468
M
embers of the family Pomacanthidae, which contain at least 88 species, are generally known as angelfishes and are associated with coral reefs around the world. The blue-striped angelfish Chaetodontoplus septentrionalis is distributed throughout the northwestern Pacific, north in Japan through southern Korea and south to Taiwan and Hong Kong (Debelius et al. 2003). It lives at 5-60 m in depth (Allen et al. 1998). In the post-larval to juvenile stage, the body of the fish is almost completely black with a yellow band behind the eye that disappears with growth. The fish are caught as both juveniles and adults for sale in the ornamental fish trade because of their colorful pattern. Individuals yield a commercialReproductive Mode of the Blue-striped Angelfish Chaetodontoplus
septentrionalis in Northeastern Taiwan
Kuan-Yu Chen1 and Wann-Nian Tzeng1,2,*
1Institute of Fisheries Science, College of Life Science, National Taiwan University, Taipei 106, Taiwan 2Department of Life Science, College of Life Science, National Taiwan University, Taipei 106, Taiwan (Accepted December 3, 2008)
Kuan-Yu Chen and Wann-Nian Tzeng (2009) Reproductive mode of the blue-striped angelfish
Chaetodontoplus septentrionalis in northeastern Taiwan. Zoological Studies 48(4): 468-476. The blue-striped angelfish Chaetodontoplus septentrionalis Temminck and Schlegel 1844 is a coral reef fish distributed from Hong Kong through Taiwan to southern Japan. This species has potential in the future aquaculture industry of marine ornamental fishes. However, fundamental knowledge of the reproductive biology of this species is scant. To understand its reproductive mode, the gonadosomatic index (GSI) and gonadal histology of the fish were examined based on 155 individuals collected monthly from the reef zone of Ilan County, northeastern Taiwan between Jan. 2003 and Jan. 2004. The GSI of females significantly increased from late spring to mid summer (May-July), and that of males increased earlier and declined later (Mar.-Aug.) than females. Seasonal changes in the ovarian maturity stage composition indicated that the peak spawning season of C. septentrionalis occurs in summer. The reproductive activity of C. septentrionalis drastically decreased when water temperatures exceeded 28°C. The histological analysis of the ovaries indicated that this fish is a partial spawner with synchronous occurrence of previtellogenic and vitellogenic oocytes and secondary oocytes during the spawning season. The total length of the fish ranges 143.1-215.4 mm. The mean length of males (180.7 ± 13.9 mm) is larger than that of females (172.8 ± 12.0 mm), and the proportion of males increased in large size classes indicating sexual size dimorphism in C. septentrionalis. The presence of a male with a few oogonia in its testes suggests that C. septentrionalis exhibits hermaphroditism. http://zoolstud.sinica.edu.tw/Journals/48.4/468.pdf Key words: Reproductive cycle, Gonadosomatic index, Gonadal histology, Hermaphroditism,
Blue-striped angelfish.
value of as high as US$200 for each individual in the international ornamental fish market. Although individuals are important commercially, biological information, such as the reproductive cycle, maturation, and sex ratio, is still little known.
Reproductive cycles of other species of angelfish have been studied in both the eastern and central Pacific Ocean regions. The king angelfish Holocanthus passer spawns from June to Aug. at Cueva de Leon in the Gulf of California (24°02'N, 110°24'W) (Arellano-Martinez et al. 1999). Similarly, Potter,s angelfish Centropyge
potteri in lower-latitude Hawaii (21°04'N) also
spawns seasonally, from Dec. to May (Collier et al. 2003). The reproductive cycle of a fish * To whom correspondence and reprint requests should be addressed. Tel: 886-2-33662887. Fax: 886-2-23639570.
E-mail:wnt@ntu.edu.tw
represents a seasonal pattern, and the duration of the reproductive period is longer at lower latitudes. The northern part of Taiwan is a subtropical area where water temperatures change seasonally, and the latitude (24°06'N) is similar to that of Cueva de Leon in the Gulf of California. We expected that the reproductive cycle of C. septentrionalis should also have a seasonal pattern and be influenced by seasonal changes in water temperature. To determine this, blue-striped angelfish were collected monthly, and its reproductive cycle was examined.
