ARTICLE NO.CS975021
The Electric Conductivity of Dilute Suspensions
of Charged Porous Spheres
Yung C. Liu and Huan J. Keh1
Department of Chemical Engineering, National Taiwan University, Taipei 106-17, Taiwan, Republic of China
Received March 4, 1997; accepted June 2, 1997
potential but also the local ionic densities and fluid velocity. The effective electric conductivity of a dilute suspension of poly- That is, one must first solve a set of coupled electrokinetic electrolyte molecules or charged flocs in an electrolyte solution is
equations to obtain the distributions of electric potential, analytically studied. The model used for the particles is a porous
ionic concentrations and fluid velocity in the electrolyte solu-sphere in which the density of hydrodynamic frictional segments,
tion, and then compute the average electric current and con-and therefore also that of the fixed charges, is constant. The
equa-ductivity in the suspension. tions which govern the electrochemical potential distributions of
Theoretical studies on the effective electric conductivity ionic species and the fluid flow field inside and outside a porous
of a suspension of impermeable charged spheres have been particle migrating in an unbounded solution are linearized
assum-made by Saville ( 1 ) and O’Brien ( 2 ) , assuming that the ing that the system is only slightly distorted from equilibrium.
Using a perturbation method, these linearized equations are solved particles and their electric double layers occupy only a small for a porous sphere in a uniform applied electric field with the fraction of the total volume. Approximate formulas for the density of the fixed charges as the small perturbation parameter. conductivity were obtained using a perturbation method for An analytical expression for the effective conductivity of a dilute particles with low z potential immersed in a symmetric elec-suspension of identical charged porous spheres is obtained from
trolyte correct to O ( z2
) . Their results have some discrepanc-the average electric current density calculated using discrepanc-the solution
ies with the experimental data reported by Watillon and of electrochemical potential distributions of the ions. The result
Stone-Masui ( 3 ) , who measured the surface conductances demonstrates that the presence of the fixed charges in the porous
of a number of monodisperse polystyrene latices over a range particles can lead to an augmented or a diminished electric
con-of particle volume fractions. Later, Saville ( 4 ) considered ductivity of the suspension relative to that of a corresponding
the effects of added counterions produced by the particle suspension of uncharged porous particles, depending on the
char-charging procedure and of nonspecific adsorption which al-acteristics of the electrolyte solution and the suspending particles.
When the anionic and cationic diffusion coefficients of a symmet- ters the concentrations of ions in the solution outside the ric electrolyte are the same, the correction for the effect of the double layers, and obtained better agreement between theo-fixed charges of the particles on the electric conductivity of the ries and experiments.
suspension is proportional to the square of the fixed charge density. The basic equations governing the electric conductivity
q 1997 Academic Press of a dilute suspension of colloidal particles also describe the
Key Words: polyelectrolyte particles; porous spheres; electric
electrophoretic phenomena. O’Brien ( 5 ) derived the analyti-conductivity; dilute suspensions.
cal formulas for the electrophoretic mobility and the electric conductivity of a dilute suspension of dielectric spheres with thin but polarized double layers in a general electrolyte solu-1. INTRODUCTION
tion. Using a similar analysis, O’Brien and Ward ( 6 ) also determined the electrophoretic mobility and the effective When an electric field is applied to charged colloidal
parti-conductivity of a dilute suspension of randomly oriented cles suspended in an electrolyte solution, the particles and
spheroids with thin polarized diffuse layers at the particle the surrounding ions are driven to migrate. As a
conse-surfaces. On the other hand, approximate analytical expres-quence, the fluid is dragged to flow by the motion of the
sions for the electrophoretic mobility and the conductivity particles and the ions, and there is an electric current through
of dilute suspensions of colloidal spheres in symmetric elec-the suspension to produce. To determine elec-the current density
trolytes were obtained by Ohshima et al. ( 7 ) . These expres-distribution and transport properties such as the electric
con-sions are correct to order ( ka )01
, where k is the Debye – ductivity, it is necessary to find out not only the local electric
Huckel parameter ( defined by Eq. [ 34 ] ) and a is the particle radius. When the zeta potential of the particles is small, their
1
To whom correspondence should be addressed. reduced result is in agreement with O’Brien’s ( 2 ) .
