Curcumin Inhibits Human Lung Large Cell
Carcinoma Cancer Tumour Growth in a Murine
Xenograft Model
Chin-Cheng Su,1† Jai-Sing Yang,2† Chi-Cheng Lu,3 Jo-Hua Chiang,3 Chang-Lin Wu,4 Jen-Jyh Lin,5,6 Kuang-Chi Lai,7,8 Te-Chun Hsia,8 Hsu-Feng Lu,9,10 Ming-Jen Fan11 and Jing-Gung Chung4,11*
1Division of General Surgery, Buddhist Tzu Chi General Hospital, Tzu Chi University, Hualien 970, Taiwan 2Department of Pharmacology, China Medical University, Taichung 404, Taiwan
3Department of Life Sciences, National Chung Hsing University, Taichung 402 Taiwan
4Departments of Biological Science and Technology, China Medical University, Taichung 404, Taiwan 5Division of Cardiology, China Medical University Hospital, Taichung 404, Taiwan
6Department of Medicine, China Medical University Hospital, Taichung 404, Taiwan
7Department of Surgery, China Medical University Beigang Hospital, Beigang Township, Yunlin 651, Taiwan 8Department of Internal Medicine, China Medical University Hospital, Taichung 404, Taiwan
9Department of Clinical Pathology, Cheng Hsin Rehabilitation Medical Center, Taipei 112, Taiwan 10College of Human Ecology, Fu-Jen University, Taipei 510, Taiwan
11Department of Biotechnology, Asia University, Taichung 431, Taiwan
Curcumin can decrease viable cells through the induction of apoptosis in human lung cancer NCI-H460 cells
in vitro. However, there are no reports that curcumin can inhibit cancer cells in vivo. In this study, NCI-H460
lung tumour cells were implanted directly into nude mice and divided randomly into four groups to be treated with vehicle, curcumin (30 mg/kg of body weight), curcumin (45 mg/kg of body weight) and doxorubicin (8 mg/kg of body weight). Each agent was injected once every 4 days intraperitoneally (i.p.), with treatment starting 4 weeks after inoculation with the NCI-H460 cells. Treatment with 30 mg/kg and 45 mg/kg of curcumin or with 8 mg/kg of doxorubicin resulted in a reduction in tumour incidence, size and weight compared with the control group. The fi ndings indicate that curcumin can inhibit tumour growth in a NCI-H460 xenograft animal model in vivo. Copyright © 2010 John Wiley & Sons, Ltd.
Keywords: curcumin; human lung NCI-H460 cancer cells; xenograft transplantation; in vivo.
INTRODUCTION
In Taiwan, about 32.8 and 32.5 persons per 100 thou-sand die annually from lung and liver cancer respec-tively, based on reports from the People’s Health Bureau of Taiwan in year 2006 (Department of Health, Executive Yuan, R.O.C. (Taiwan) Taipei; http:// www.doh.gov.tw/EN2006/index_EN.aspx.). Currently, the treatment of these cancers involves radiotherapy, chemotherapy, or a combination of both, but mortality in both types of cancer patient remains high. Many studies have shown that certain phytochemicals can act as chemopreventive or chemotherapeutic agents in human cancer and many prescription drugs in use for cancer treatment are derived from plants (Craig, 1997; Kucuk, 2002).
Curcumin (diferuloylmethane), a phenolic compound obtained from turmeric, the rhizome of Curcuma longa (L.), is commonly used in food (Huang et al., 1998). It has been reported that curcumin inhibits cell
prolifera-tion, induces apoptosis and has antitumour activity in many human cancer cell lines, including non-small cell lung cancer cells (Kawamori et al., 1999; Rao et al., 1995; Verma et al., 1997). Curcumin also exhibits anticancer activities in vitro and in vivo in leukemia WEHI-3 cells (Gajate et al., 2003; Su et al., 2008) and has been shown to act by regulating a variety of antitumour signalling pathways (Kuttan et al., 2007; Lin, 2007). It has been suggested that curcumin acts as an oral cancer preventa-tive agent (Sharma et al., 2004) although few in vivo studies have yet been reported. The present study focused on the anticancer effect of curcumin in vivo in mice, using a human lung cancer xenograft model of NCI-H460 cells.
