965, 3, 2653.
[11] Kato, C.; Kuroda, K.; Takahara, H. Clay and Clay Minerals 1981, 29, 294. [12] Kelly, P.; Moet, A.; Qutubuddin, S. J. Mater. Sci. 1994, 29, 2274.
[13] Akelah, A.; Kelly, P.; Qutubuddin, S.; Moet, A. Clay Minerals 1994, 29, 169. [14] Akelah, A.; Moet, A. J. Mater. Sci. 1996, 31, 3189.
[15] Akelah, A.; Moet, A. Mater. Lett. 1993, 18, 97. [16] Giannelis, E. P.; Adv. Mater. 1996, 8, 29.
[17] Vaia, R.; Ishii, H.; Giannelis, E. Chem. Mater. 1993, 5, 1694. [18] Doh, J. G.; Cho, I. Polymer Bulletin 1998, 41, 511.
[19] Takekoshi, T.; Fouad, F.; Mercx, F. P. M.; De Moor, J. J. M. US patent 5,773,502. Issued to General Electric Co., 1998.
[20] Okada, K. (Sekisui) Japan Patent 11-228748, 1999.
[21] Zhu, J.; Alexander, B. M.; Frank, J. L.; Charles, A. W. Chem. Mater. 2001, 13, 3774
[23] Dongyan, W.; Charles, A. W. Polym. Deg. Stab. 2003, 82, 309. [24] Fu, X.; Qutubuddin, S. Polymer 2001, 42, 807.
[25] Lin, J. J.; Cheng, I. J.; Chou, C. C. Macromol. Rapid Commun. 2003, 24, 492. [26] Yei, D. R.; Kuo, S. W.; Fu, H. K.; Chang, F. C. Polymer 2005, 46, 741.
[27] Yei, D. R.; Kuo, S. W.; Su, Y. C.; Chang, F. C. Polymer 2004, 45, 2633. [28] Tseng, C.R.; Wu, J.Y.; Lee, H.Y.; Chang, F.C. Polymer 2001; 42: 10063.
[29] Tseng, C.R.; Lee, H.Y.; Chang, F.C. J. Polym Sci Part B: Polym Phys 2001; 39: 2097.
[30] Chen, H. W.; Chiu, C. Y.; Chang, F.C. J. Polym Sci Part B: Polym Phys 2002; 40: 1342.
[31] Tseng, C.R.; Wu, J.Y.; Lee, H.Y.; Chang, F.C. J Appl Polym Sci 2002; 85: 1370. [32] Tseng, C.R.; Wu, H.D.; Wu, J.Y.; Chang, F.C. J Appl Polym Sci 2002; 86: 2492. [33] Weimer, M. W.; Chen, H.; Giannelis, E. P.; Sogah, D. Y. J. Am. Chem. Soc. 1999,
121, 1615.
[34] Zhou, Q.; Fan, X.; Xia, C.; Mays, J.; Advincula, R. Chem. Mater. 2001, 13, 2465.
[35] Fan, X.; Zhou, Q.; Xia, C.; Crsitopholi, W.; Mays, J.; Advincula, R. C. Langmuir 2002, 18, 4511.
[36] Fan, X.; Xia, C.; Advincula, R. C. Langmuir 2003, 19, 4381.
[37] Meier, L.; Shelden, R.; Caseri, W.; Suter, U. Macromolecules 1994, 27, 1637. [38] Pruker, O.; Ruhe, J. Macromolecules 1998, 31, 592.
[39] Pruker, O.; Ruhe, J. Macromolecules 1998, 31, 602.
[40] Yano, K.; Usuki, A.; Okada, A.; Kuraychi, T.; Kamigaito, O. Polym. Prepr. 1991, 32, 65.


![Figure 1-16 Schematic of the synthesis of polyimide-clay hybrid film. [40]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8360226.176784/41.892.235.660.576.945/figure-schematic-synthesis-polyimide-clay-hybrid-film.webp)
![Figure 1-19. Peak heat release rates for polystyrene and the three nanocomposites. [21]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8360226.176784/49.892.210.680.541.907/figure-peak-heat-release-rates-polystyrene-nanocomposites.webp)