Moreover, sexual dimorphism is a well-known phenomenon in angelfishes, which exhibits pronounced sexual differences in size, shape, and coloration, particularly in the genus Genicanthus and several species of Centropyge. However, the sex of C. septentrionalis is not distinguishable from its appearance. Although mating and spawning behavior and sex change have been described for a few angelfish species (Lobel 1978, Moyer and Nakazono 1978, Bruce 1980, Bauer and Bauer 1981, Moyer 1984, Hourigan and Kelley 1985, Aldenhoven 1986, Lutnesky 1994, Arellano-Martinez et al. 1999, Hamaguchi et al. 2002), it remains unclear whether hermaphroditism exists in
C. septentrionalis. Little effort has been directed to
understanding the reproductive biology of marine angelfishes, whose sales are almost entirely generated from wild-caught specimens (Collier et al. 2003). Knowledge of the reproductive biology of C. septentrionalis is very important for its artificial propagation and for general knowledge of the species biology. The objective of this study was to understand the reproductive cycle and seasonal changes in gonadal development of this species.
MATERIALS AND METHODS
In total, 164 adult-colored blue-striped angelfish were collected at Nanfangao fish market in Ilan County, northeastern Taiwan during the period from Jan. 2003 to Jan. 2004. These specimens were by-catch and were caught in benthic gill nets of local fishing boats in coastal reef areas off the Ilan coast. The nets were deployed in waters around 40 m deep. The captured specimens were immediately frozen, and their gonads were examined within 2 wk. The total length (TL) and body weight of the fish were respectively measured to the nearest 0.1 mm and 0.01 g.
The reproductive cycle of the fish was determined from monthly changes in the gonadosomatic index (GSI) which was calculated as follows:
GSI = gonad weight (g)body weight (g) x 100%
Gonadal tissues were treated in the following stepwise process: (1) fixed in Bouins, solution overnight and then transferred to 70% alcohol; (2) dehydrated and cleared with a series of ethanol-xylene solutions and then embedded in paraffin; and (3) sections (8 μm) of the gonad were placed on slides and stained with hematoxylin and counterstained with eosin (West 1990). The development of oocytes was classified into 7 stages following Collier et al. (2003). In total, 41 histological sections of the ovaries of C.
septentrionalis were analyzed for ovarian maturity
and seasonal composition. The ovarian maturation status of the fish was divided into 5 stages, and temporal changes in the stage composition were represented by season. The monthly mean water temperature in the study area was calculated according to data measured by the Water Resources Agency, Ministry of Economic Affairs, Taipei, Taiwan. Correlations between the monthly mean water temperature and GSI of both male and female fish were analyzed by linear correlations.
The sex ratio was classified in length intervals of 20 mm, and a χ2 test of a 2 x K contingency
table was used to test whether the sex ratio of the fish differed from 1: 1. If it did, then a χ2 test was
further conducted for each length interval. The significance level was set to 0.05. The absolute weights of the testes or ovaries vs. the total length of the fish were plotted for spawning (Mar.-Aug. 2003) and non-spawning seasons (Jan. and Feb. 2003 and Sept. 2003-Jan. 2004) to understand differences in the maturation status between sexes and possible mating systems.
RESULTS The sex ratio
Among the examined 155 specimens, 91 were males (58.7%) and 64 were females (41.3%). The sex ratio of the overall specimens was 1.42: 1 which slightly significantly differed from the expected ratio of 1: 1 (χ2 = 4.703, p = 0.03).
However, the sex ratio did not significantly differ from 1: 1 for each month (p > 0.05) (Table 1).
The TL of the fish ranged 143.1-215.4 mm. The mean length of males (180.7 ± 13.9 mm) was larger than that of females (172.8 ± 12.0 mm). The distribution of the sex ratio for the different size classes indicated that the proportion of males increased in larger size classes (Fig. 1). The 2 x K contingency table of the χ2 test also indicated
that the sex ratio of the fish significantly differed
among size classes (χ2 = 12.1, p = 0.007) (Table 2).