MATERIALS AND METHODS
Chemicals. Curcumin and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical (St Louis, MO, USA).
Cell culture. Human lung large cell carcinoma cancer NCI-H460 cells were obtained from the Food Industry * Correspondence to: Jing-Gung Chung, Department of Biological
Science and Technology, China Medical University, No 91, Hsueh-Shih Road, Taichung 404, Taiwan.
E-mail: jgchung@mail.cmu.edu.tw (J.-G. Chung) †Both authors contributed equally.
Received 10 January 2009 Revised 31 March 2009
Copyright © 2010 John Wiley & Sons, Ltd. Accepted 28 April 2009
PHYTOTHERAPY RESEARCH
Phytother. Res. 24: 189–192 (2010)
Published online 13 January 2010 in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/ptr.2905
Copyright © 2010 John Wiley & Sons, Ltd. Phytother. Res. 24: 189–192 (2010)
190 C.-C. SU ET AL.
Research and Development Institute (Hsinchu, Taiwan), and maintained at 37 ºC in a humidifi ed 5% CO2 and 95% air in RPMI-1640 medium (Gibco-BRL,
Grand Island, NY, USA) supplemented with 10% FBS (Hyclone Laboratories, Logan, UT, USA), 1% penicillin–streptomycin (100 units/mL penicillin and 100 μg/mL streptomycin) and 2 mm l-glutamine.
In vivo NCI-H460 tumour xenograft model. Female athymic nude (BALB/cnu/nu mice) were obtained from
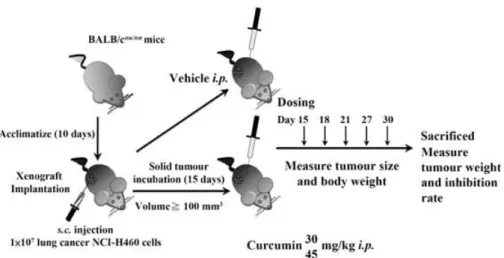
Laboratory Animal Center of National Applied Research Laboratories (Taipei, Taiwan). All animals were maintained in standard vinyl cages with air fi lter tops in a fi ltered laminar air fl ow room at 25 ºC on a 12 h light/dark cycle; water and food were autoclaved and provided. The experimental design for this study is shown in Fig. 1.
NCI-H460 cells (1 × 107) in RPMI-1640 medium were
injected subcutaneously into the fl anks of mice. Tumour-bearing mice were then divided randomly into treat-ment groups (six mice per group) and treattreat-ment initiated when the xenografted solid tumours reached a volume of about 100 mm3. Each mouse was injected i.p. every
3 days with either 30 μL of control vehicle (DMSO), curcumin (30 and 45 mg/kg) or doxorubicin (8 mg/kg). All experiments were conducted according to institu-tional guidelines and approved by the Animal Care and Use Committee of the China Medical University, Taichung, Taiwan. The doses of curcumin (30 and 45 mg/kg) used here are close to those used in other reports, for example human PC-3 prostate cancer (Khor et al., 2006) and pancreatic cancer (Kunnumakkara et al., 2007) xenografts in immunodefi cient mice.
After xenograft transplantation, mice exhibiting tumours were monitored and tumour size was measured once every 3 days using calipers. The tumour volume in each animal was estimated according to the formula: tumour volume (mm3) = L × W2/2 (where L is the length
and W is the width) with the fi nal measurement taken 4 weeks after tumour cell inoculation. At the same time, the body weight of each animal was measured once every 3 days, although they were more frequently checked during the fi rst 3 weeks to monitor potential drug-related toxicity. At the end of the experiment (4 weeks after cell inoculation), the animals were
anaes-thetized by CO2 and killed. Tumours from each animal
were removed, measured and weighed individually (Kuo et al., 2006; Yang et al., 2008).