In most practical applications, the electric conductivity of cles is given as an integral over a large surface enclosing a single particle and its adjacent double layer, and the a suspension is known from direct measurement and then
the zeta potential of dielectric particles in the suspension effective conductivity of the suspension is related to the electrochemical potential energies of the ionic species. can be calculated. Similarly, one can also measure the
elec-trophoretic mobility of a particle in order to get the zeta In Section 3, we present the fundamental electrokinetic equations and boundary conditions which govern the elec-potential. O’Brien and Perrins ( 8 ) derived a formula for the
electric conductivity of a porous plug composed of closely trochemical potential distributions, the electrostatic poten-tial profile, and the fluid flow field inside and outside a packed spheres and compared it with the conductivity data
for dilute and concentrated dispersions of monodisperse porous particle migrating in an unbounded solution when a constant electric field is applied. These basic equations polystyrene particles reported by van der Put and
Bijster-bosch ( 9 ) . They found significant differences between the are linearized assuming that the ionic concentrations, the electric potential, and therefore the electrochemical poten-zeta potentials evaluated from measurements of the electric
conductivity and of the electrophoretic mobility. Similar dif- tials have only slight deviations from equilibrium due to the imposed field. The axisymmetric electrophoretic mo-ferences were also found by another work ( 10 ) in which the
conductivities and electrophoretic mobilities of polystyrene tion of a charged porous sphere in an unbounded electro-lyte solution is considered in Section 4. Using the Debye – latex systems were measured. On the other hand, Stigter
( 11 ) developed a theory based on the concepts used to de- Huckel approximation, we first get the solution of the equilibrium electric potential distribution. Then the linear-scribe the conductivity of strong electrolyte solutions, in
which the specific conductance of the suspension was com- ized electrokinetic equations are transformed into a set of differential equations by using a regular perturbation puted by summing the individual contributions of the
parti-cle – ion interactions expressed in terms of equivalent con- method with the density of the fixed charges inside the porous particle as the small perturbation parameter. The ductances. The differences between the kinetic charges
cal-culated from electrophoresis and from conductance in this perturbed electrochemical potentials of ions and the fluid velocity are determined by solving this set of differential theory were found to be small and within the errors of the
experiments and the theoretical models ( 12 ) . equations subject to the appropriate boundary conditions. An analytical expression for the electric conductivity of A theoretical investigation of the electrokinetic
phenom-ena of charged porous particles was first performed by Her- a dilute suspension of identical charged porous spheres is obtained. Finally, in Section 5, typical numerical results mans and Fujita ( 13, 14 ) . They derived formulas for the
electrophoretic mobility of a porous sphere by introducing for the effects of the fixed charges of porous spheres on the effective conductivity of the suspension are presented. the Brinkman equation for the internal flow field of the
parti-cle and assuming that the electric double layer remains
spherically symmetric in the presence of the applied electric 2. AVERAGE CURRENT DENSITY IN A SUSPENSION field. The effect of the distortion of the counterion atmo- OF CHARGED PARTICLES
sphere around a nearly free-drained polyelectrolyte coil un-der an applied electric field was examined theoretically by
We consider a dilute suspension of identical charged parti-Imai and Iwasa ( 15 ) , and their numerical results agree well
cles immersed in a solution containing M ionic species. The with the experimental data ( 16 ) . Recently, analytical
expres-particles may be either porous or impermeable to the fluid. sions were derived by Liu and Keh ( 17 ) for the
sedimenta-It is assumed that the suspension is statistically homogeneous tion velocity and potential in a dilute suspension of charged
and all effects of its boundaries are ignored. When the iso-porous spheres under the situation that the electric potentials
tropic suspension is subjected to a uniform applied electric are low. However, the effects of particle charges on the
field E`, one has effective conductivity of a suspension of porous particles
have not yet been theoretically investigated.
In this work, we study analytically the effective
conduc-E`Å 0 1
V
*
VÇcd x , [1]
tivity of a suspension of charged porous particles. The density of charged segments of each porous particle is assumed to be uniform, but no assumption is made as to
where c( x ) is the electric potential at position x and V the thickness of the electric double layer relative to the
denotes a sufficiently large volume of the suspension. There dimension of the particle. The suspension is sufficiently
is a resulting volume-averaged current density, which is col-dilute that the suspended particles occupy only a small
linear with E`, defined by
fraction of the total volume of the suspension and the double layer surrounding each particle does not overlap
with the others. In Section 2, the average electric current »i… Å 1
V
*
Vi d x , [ 2 ]
parti-where i ( x ) is the current density distribution. The effective of concentration of species m , electric potential, and electro-chemical potential energy of species m , respectively, and electric conductivity L of the suspension can be assigned
by the linear relation dnm, dc, and dmmare the small perturbations to the
equilib-rium state ( in which no external field is applied ) . The equi-librium concentration of any species is related to the
equilib-»i… ÅLE`. [ 3 ]
rium potential by the Boltzmann distribution, Since the measured electric field and current density are
equal to E` and »i…, respectively, Eq. [ 3 ] reduces to the
n( eq )
m Ån`mexp
S
0zme c( eq )
kT
D
, [ 8 ]usual experimental definition of conductivity, provided that the suspension is everywhere homogeneous.
The current density i can be written as
where n`mis the constant bulk concentration of type-m ions.
The perturbation quantity dmmis linearly related to the others
i Å
∑
M
mÅ1
zmeJm, [ 4 ] by
where Jm( x ) and zmare the number flux distribution and the dmmÅkT dnm
n( eq )
m
/zmedc. [ 9 ]
valence of species m , respectively, and e is the charge of a proton. If the solution is dilute, the flux Jmis given by
Substituting Eqs. [ 5 ] – [ 9 ] into Eq. [ 4 ] , using the fact thatÇm( eq )
m Å0 , and neglecting products of the small
quanti-Jm Ånmu0nm
Dm
kT
Çmm, [ 5 ]
ties u , dnm, and dmm, one has
with the electrochemical potential energy field of the mth
i Å
∑
M mÅ1 zmen ( eq ) mS
u0 Dm kT ÇdmmD
. [10 ] species mm( x ) defined as ( 7 ) mmÅm0m/kT ln nm/zme c. [ 6 ]Far from any particle ( beyond the double layer ) , n( eq )
m r
n`mand Eq. [10 ] becomes
Here, u ( x ) is the fluid velocity field; nm( x ) is the
concentra-tion distribuconcentra-tion of species m ; Dmis the diffusion coefficient
of species m which is assumed to be constant both inside and ir 0
∑
M mÅ1 zmeDm
S
Çdnm/ zmen`m kT ÇdcD
. [11]outside the particles if they are porous; k is the Boltzmann constant; T is the absolute temperature; and m0
mis a constant.
The first term on the right-hand side of Eq. [ 5 ] represents By adding and subtracting the current density given by the the convection of the ionic species by the fluid and the above equation in the integrand of Eq. [ 2 ] , one obtains second term denotes the diffusion and electrically induced
migration of the ions.