The sex ratio of each size class further analyzed by the χ2 test also indicated that the larger
size classes (180-220 mm) were significantly dominated by males (χ2 = 5.25 and 6.4, both p <
0.05), and the sex ratios in the medium (160-180 mm) and smaller size class (140-160 mm) did not significantly differ from 1: 1 (χ2 = 1.05 and 3.77,
Table 1. Monthly changes in the sex ratio of the blue-striped
angelfish collected in northeastern Taiwan
Month No. of fish Sex ratio χ2 p
Male (M) Female (F) Total (M:F)
Jan. 2003 8 2 10 04:1 3.6 0.058 Feb. 0 1 1 Mar. 7 4 11 1.75:1 0.818 0.366 Apr. 6 7 13 01:1.8 0.077 0.782 May 3 2 5 1.5:1 0.2 0.655 June 10 5 15 02:1 1.667 0.197 July 8 8 16 01:1 0 1 Aug. 4 6 10 01:1.5 0.4 0.527 Sept. 21 12 33 1.75:1 2.455 0.117 Oct. 5 4 9 1.25:1 0.111 0.739 Nov. 9 7 16 1.29:1 0.25 0.617 Dec. 6 3 9 02:1 1 0.317 Jan. 2004 4 3 7 1.33:1 0.143 0.705 Total 91 64 155 1.42:1 4.703* 0.03
Fig. 1. Sex ratio changes with total length of Chaetodontoplus septentrionalis (n = sample size). 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 Sex ratio Total length (mm) 140-150 150-160 160-170 170-180 180-190 190-200 200-210 210-220 Female Male 30 39 16 7 3 9 4 = n 47
both p > 0.05) (Table 2).
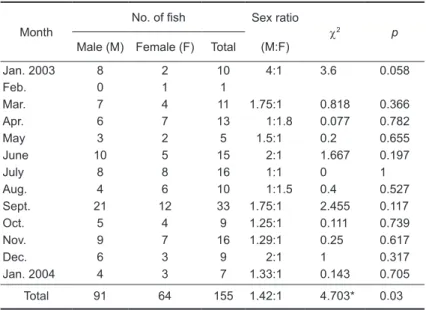
Monthly changes in the GSI
Temporal changes in the GSI slightly differed between males and females. GSI values for females remained below 2% during the period
from Jan. to Feb. Larger GSI values were then observed in Mar. and Apr. which varied 1.1%-4.9%. The GSI values were significantly higher from May until July (5.6%-8.1%), and then drastically decreased to as low as 3.3% in Aug. After that, GSI values for females remained < 1% until Jan. On the other hand, GSI values for males increased
Table 2. χ2 test of the sex ratio in 4 size classes. A 2 x K contingency
table of χ2 test results was used for all samples
Total length (mm) No. of fish Sex ratio p
Male (M) Female (F) Total (M:F)
140-160 3 10 13 0.3:1 3.77 0.052
160-180 43 34 77 1.26:1 1.05 0.305
180-200 36 19 55 1.89:1 5.25* 0.021
200-220 9 1 10 9:1 6.4* 0.011
Total (2 x K) 91 64 155 1.42:1 12.1* 0.007
Fig. 2. Monthly changes in the mean (± SD) gonadosomatic index (GSI) of Chaetodontoplus septentrionalis. (A) Females, (B) males. 0.3 0.25 0.2 0.15 0.1 0.05 0 2003 Jan.
Feb. Mar. Apr. May June July Aug. Sept. Oct. Nov. Dec. 2004 Jan. (B) GSI (%) 9 8 7 6 5 4 3 2 1 0 2003 Jan.
Feb. Mar. Apr. May June July Aug. Sept. Oct. Nov. Dec. 2004 Jan.
(A)
to a higher level in Mar. (0.19%), remained high until Aug. (0.2%), and decreased in Sept. (0.09%) (Fig. 2). Drastic changes in the GSI indicate that
C. septentrionalis spawns during summer in
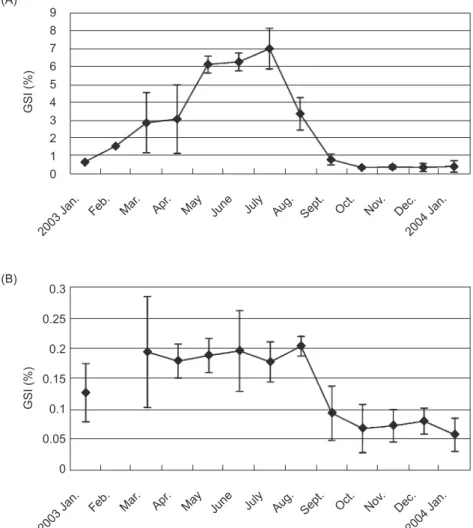
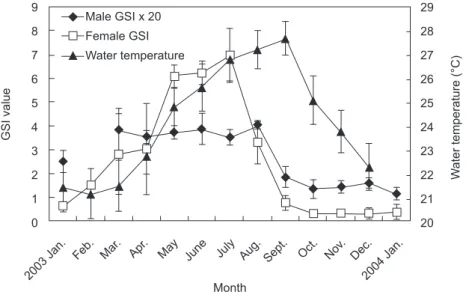
July-Aug. The mean water temperature was lowest in Feb. (20°C), began to increase in Apr., reached the highest in Sept. (28°C), and then continuously decreased from Oct. (Fig. 3). The GSI and water temperature were positively correlated for both sexes. Correlation coefficients of the GSI and water temperature were 0.0027 and 0.41 for males and females, respectively. However, there was no significant correlation (p > 0.05) between the GSI of either sex and water temperature.