Statistical analysis. Each value represents mean ± SD. The control and experimental animal groups were com-pared by Student’s t-test, ***p < 0.001 was considered signifi cant.
RESULTS AND DISCUSSION
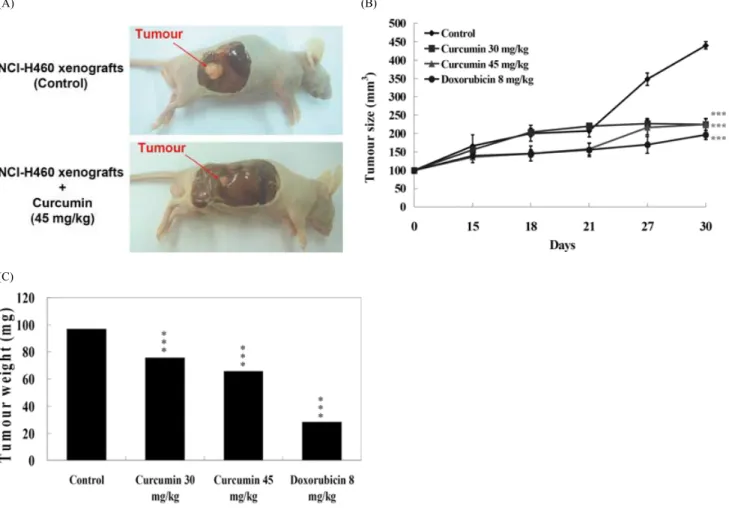
The results indicated that curcumin and doxorubicin decreased tumour size signifi cantly. An illustration of a representative animal treated with curcumin relative to the control is shown in Fig. 2A. Curcumin treatment decreased signifi cantly both tumour volume (Fig. 2B) and tumour weight (Fig. 2C) compared with the control. The percentage inhibition of each is shown in Table 1. None of the treatments, i.e. vehicle (DMSO), 30 mg/kg curcumin, 45 mg/kg curcumin or 8 mg/kg doxorubicin, altered the body weight signifi cantly (data not shown). All tumours appeared only at the inoculation sites.
Based on these in vivo experiments, it can be seen that curcumin at 30 mg/kg can inhibit tumour growth in a NCI-H460 xenograft mice model. However, other inves-tigators have shown that in human clinical trials, cur-cumin can safely be administered at doses up to 10 g/day. When given at 8 g/day, the serum concentration of curcumin was 1.77 ± 1.87 μmol/L, and there was no indi-cation of dose-limiting toxicity (Cheng et al., 2001). In the present study, serum concentrations of curcumin
Figure 1. Experimental design of the xenograft animal model. Animals are implanted s.c. with NCI-H460 cells and when the tumour
volume is around 100 mm3, randomly divided into four groups and treated as described in Materials and Methods.
Table 1. Inhibitory effect of curcumin on growth of H460 tumour xenografts in BALB/cnu/nu mice
Treatment Tumour weight (g) Inhibition (%) Control 0.097 – Curcumin 30 mg/kg 0.076 21.60 Curcumin 45 mg/kg 0.066a 31.96
Doxorubicin 8 mg/kg 0.028a 71.13
Doxorubicin and curcumin groups were compared and analysed using Student’s t-test. a p < 0.001.
CURCUMIN INHIBITS THE GROWTH OF LUNG CANCER NCI-H460 CELLS IN VIVO 191
Copyright © 2010 John Wiley & Sons, Ltd. Phytother. Res. 24: 189–192 (2010)
and its metabolites were not measured, but despite the low bioavailability of curcumin, tumours in mice that received curcumin alone were about 55% smaller than those of the control group (Fig. 2B).