»i… Å 0
∑
M mÅ1 zmeDm V*
VS
Çdnm/zmen ` m kT ÇdcD
d xThe linear relation [ 3 ] between the volume-average cur-rent density and the electric field for a suspension will be exact in the limit as E` r 0 . To calculate the effective
/ 1 V
*
VF
i/∑
M mÅ1 zmeDmS
Çdnm/ zmen`m kT ÇdcDG
d x .conductivity of the suspension we may assume that the inten-sity of the applied electric field is not high, and hence that
the electric double layer surrounding each particle is only [12 ]
slightly distorted from equilibrium by the application of the
field. Therefore, the concentration distribution of each ionic In a statistically homogeneous suspension, the volume species and the electric potential distribution have small de- average ofÇdn
mis zero. According to the definition of Eq.
viations from equilibrium, and one can write [1] the first term on the right-hand side of Eq. [12 ] equals L`E`, where nmÅn ( eq ) m /dnm, [ 7a ] cÅc( eq ) /dc, [ 7b ] L`Å
∑
M mÅ1 z2 me 2 n`mDm kT , [13 ] mmÅm( eq )m /dmm, [ 7c ] where n( eq )in the absence of the particles. The integral in the second all species, which do not react with one another, in the steady state requires that
term on the right-hand side of Eq. [12 ] can be calculated by first considering for a single particle as if the others were
absent and then multiplying the result by the particle number Ç r
JmÅ0, m Å1, 2, . . . , M . [16 ]
N in the volume V, since the integrand vanishes beyond the
double layers surrounding the particles and the suspension
Here, the species fluxes Jmare defined by Eq. [ 5 ] taking u
is assumed to be sufficiently dilute that the double layers do
as the fluid velocity relative to the particle. not overlap with one another. Also, the volume integral can
We assume that the Reynolds number for the fluid motion be transformed into a surface integral over a spherical
bound-is very small, so the inertial effect on the fluid momentum ary of infinite radius containing the single particle at its
balance can be neglected. The fluid flow is governed by the center. With this arrangement, the second term becomes
modified Stokes equations,
N V
*
rr`S
nrir /∑
M mÅ1 zmen`mDm kT dmmnD
dS hÇ2 u0h ( x ) f uÅ Çp/∑
M mÅ1 zmenmÇc, [17 ] Å 0 N V∑
M mÅ1 zmen`mDm kT*
rr ` ( nrÇdmmr 0dmmn ) dS , Ç r uÅ0, [18 ] [14 ]where h is the viscosity of the fluid; f is the friction coeffi-cient inside the porous particle per unit volume of the fluid; where r is the position vector relative to the particle center,
p ( x ) is the dynamic pressure distribution; and h ( x ) is a unit r Å ÉrÉ, and n is the unit vector outwardly normal to the
step function which equals unity if x is inside the particle, surface of the boundary. To obtain Eq. [14 ] , the requirement
and zero otherwise. In Eq. [17 ] , h and f are assumed to be of the conservation of electric charge (Çri Å 0 ) and Eq.
constant. Note that f can be expressed as 6phasNs, where [11] have been used. Therefore, the average current density
Ns and as are the number density and the Stokes radius, given by Eq. [12 ] can be expressed as
respectively, of the particle segments.
The electric potential c and the space charge density are
»i… ÅL`E` 0 N
V
∑
M
mÅ1
zmen`mDm
kT related by Poisson’s equation:
1
*
rr ` ( rÇdmmrn0dmmn ) dS . [15 ] Ç2 c Å 04p e [∑
M mÅ1 zmenm/h ( x ) Q ] . [19 ]The determination of dmm in the above equation is
con-Here, Q is the fixed charge density inside the porous particle cerned with the solution of a set of basic electrokinetic
equa-and eÅ 4pe0er, where er is the relative permittivity of the tions for the electrolyte around a single particle. These
elec-electrolyte solution, which is assumed to be the same inside trokinetic equations for the case of a charged porous particle
and outside the particle, and e0 is the permittivity of free are described in the next section and their analytical solution
space. Experimental data for layers of poly ( methacrylic for a charged porous sphere with low fixed charge density
acid ) , poly ( 4-vinylpyridine ) ( 18 ) , and poly ( N-isopropyla-is presented in Section 4. From thN-isopropyla-is solution we shall derive
crylamide ) ( 19 ) on the surfaces of polymer particles indicate the effective conductivity of a dilute suspension of identical
that the magnitude of Q ranges from very low to as high as porous spheres.
9 1 106 C /m3
, depending on the temperature, pH value, and ionic strength of the electrolyte solution.