Oocyte development
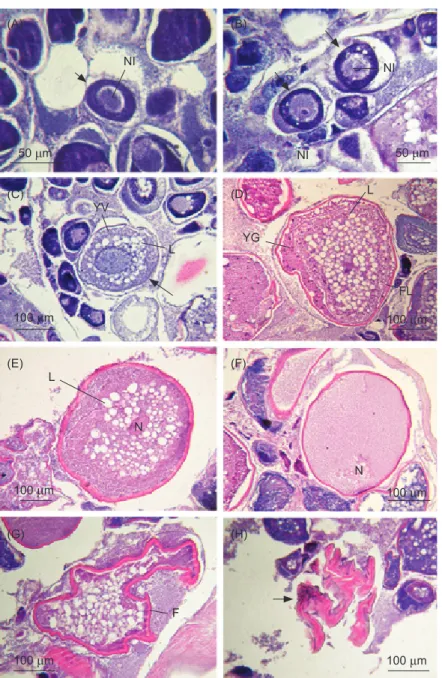
The development of oocytes in the ovaries of female C. septentrionalis was classified into 7 stages (Fig. 4). (1) In the chromatin nucleolus stage (Fig. 4A), oocytes were 15- 70 μm in diameter. The cytoplasm of oocytes was dark purple with hematoxylin staining, and more basophilic nuclei occurred. One or more darkly stained nucleoli were visible. (2) In the perinucleolus stage (Fig. 4B), oocytes were 50- 110 μm. The oocytes became larger than those in the chromatin nucleolus stage with strongly basophilic cytoplasm. The nucleus was less basophilic and had multiple nucleoli located centrally and peripherally. (3) In the yolk vesicle stage (Fig. 4C), oocytes were 100-200 μm. The cytoplasm was still basophilic, and began to
produce lipid droplets that assembled around the periphery of the nucleus. A thin follicle layer was observed surrounding the oocyte. (4) In the early vitellogenesis stage (Fig. 4D), oocytes were 190-350 μm. Eosinophilic yolk granules occurred around the periphery of the cytoplasm which made the cytoplasm become less basophilic. Lipid droplets continued to aggregate around the nucleus. The follicle layer became thicker than that in the yolk vesicle stage, and the zona radiata became clear. (5) In the late vitellogenesis stage (Fig. 4E), oocytes were 300-450 μm. Yolk granules, nearly filling the interior of oocytes, continued to be deposited which made the cytoplasm become entirely eosinophilic, and it began to fuse into larger globules. Lipid droplets also began to fuse around nuclei. (6) In the final maturation stage (Fig. 4F), oocytes were 400-650 μm. Yolk granules and globules fused together to make oocytes which appeared wavy and fluid-like. The coalescence of lipid droplets formed one large, centrally located lipid drop. The nucleus migrated towards the periphery and broke down. During hydration, the cytoplasm became strongly translucent. Just prior to ovulation, the egg membrane separated from the follicle. (7) In the postovulation stage (Fig. 4G), the ovary was composed of many postovulatory follicles, immature oocytes, and unspawned mature eggs. The egg follicle remained (Fig. 4H) in the ovarian lamellae after hydrated oocytes had ovulated into the ovarian lumen. It appeared as a hollow mass
Fig. 3. Monthly changes in the gonadosomatic index (GSI) of both males and females and mean water temperature. The male GSI was multiplied by 20. 9 8 7 6 5 4 3 2 1 0 Male GSI x 20 Female GSI Water temperature 2003 Jan.
Feb. Mar. Apr. May June July Aug. Sept. Oct. Nov. Dec. 2004 Jan. 29 28 27 26 25 24 23 22 21 20 W ater temperature ( °C ) GSI value Month
of granulose cells that seemed to be crumpled inwards.