Even in the curcumin treatment (30 and 45 mg/kg) and doxorubicin (8 mg/kg) groups, tumours continued to grow slowly compared with the control group, indi-cating that complete regression of NCI-H460 cells xeno-grafts was not achieved using a single treatment agent, suggesting that multiple treatments may be necessary to achieve a complete response. However, several recent reports have shown that combinations of curcumin with other agents can produce enhanced effects. Curcumin and genistein show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides (Verma et al., 1997), and curcumin and phenethyl isothiocyanates, either alone or in com-bination, possess signifi cant cancer-preventive activities
(A) (B)
(C)
Figure 2. Effect of treatment on tumour growth. (A) Illustration of a representative tumour after treatment with curcumin and control.
(B) The effect of curcumin and doxorubicin on tumour size. (C) The effect of curcumin and doxorubicin tumour weight. Data presented are the mean ± SD at 10–34 days post-tumour implantation; groups were compared and analysed using Student’s t-test. *** p < 0.001.
REFERENCES
Cheng AL, Hsu CH, Lin JK et al. 2001. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21: 2895–2900.
Craig WJ. 1997. Phytochemicals: guardians of our health. J Am
Diet Assoc 97: S199–S204.
Dujic J, Kippenberger S, Ramirez-Bosca A et al. 2009. Curcumin in combination with visible light inhibits tumor growth
in a xenograft tumor model. Int J Cancer 124: 1422– 1428.
Gajate C, An F, Mollinedo F. 2003. Rapid and selective apoptosis in human leukemic cells induced by aplidine through a Fas/ CD95- and mitochondrial-mediated mechanism. Clin Cancer
Res 9: 1535–1545.
Huang MT, Lou YR, Xie JG et al. 1998. Effect of dietary curcumin and dibenzoylmethane on formation of 7,12-
in the PC-3 prostate tumour xenografts (Khor et al., 2006). Curcumin potentiates the antitumour effects of gemcitabine in pancreatic cancer by suppressing prolif-eration, angiogenesis, NF-κB and NF-κB–regulated gene products (Kunnumakkara et al., 2007). A combina-tion of curcumin and light therapy increases the effi cacy of curcumin in a human epithelial carcinoma A431 xenograft tumour model (Dujic et al., 2009) and offers a new therapeutic concept. The present study provides the fi rst report of the effi cacy of curcumin against tumours in an in vivo xenograft of human lung cancer NCI-H460 cells in mice.
Acknowledgement
This work was supported by grant CMU96-086 from the China Medical University and grant NSC95-2745-B-039-002-URD from the National Science Council, Taiwan.
Copyright © 2010 John Wiley & Sons, Ltd. Phytother. Res. 24: 189–192 (2010)
192 C.-C. SU ET AL.
dimethylbenz[a]anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis 19: 1697–1700.
Kawamori T, Lubet R, Steele VE et al. 1999. Chemopreventive effect of curcumin, a naturally occurring anti-infl ammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 59: 597–601.
Khor TO, Keum YS, Lin W et al. 2006. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC3 prostate xenografts in immunodefi -cient mice. Cancer Res 66: 613–621.
Kucuk O. 2002. Chemoprevention of prostate cancer. Cancer
Metastasis Rev 21: 111–124.
Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. 2007. Curcumin potentiates anti-tumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB- regulated gene products. Cancer Res 67: 3853–3861. Kuo PL, Hsu YL, Cho CY. 2006. Plumbagin induces G2-M arrest
and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther
5: 3209–3221.
Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. 2007. Anti-tumor, anti-invasion, and antimetastatic effects of cur-cumin. Adv Exp Med Biol 595: 173–184.
Lin JK. 2007. Molecular targets of curcumin. Adv Exp Med Biol
595: 227–243.
Rao CV, Rivenson A, Simi B, Reddy BS. 1995. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 55: 259–266.
Sharma RA, Euden SA, Platton SL et al. 2004. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10: 6847–6854.
Su CC, Yang JS, Lin SY et al. 2008. Curcumin inhibits WEHI-3 leukemia cells in BALB/c mice in vivo. In Vivo 22: 63–68.
Verma SP, Salamone E, Goldin B. 1997. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem Biophys Res Commun
233: 692–696.
Yang SF, Yang WE, Chang HR, Chu SC, Hsieh YS. 2008. Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res