3. BASIC ELECTROKINETIC EQUATIONS FOR
If the strength of the imposed electric field is weak, the A CHARGED POROUS PARTICLE
deviations in the ionic concentrations and electric poten-IN AN ELECTRIC FIELD
tial from equilibrium are so slight that Eqs. [16 ] and [17 ] can be linearized. It has been found that the perturbed In this section we consider a charged porous particle ( such electrochemical potential energies dm
mand the fluid
veloc-as a polyelectrolyte molecule or an aggregate of fine particles ity u satisfy the following set of electrokinetic equations bearing surface charges ) of arbitrary shape in an unbounded ( 5, 7 ) :
liquid solution containing M ionic species when a constant electric field E` is applied. In this solvent-permeable and
ion-penetrable particle, idealized charged segments are as- n( eq )
m Ç2dmm/ Çn( eq )m rÇdmmÅ
kT Dm
Çn( eq )
m ru , [ 20 ]
cr 0E`rx . [ 27c ] hÇ2Ç 1 u0h ( x ) fÇ 1uÅ
∑
M mÅ1 Çn( eq ) m 1 Çdmm. [ 21]Here, mE is the electrophoretic mobility of the charged Substituting Eq. [ 8 ] into Eqs. [ 20 ] and [ 21] , one has porous particle, and its expression for a porous sphere will be given in the next section. Because the equilibrium electric potential and concentration of type-m ions in the
Ç2dm mÅ zme kT
S
Çc( eq )rÇdm m0 kT Dm Çc( eq )ru
D
, [ 22 ] bulk solution have been set equal to zero and n`m,
respec-tively, from Eqs. [ 7 ] , [ 9 ] , [ 27b ] , and [ 27c ] one obtains
Ç2
Ç 1u0h ( x ) l2
Ç 1uÅ the following condition for dmm at large distances from
the particle: 01 h
∑
M mÅ1F
zmen`m kT expS
0 zme c( eq ) kTD
Çc( eq )1 Çdm mG
, [ 23 ] dmmr 0zmeE`rx . [ 28 ] where lÅ( f / h )1 / 2, and the solution of c( eq )
for the case
Equations [17 ] , [18 ] , and [ 27a ] take a reference frame that of a charged porous sphere will be given in the following
the porous particle is at rest and the velocity of the fluid at section. Note that the reciprocal of the parameter l is the
infinity is the particle velocity in the opposite direction. shielding length characterizing the extent of flow
penetra-tion inside the porous particle. For some model porous
4. SOLUTION FOR THE EFFECTIVE CONDUCTIVITY particles made of steel wool ( in glycerin – water solution )
OF A DILUTE SUSPENSION OF CHARGED ( 20 ) and plastic foam slab ( in silicon oil ) ( 21 ) ,
experi-POROUS SPHERES mental values of 1 / l can be as large as 0.4 mm, while in
the charged or uncharged temperature-sensitive poly (
N-We now consider a charged porous sphere of radius a isopropylacrylamide ) hydrogel layers on latex particles
immersed into an unbounded electrolyte solution under a in electrolyte solutions, values of 1 / l were found to be
uniform applied electric field E`. The electrophoretic
veloc-about 1 – 50 nm ( 19 ) .
ity of the particle is mEE`ez, where ez is the unit vector in
The conditions inside the particle are
the axial direction and E`Å ÉE`É. The origin of the spherical
coordinate system ( r , u, f ) is taken to be the center of the dmmand u are finite. [ 24 ]
particle.
We first seek the solution of c( eq )
which appears in the The boundary conditions at the surface of the particle ( S{
)
governing equations [ 22 ] and [ 23 ] for a charged porous are
sphere. Substituting the Boltzmann distribution, Eq. [ 8 ] , into Poisson’s equation [19 ] at equilibrium, one can get the dmmÉS/ÅdmmÉS0, [ 25a ] equilibrium Poisson – Boltzmann equation,
ÇdmmÉS/Å Çdm mÉS0, [ 25b ] uÉS/ ÅuÉS0, [ 25c ] Ç2c( eq )Å 04p e nrsÉS/ ÅnrsÉS0. [ 25d ] 1
F
∑
M mÅ1 zmen`mexpS
0 zme c( eq ) kTD
/h ( r ) QG
, [ 29 ]Here, s is the hydrodynamic stress of the fluid given by
s Å 0pI/h[Çu/(Çu )T
] , [ 26 ] where h ( r ) equals unity if rõa and is zero if rúa .
The appropriate boundary conditions for the equilibrium potential are
where I is the unit dyadic. Equations [ 25a ] and [ 25b ] indi-cate that the concentration and flux of species m and the
potential and electric field must be continuous at the particle c( eq )É
r Å0is finite, [ 30a ]
surface. Equations [ 25c ] and [ 25d ] are the continuity
re-c( eq )
Ér Åa/Å c( eq )Ér Åa0, [ 30b ] quirement of the fluid velocity and stress tensor.
The conditions far from the particle are dc( eq ) dr
Z
r Åa/ Å dc ( eq ) drZ
r Åa0 , [ 30c ] uÅ 0mEE`, [ 27a ] nm rn`m, [ 27b ] c( eq )Érr ` r0. [ 30d ]The solution to Eq. [ 29 ] satisfying the above boundary con- equations [ 22 ] and [ 23 ] and the boundary conditions [ 24 ] , [ 25 ] , [ 27a ] , and [ 28 ] , and equating like powers of QV on ditions is
both sides of the respective equations, we obtain a set of differential equations and boundary conditions for each set c( eq )
Åceq1QU /O ( QU2
) . [ 31]
of the functions mm j, uj, and mEjwith j Å1, 2, . . . .