Development of the testes
Testes of C. septentrionalis appeared pale pink, and exhibited a lamellar structure during active reproduction, but were thread-like at rest. The spermatogenic tissue was strongly basophilic. One specimen (with a TL of 170 mm) was found to
have few oogonia within an asophilic mature testis (Fig. 5).
Seasonal changes in the ovarian maturity stage composition
Ovarian maturity of C. septentrionalis was divided into the following 5 stages. (1) In the resting stage, the ovary had predominant oocytes with chromatin nucleoli and perinucleolus stage
Fig. 4. Seven developmental stages of oocytes in the ovary of Chaetodontoplus septentrionalis. (A) Chromatin nucleolus stage, (B) perinucleolus stage, (C) yolk vesicle stage, (D) early vitellogenesis stage, (E) late vitellogenesis stage, (F) final maturation stage, (G) postovulation stage with a follicle, and (H) postovulation stage with an atretic oocyte. NI, nucleolus; YV, yolk vesicle; L, lipid droplet; YG, yolk granule; FL, follicle layer; N, nucleus; F, follicle.
NI (A) (B) (C) (D) (E) (F) (G) (H) NI NI YV L L L N F N YG FL 50 μm 100 μm 100 μm 100 μm 100 μm 100 μm 100 μm 50 μm
oocytes. (2) In the developing stage, vitellogenic oocytes occurred in the ovary. Oocytes at the yolk vesicle, early, and late vitellogenesis stage were dominant. (3) In the ripe stage, the composition of oocytes was similar to that in the developing stage, but more mature oocytes were present. (4) In the spawning stage, the appearance was similar to the ripe stage but the distinctive characteristic at this stage was the presence of postovulatory follicles. (5) In the spent stage, the abundance of early oocytes had increased. Vitellogenic and mature oocytes had been reabsorbed, and only abundant follicles remained.
Seasonal variations in the ovarian maturity stage composition of C. septentrionalis are
shown in figure 6. In spring (Mar.-May 2003), 50%, 33.33%, and 16.67% of ovaries were in the resting, developing, and ripe stages, respectively. In summer (June-Aug. 2003), ovaries of the fish were dominated by the spawning stage (46.15%) and ripe stage (23.08%), with both the developing and spent stages representing 15.38% each. In autumn (Sept.-Nov. 2003), most ovaries of the fish were in the spent stage (54.56%), with the spawning and resting stages respectively representing 18.18% and 27.27%. In winter (Dec. 2003-Jan. 2004), ovaries of the fish were all in the resting stage (100%). This indicates that the peak spawning season of C. septentrionalis occurs in summer.
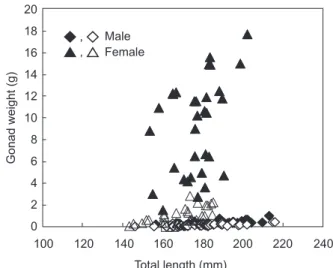
The relationship between gonadal weight and TL of C. septentrionalis indicated that the relative weights greatly differed between testes and ovaries (Fig. 7). Weights of the testes increased from < 0.1 g in the non-spawning season to 1.0 g in the spawning season, while weights of the ovaries increased from < 0.1 g in the non-spawning season to 18.0 g in the spawning season.
DISCUSSION
Sex change behavior has been well studied in the genus Centropyge of smaller angelfishes. At least some, and possibly all, species in that genus are protogynous (female to male) hermaphrodites (Thresher 1984, Sakai et al. 2003a b). In all investigated species of Centropyge, males appear to be derived from females. This leads to the Fig. 5. Basophilic spermatogenic tissue (T) and oogonium
(dark purple) in a mature testis of an individual at 170 mm in total length. The inset photo shows a magnified oogonium (O), primary spermatocyte (PSc), and secondary spermatocyte (SSc). 50 μm PSc SSc O O T 100 μm
Fig. 6. Seasonal variations of the ovarian maturity stage composition of Chaetodontoplus septentrionalis.
100 90 80 70 60 50 40 30 20 10 0 Relative frequency (%) Spent Spawning Ripe Developing Resting
Spring Summer Autumn Winter
Fig. 7. Relationship between gonadal weights and total lengths for male and female Chaetodontoplus septentrionalis (solid marks, spawning season; open marks, non-spawning season).