After collecting the O ( QV ) terms in the procedure of this Here,
regular perturbation, one obtains the following equations:
QUÅ 4peQ ek2 kT, [ 32 ] Ç2mm 1Å 0 z2 me2E` kT
Çceq1rÇ( r cos u ) , [ 36a ] Ç2
Ç 1u10h ( r ) l 2
Ç 1u1 which is the nondimensional charge density of the porous
sphere, and ( 13 ) Å 1 h
∑
M mÅ1 z2 me 2 n`mE` kT Çceq11 Ç( r cos u ) . [ 36b ] ceq1( r )Å kT eF
10( ka/1 ) e 0 kasinh ( kr ) krG
, if rõa ,The boundary conditions for mm 1and u1are
[ 33a ]
rÅ0: mm 1and u1are finite; [ 37a ]
ceq1( r )Å kT
e [ ka cosh ( ka )0sinh ( ka ) ] e0 kr
kr , if rúa , r Åa : mm 1and u1are continuous, [ 37b ]
Çmm 1 and nrs1are continuous; [ 37c ]
[ 33b ]
r r `: mm 1are zero, [ 37d ]
where k is the reciprocal of the Debye screening length,
u1r 0mE1E`ez. [ 37e ]
defined by
The solutions for mm 1 and the r and u components of u1
subject to Eqs. [ 37 ] are kÅ
S
4pe 2 ekT∑
M mÅ1 z2 mn`mD
1 / 2 . [ 34 ] mm 1ÅE`Fm( r ) cos u; [ 38 ] Note that c( eq )is a function of r only. Expression [ 31] for
u1rÅE`Fu( r ) cos u, [ 39a ]
c( eq )
as a power series in the fixed charge density of the porous sphere up to O ( QV ) is the equilibrium solution for
u1uÅ 0E` 1 2r d dr[ r 2 Fu( r ) ] sin u, [ 39b ]
the linearized Poisson – Boltzmann equation that is valid for small values of the electric potential ( the Debye – Huckel approximation ) . That is, the charge density Q of the porous
with particle must be small enough for the potential to remain small.
To solve the small quantities dmm, u , and mEfor the case
Fm( r ) Åz2mea
F
0 1 3 r a/S
1/ 1 kaD
e 0 ka a( kr ) ( kr )2G
, of small parameter QV , these variables can be written asperturbation expansions in powers of QV ,
if rõa , [ 40a ] dmmÅ 0zmeE`r cos u/ mm 1QU /mm 2QU 2/ rrr, [ 35a ] Fm( r ) Åz2mea
F
0 1 3S
a rD
2 / a( ka ) ka kr/1 ( kr )2 e 0 krG
, uÅu1QU /u2QU 2/ rrr, [ 35b ] if rúa ; [ 40b ] mEÅmE1QU /mE2QU 2/ rrr , [ 35c ] Fu( r ) Å 0 ekT 4pheHS
k lD
2 /2 ( ka /1 ) e0 ka k 2 l2 0k2 where the functions mm j, uj, and mEj, with j Å 1, 2, . . . ,are independent of QV . The zeroth-order terms of u and mE disappear because an uncharged particle will not move by
1
F
a( ka ) a( la ) 0 a( kr ) a( lr )G
a( lr ) ( kr )3J
, if rõa , [ 41a ] applying an electric field. Substituting the expansions givenby Eq. [ 35 ] and c( eq )
The solution for mm 2is Fu( r ) Å 0mE1/
F
mE10 ekT 4pheS
k lD
2GS
a rD
3 mm 2Å 0 zmeE` 3kTF
1 r2*
r 0 r3S
dFm dr 0 kT Dm FuD
dceq1 dr dr 0 ekT 2phea( ka ) [ ( kr /1 ) e 0 kr /r*
` rS
dFm dr 0 kT Dm FuD
dceq1 dr drG
cos u, [ 45 ] 0( ka/1 ) e0 ka] 1 ( kr )3, if rúa , [ 41b ]where functions Fm( r ) and Fu( r ) are given by Eqs. [ 40 ]
and [ 41] . Note that mm 2 takes the same forms both in the
where regions rõa and rúa .
By substituting Eqs. [ 35a ] , [ 38 ] , and [ 45 ] into Eq. [15 ] , making relevant calculations, and then comparing the result with Eq. [ 3 ] , the effective conductivity of a dilute suspen-a( x )Åx cosh x0sinh x , [ 42 ]
sion of identical charged porous spheres is obtained as mE1Å ekT 4phe
HS
k lD
2 /1 3S
1/e 02k a 010e 02k a kaD
LÅL`H
10 w 10 ep[ L1( ka ) 2 QU /1 3S
k2 l2 0k2DS
1/ 1 kaD
/L2( ka )4QU 2 /O ( QU 3) ]J
. [ 46 ] 1FS
l kD
2 ka ( 1/e02k a )01/e02k a la coth ( la )01 01/e 02k aGJ
. [ 43 ] Here, epis the porosity of each particle; wÅN ( 4 / 3 ) pa3 ( 1
0ep) /V is the true volume fraction of the porous particles; Here, the fact that the sum of the hydrodynamic frictional
L1 Å( M mÅ1z3mn`mDm (M mÅ1z 2 mn`mDm 1 ( ka )2 [ 47 ] force and electric force exerted on the porous sphere must
be zero has been used to determine mE1. Equation [ 43 ] for the electrophoretic mobility of a charged porous sphere at
for a general electrolyte; and low electric potential is the same as that obtained by
Her-mans and Fujita ( 13 ) . This formula was also derived by
Ohshima ( 22 ) who considered the electrophoresis of a soft L1Åz ( D/0D0)
D// D0
1
( ka )2, [ 48a ] spherical particle which tends to a charged porous sphere in
the absence of the particle core.
L2Å 0 ek 2 T2 2ph( D//D0) e 2A0 z 2 B [ 48b ] Among the O ( QV2
) terms in the perturbation procedure, the only distribution we need in the following calculations
is the electrochemical potential energy mm 2. If the solution for a symmetric electrolyte ( MÅ 2, z
/ Å 0z0Å z , n`/ Å
contains only a symmetrically charged, binary electrolyte n`
0Ån`, L`Å( D//D0) z2e2n`/ kT , where subscripts/
the equation governing mm 2is and 0 refer to the cation and anion, respectively ) . In
Eq. [ 48b ] , Ç2m m 2Å zme kT Çceq1r
S
Çmm 10 kT Dm u1D
. [ 44 ] AÅ 1 ( la )2 ( ka )2/ 2/ka ( ka )8 [ a( ka ) ] 2 e02k a 0S
k 2 l2 0k2D
( 1/ka )2 ( ka )8 e 02k a [ a( ka ) sinh ( ka ) The boundary conditions for mm 2 are also given by Eq.[ 37 ] with the subscript 1 of mm 1being replaced by 2. For
a general electrolyte there is an extra term on the right-hand side of Eq. [ 44 ] involving the O ( QV 2
) correction to 0sinh2 ( ka )/( ka )2 ]/ 2l 2k2 ( l2 0k2 )2 ( 1/ka )2 ( ka )8 the equilibrium potential as expressed by Eq. [ 31] . This
extra term considerably complicates the problem. So we
1a( ka ) e02k a
FS
lk
D
2a( ka )
a( la )sinh ( la )0sinh ( ka )
G
, consider here only the case of a symmetric electrolyte, inwhich the QV 2
term in Eq. [ 31] vanishes and the leading correction to ceq1QV is O ( QV3
B Å2 ( ka ) 2 /3ka /3 3 ( ka )7 a( ka ) e 0 ka 0ka/1 3 ( ka )5 e 0 ka AÉ 1 ( la )2 ( ka )2/ 1 4
F
1 ( ka )50 3 ( ka )7GS
l2 02k2 l20k2D
1sinh ( ka )03 4 ka /2 ( ka )8 [ a( ka ) ] 2 e02k a /l ( ka ) 2 0k 02l 2a ( ka )6F
l ( l2 0 k2 ) ( l /k )G
. [ 52 ] 03 4 ( 1 /ka )2 ( ka )8 e 02k a[ a( ka ) sinh ( ka ) The above equation leads to Eq. [ 51a ] when ka@ la . If la@ka , then Eq. [ 52 ] is simplified as
0sinh2 ( ka )/( ka )2 ] . [ 49b ] A É1 4
F
1 ( ka )50 3 ( ka )7G
/ 1 2F
1 ( ka )50 2 ( ka )7G
Note that Eq. [ 46 ] is correct to order w, ( ka )2QV and ( ka )4
QV 2
are independent of k or n`, and the result of the
QV 2
term is limited to a symmetric electrolyte. As ex- 1
S
kl
D
/ 1 4F
4 ( ka )40 3 ( ka )5GS
k lD
2 . [ 53 ] pected, the electric conductivity of a suspension ofun-charged porous particles penetrable to electrolyte ions is
When la! 1 and ka is moderate, parameter A becomes the same as that in the absence of the particles ( LÅL`
if Q Å 0 ) . The coefficient L1 of the QV term, which is independent of the shielding parameter l, is proportional
A É 1 ( la )2 ( ka )2/ 2/ka ( ka )8 [ a( ka ) ] 2 e02k a
to ( ka )02, and disappears for a symmetric electrolyte when the diffusivities of the cation and anion take the same value. As to the coefficient L2of the QV 2
term for a
/( 1 /ka )
2
( ka )8 [ a( ka ) sinh ( ka ) symmetric electrolyte, the first term of the right-hand side
of Eq. [ 48b ] ( which depends on parameters ka and la )
0sinh2
( ka )/( ka )2 ] e02k a
. [ 54 ]
represents the effect due to the convection of the fluid, while the second term ( which is a function of ka only )
denotes the effect due to the deviations of the electro- When both la and ka are much smaller than unity, A reduces chemical potential distributions from their combined to
equilibrium and applied values. It is understood that the result given by Eqs. [ 46 ] – [ 49 ] is only valid with the requirements that w! 1 and ka[ ( 10 ep) / w]1 / 3@
1. AÉ 1 ( la )2 ( ka )2/ 1 15
F
4 ( ka )20 5 kaG
. [ 55 ] When ka!1 and la is moderate, parameters A and B inEq. [ 49 ] have the approximate forms
5. RESULTS AND DISCUSSION
A É1 9
F
2/ 3 ( la )2/ 2 sinh ( la ) a( la )G
1 ( ka )20 13ka, [ 50a ] For an aqueous solution of KCl in which the cation and anion mobilities are nearly equal, the coefficient L1 of the QV term in Eq. [ 46 ] is small in magnitude and equal
B É 1 36ka0 1 35. [ 50b ] t o 00 . 0 17 ( k a) 0 2 (c a l cu l a te d u si n g th e v al u e s o f ek2 T2
/ 4phDmz2me2for ions at room temperature listed in Ref.
( 23 ) ) . For other electrolytes in which the ionic mobilities are very different, the magnitude of L1can be quite large; When ka@ 1 and la is still moderate, A and B become
e.g., L1 Å 00.206 ( ka )02
for NaCl and L1Å0.672 ( ka )02
for HClO4. Note that the value of electric conductivity of a suspension of charged porous particles ( L ) can be smaller or larger than that of the suspension when the porous
parti-A É 1
( la )2
( ka )2/ 1
2 ( ka )5, [ 51a ]
cles are uncharged or in the absence of the particles ( L`) , depending on the signs of L1and Q . Also, the influence of
B É 1
24
F
4 ( ka )409
( ka )5
G
. [ 51b ] the presence of the charged particles on the electric conduc-tivity of a suspension is reduced if the concentration of the electrolyte is increased ( i.e., k is increased ) assuming all When both ka and la are large compared with unity, Eq. other conditions are constant.The parameters A and B in association with the coefficient [ 49a ] reduces to
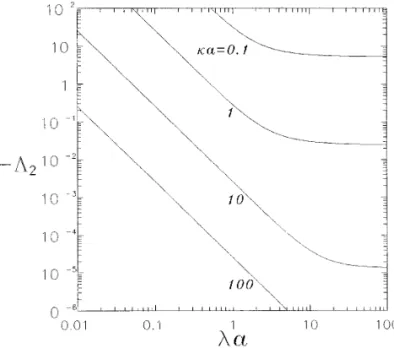
FIG. 3. Plot of the coefficient L2in Eq. [ 46 ] for the effective
conductiv-ity of a dilute suspension of identical charged porous spheres in an aqueous FIG. 1. Plot of the parameter A given by Eq. [ 49a ] for the coefficient
L2 versus ka at fixed values of la . The dotted curve is plotted for the solution of KCl versus ka at fixed values of la .