20 18 16 14 12 10 8 6 4 2 0 Total length (mm) 240 220 200 180 160 140 120 100 Male Female Gonad weight (g) ◆ , ◇ ▲ , △
size at first maturity of the species being larger in males than females (Moyer and Nakazono 1978, Randall and Yasuda 1979). A larger body size of males is one of the characters of protogynous hermaphroditism among many reef fishes (Warner 1988, Sadovy 1996, Jennings et al. 2001). It was also found that larger individuals of Holacanthus
passer are males (Arellano-Martinez et al. 1999).
Although its sex change behavior is still unknown, the larger body size of males of C. cententrionalis indicates sexual dimorphism.
The sex ratio of C. septentrionalis among the 8 different size classes indicated that > 50% of the fish were males at a TL of > 160 mm, and 100% were males at a TL of > 202 mm. The sex ratio analyzed by the χ2 test in the 4 size
classes also showed that the number of males was significant greater than that of females in the 180-220 mm size class but was not significant in the 140-180 mm size class. The percentages of males increased with TL, which coincides with the characteristic of protogynous hermaphroditism among other reef fishes. One individual at 170 mm in TL found to have both testicular and ovarian tissues indicates that hermaphroditism possibly exists in this species. However, more histological evidence is needed to validate whether protogyny exists in the species, or males are derived from a bisexual gonad. Moreover, the result of the larger body size of males suggests possible sexual size dimorphism in C. septentrionalis.
In general, some indications showed that weights of both testes and ovaries increase with body size in group spawners. However, weights of ovaries are usually much larger than
those of testes in haremic fishes. The pattern of gonadal weights against TL for each sex in
C. septentrionalis was similar to that of haremic
species. One male having many partners could result in a population with more females. In this study, however, the total number of males was larger than that of females. This result may have been caused by size-selective fishing gear, with gill nets meant to take larger species capturing larger individuals, and less likely taking smaller size classes that are dominated by females. Therefore, smaller fish that are supposedly dominated by females were less often caught.
The GSI of female C. septentrionalis rapidly increased from May and reached a peak in July. This indicates that the spawning season of the fish is in mid-summer. The coastal waters of subtropical Taiwan are influenced by the prevailing northeasterly trade winds. Water temperatures in the studied area of Ilan County significantly increased from Apr. and reached a peak in Sept. (Fig. 3). The linear correlation analysis showed that the positive but insignificant correlation between water temperatures and GSI values was due to the water temperature beginning to decrease 1 and 2 months later than the GSI values of males and females, respectively. This indicates that the highest water temperature (of > 28°C) might not be the optimal temperature for reproduction by C. septentrionalis.
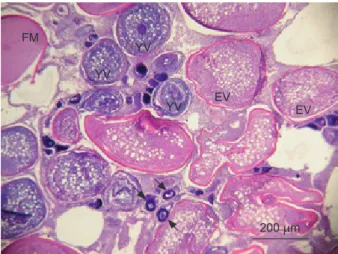
In terms of gonad development, mature females during the spawning season were found to have oocytes of different stages in the same section (Fig. 8). This strongly suggests that
C. septentrionalis spawns multiple times in a
season. A clear developing-stage oocyte was found in an ovary with follicles in the postovulation stage, which may validate C. septentrionalis being a partial spawner. Although the GSI is a suitable index to provide information about annual trends in the reproductive activity, it has some disadvantages for determining the maturation status since similar GSI values can be determined for 2 different stages of gonad development (e.g., resting and spent). In this study, the GSI values in autumn and winter were similar, but the ovarian histology sections indicated that in autumn, more than 50% of ovaries were in the spent stage; while in winter, 100% of ovaries were in the resting stage. Seasonal changes in ovarian maturity stage compositions indicate that C. septentrionalis ripens in spring and early summer, and spawns in mid-summer. This result agrees with the annual reproductive activity indicated by the GSI.
Fig. 8. A mature ovary containing oocytes of different stages. Arrow, perinucleolus stage; YV, yolk vesicle stage; EV, early vitellogenesis stage; FM, final maturation stage.
FM
YV YV
YV EV EV
In conclusion, according to the GSI and ovarian maturity stage composition, C.
septentrionalis might spawn in a single season
annually, and its reproductive activity might peak in summer. The reproductive activity of
C. septentrionalis drastically decreases when
the water temperature exceeds 28°C. Ovarian histological results showed that C. septentrionalis is a partial spawner, which indicates that each female individual likely spawns more than once in a reproductive season. The sex ratio among different size classes and the histology indicated that C. septentrionalis may exhibit hermaphroditism and sexual size dimorphism.
Acknowledgments: This study was financially
supported by the National Science Council of Taiwan (NSC95-2313-B-002-016). We are grateful to Mr. and Mrs. Chu in the Nanfangao Fish Market for kindly providing us with samples, and Mr. S.G. Chen for assisting with delivery of the samples. We also thank Dr. Y.S. Han and 2 anonymous reviewers for their valuable comments on early drafts of the manuscript.
REFERENCES
Aldenhoven JM. 1986. Different reproductive strategies in a sex-changing coral-reef fish Centropyge bicolor (Pomacanthidae). Aust. J. Mar. Freshw. Res. 37: 353-360.
Allen GR, R Steen, M Allen. 1998. A guide to angelfishes and butterflyfishes. Odyssey Publishing (USA)/Tropical Reef Research (Aust.).
Arellano-Martinez M, BP Ceballos-Vazquez, F Garcia-Dominguez. 1999. Reproductive biology of the king angelfish Holacanthus passer Valenciennes 1846 in the Gulf of California, Mexico. Bull. Mar. Sci. 65: 677-685. Bauer JA, SE Bauer. 1981. Reproductive biology of pigmy
angelfishes of the genus Centropyge (Pomacanthidae). Bull. Mar. Sci. 31: 495-513.
Bruce RW. 1980. Protogynous hermaphroditism in 2 marine angelfishes. Copeia 2: 353-355.
Collier JT, T Kaneko, T Hirano, EG Grau. 2003. Seasonal changes in reproductive activity in the Potter,s angelfish (Centropyge potteri) in Kaneohe Bay, Hawaii. Environ. Biol. Fishes 68: 49-57.
Debelius H, H Tanaka, RH Kuiter. 2003. Angelfishes - a comprehensive guide to Pomacanthidae. Chorleywood, UK: TMC Publishing.
Hamaguchi Y, Y Sakai, F Takasu, N Shigesada. 2002. Modeling spawning strategy for sex change under social control in haremic angelfishes. Behav. Ecol. 13: 75-82. Hourigan TF, CD Kelley. 1985. Histology of the gonads and
observations on the social behavior of the Caribbean angelfish Holocanthus tricolor. Mar. Biol. 88: 311-322. Jennings S, MJ Kaiser, JD Reynold. 2001. Marine fisheries
ecology. Oxford UK: Blackwell Publishing.
Lobel PS. 1978. Diel, lunar, and seasonal periodicity in the reproductive behavior of a pomacanthid fish, Centropyge
potteri, and some other reef fishes in Hawaii. Pac. Sci.
32: 193-207.
Lutnesky MMF. 1994. Density-dependent protogynous sex-change in territorial haremic fishes-models and evidence. Behav. Ecol. 5: 375-383.
Moyer JT. 1984. Reproductive behavior and social organization of the pomacanthid fish Genicanthus lamarck at Mactan Island, Philippines. Copeia 1984: 194-200. Moyer PB, A Nakazono. 1978. Population structure
reproductive behavior and protogynous hermaphroditism in the angelfish Centropyge interruptus at Miyake-jima, Japan. Jpn. J. Ichthyol. 25: 25-39.
Randall JE, J Yasuda. 1979. Centropyge shepardi, a new angelfish from the Mariana and Ogasawara Islands. Jpn. J. Ichthyol. 26: 55-61.
Sadovy YJ. 1996. Reproduction of reef fishery species. In Polunin VC, Roberts CM, eds. Reef fisheries. London: Chapman and Hall, pp. 15-59.
Sakai Y, K Karino, T Kuwamura, Y Nakashima, Y Maruo. 2003a. Sexually dichromatic protogynous angelfish
Centropyge ferrugata (Pomacanthidae) males can change
back to females. Zool. Sci. 20: 627-633.
Sakai Y, C Tsujimura, Y Nakata, H Tanabe, G Maejuma. 2003b. Rapid transition in sexual behaviors during protogynous sex change in the haremic angelfish Centropyge vroliki (Pomacanthidae). Ichthyol. Res. 50: 30-35.
Thresher RE. 1984. Reproduction in reef fishes. Neptune City, NJ: TFH Publications.