parameter B given by Eq. [ 49b ] .
01 when ka!1 and04 when ka@1, as required by Eqs. [ 50b ] and [ 51b ] . Likewise, Fig. 2 illustrates that the slope L2by Eq. [ 48b ] can be evaluated for given values of
parame-ters ka and la using Eq. [ 49 ] with the aid of a basic hand of each curve for A with constant ka equals02 when la! 1 and 0 when la@ 1, consistent with Eqs. [ 54 ] and [ 53 ] . calculator, and their results are plotted in Figs. 1 and 2
logarithmically. Figure 1 indicates that both A and B decrease Note that the situation of Bú A is possible only when la
@1 and ka) 3. monotonically with increasing ka for a given value of la ,
and Fig. 2 shows that A is a monotonic decreasing function Figures 3 and 4 show plots of the coefficient L2of the
QV 2
term in Eq. [ 46 ] as a function of parameters ka and of la for a fixed value of ka . It can be seen in Fig. 1 that
the slope of each curve for A with a constant value of la la in an aqueous solution of KCl where the value of ek2
T2
/ 2ph( D// D0) z2e2Å 0.2585 ( 23 ) is used in the
equals02 when ka ! 1 and when ka @ 1, in accordance
with Eqs. [ 50a ] and [ 51a ] , and that the slope of B equals calculations. As is the case for parameters A and B , the
FIG. 4. Plot of the coefficient L2for a KCl electrolyte versus la at
fixed values of ka . FIG. 2. Plot of the parameter A versus la at fixed values of ka .
magnitude of L2 also decreases monotonically with the APPENDIX: NOMENCLATURE increase of ka or la , the other parameter being kept
con-stant. Comparing Figs. 3 and 4 with Figs. 1 and 2, one a Radius of a porous sphere ( m)
can find that L2 is dominated by parameter A if laõ 5 A , B Parameters defined by Eqs. [ 48b ] , [ 49 ]
or if ka õ 1, and L2is dominated by parameter B if la D/, D0 Diffusion coefficients of cation and anion,
respec-@ 1 and kaú 3. tively ( m2
rs01)
Dm Diffusion coefficient of type-m ions ( m
2
rs01)
e Protonic charge ( C ) 6. CONCLUDING REMARKS
E`, E` Applied electric field ( Vrm01)
f Friction coefficient within the porous particle per The effective electric conductivity of a dilute suspen- unit volume of fluid ( kgrm03rs01)
sion of charged porous particles in an electrolyte solution Fm Function defined by Eq. [ 40 ] ( Crm)
is analytically studied in this work. Each porous particle Fu Function defined by Eq. [ 41] ( m2r
s01rV01)
is treated as a solvent-permeable and ion-penetrable ob- h Unit step function
ject in which fixed-charged groups and frictional seg- i Electric current density ( Arm02)
ments are distributed at uniform densities. The electric Jm Number flux of type-m ions ( s01
rm02)
double layer surrounding each particle is assumed not to k The Boltzmann constant ( 1.38 110023
JrK01)
overlap with the others. The average electric current den- n Unit normal vector outwardly to a surface sity passing through the suspension is given by Eq. [15 ] n` Bulk concentration of a symmetric electro-as an integral over a large spherical surface surrounding lyte ( m03
)
a single particle plus its double layer and is related to the nm Concentration of type-m ions ( m
03
)
electrochemical potential energies of the electrolyte ions. Equilibrium concentration of type-m ions ( m03
)
n( eq )
m
Solving the linearized electrokinetic equations applicable Bulk concentration of type-m ions ( m03
)
n`m
to the system of an isolated porous sphere by a regular N Number of the particles in volume V of a suspen-perturbation method, we have derived the electrochemical sion
potential distributions of ionic species and the fluid veloc- p Pressure distribution of the fluid ( Nrm02)
ity field under the application of a uniform electric field. Q Fixed charge density inside the porous par-An analytical expression, Eq. [ 46 ] , is obtained as a power ticle ( Crm03)
series in the fixed charge density of the particles up to QV Å4peQ / ek2
kT O ( QV 2
) for the electric conductivity of a dilute suspension r Position vector relative to the center of a particle
of identical charged porous spheres. ( m)
It is worth repeating that Eq. [ 46 ] with Eqs. [ 47 ] and r Radial spherical coordinate ( m) [ 48 ] is obtained on the basis of the Debye – Huckel approxi- T Absolute temperature ( K ) mation for the equilibrium potential distribution around a u Fluid velocity ( mrs01)
porous sphere. A similar formula for the electric conductivity u1 Fluid velocity of the order QV (mrs01)
of a dilute suspension of identical impermeable spheres with u1r, u1u The r and u components of fluid velocity u1 low z potential was shown to give a good approximation ( mrs01)
for the case of reasonably high z potential ( with an error of V A sufficiently large volume of the dilute suspen-about 5% in a KCl solution and less than 2% in a HClO4 sion ( m3
)
solution for the case of ze / kT Å 02 ) ( 2 ) . Therefore, our x Position vector in the suspension ( m) results might be used tentatively for the situation of reason- z Valence of a symmetric electrolyte ably high electric potentials. On the other hand, the decay zm Valence of type-m ions
of the density distributions of frictional segments and fixed
charges in the porous particle with the distance from the Greek Letters particle center has not been considered in our calculations.
In order to see whether our theory can be reasonably ex- a Function defined by Eq. [ 42 ]
dnm Perturbed concentration of type-m ions relative to
tended to the higher values of electric potential or to the
nonuniform density distributions of segments and fixed equilibrium ( m03
)
dmm Perturbed electrochemical potential energy of
charges, we propose to obtain a numerical solution of the
electrokinetic differential equations with no assumption on type-m ions relative to equilibrium ( J ) dc Perturbed potential relative to equilibrium ( V ) the magnitude of electric potential allowing the use of
arbi-trary distributions of charge and fluid drag components in e Å4pe0er ( C2r J01r
m01
)
e0 Permittivity of a vacuum ( 8.854 1 10012
the porous particle and compare it with the approximate
solution. C2r
ep Porosity of a porous particle REFERENCES er Dielectric constant of the fluid
h Fluid viscosity ( kgrm01rs01)
1. Saville, D. A., J. Colloid Interface Sci. 71, 477 ( 1979 ) . u, f Angular spherical coordinates 2. O’Brien, R. W., J. Colloid Interface Sci. 81, 234 ( 1981 ) . k The Debye – Huckel parameter ( m01
) 3. Watillon, A., and Stone-Masui, J., J. Electroanal. Chem. 37, 143 ( 1972 ) .
l Å( f / h )1 / 2 ( m01)
4. Saville, D. A., J. Colloid Interface Sci. 91, 34 ( 1983 ) . L Electric conductivity of the suspension
5. O’Brien, R. W., J. Colloid Interface Sci. 92, 204 ( 1983 ) . ( V01rm01)
6. O’Brien, R. W., and Ward, D. N., J. Colloid Interface Sci. 121, 402 L1, L2 Coefficients defined by Eqs. [ 46 ] – [ 48 ] ( 1988 ) .
L` Electric conductivity of the bulk electrolyte solu- 7. Ohshima, H., Healy, T. W., and White, L. R., J. Chem. Soc., Faraday
Trans. 2 79, 1613 ( 1983 ) .
tion ( V01
rm01)
8. O’Brien, R. W., and Perrins, W. T., J. Colloid Interface Sci. 99, 20 mE Electrophoretic mobility of a charged porous
par-( 1984 ) . ticle ( m2
rs01rV01)
9. van der Put, A. G., and Bijsterbosch, B. H., J. Colloid Interface Sci. mE1 Electrophoretic mobility of a charged porous par- 75, 512 ( 1980 ) .
ticle of the order QV (m2
rs01rV01) 10. Zukoski, C. F., and Saville, D. A., J. Colloid Interface Sci. 107, 322 ( 1985 ) .
mm Electrochemical potential energy of type-m
11. Stigter, D., J. Phys. Chem. 83, 1663 ( 1979 ) . ions ( J )
12. Stigter, D., J. Phys. Chem. 83, 1670 ( 1979 ) . mm 1 Electrochemical potential energy of type-m ions
13. Hermans, J. J., and Fujita, H., Proc. Akad. Amsterdam B 58, 182
of the order QV (J) ( 1955 ) .
mm 2 Electrochemical potential energy of type-m ions 14. Hermans, J. J., J. Polymer Sci. 18, 527 ( 1955 ) .
of the order QV 2
( J ) 15. Imai, N., and Iwasa, K., Israel J. Chem. 11, 223 ( 1973 ) .
16. Noda, I., Nagasawa, M., and Ota, M., J. Amer. Chem. Soc. 86, 5075 Equilibrium electrochemical potential energy of
m( eq )
m
( 1964 ) . type-m ions ( J )
17. Liu, Y. C., and Keh, H. J., 1996. [ Paper presented at Electrokinetic
s Hydrodynamic stress ( Nrm02)
Phenomena ’96, Rome ] s1 Hydrodynamic stress of the order QV (Nrm
02
) 18. Aoyanagi, O., Muramatsu, N., Ohshima, H., and Kondo, T., J. Colloid w True volume fraction of the porous particles Interface Sci. 162, 222 ( 1994 ) .
19. Makino, K., Yamamoto, S., Fujimoto, K., Kawaguchi, H., and Ohs-c Electrical potential of the solution ( V )
hima, H., J. Colloid Interface Sci. 166, 251 ( 1994 ) . c( eq )
Equilibrium electrical potential of the solution (V)
20. Matsumoto, K., and Suganuma, A., Chem. Eng. Sci. 32, 445 ceq1 Function defined by Eq. [ 34 ] ( V )
( 1977 ) .
21. Masliyah, J. H., and Polikar, M., Can. J. Chem. Eng. 58, 299 ( 1980 ) .
ACKNOWLEDGMENT
22. Ohshima, H., J. Colloid Interface Sci. 163, 474 ( 1994 ) .
23. van de Ven, T. G. M., ‘‘Colloidal Hydrodynamics,’’ p. 86. Academic This research was supported by the National Science Council of the