©2010 Taipei Medical University
O R I G I N A L A R T I C L E
1. Introduction
Data from the Bureau of Health Promotion, Department of Health, Executive Yuan, Taiwan, indicate that the in-cidence of breast cancer in Taiwan increased from 27.9 to 49.2 per 100,000 women in the decade from 1995 to 2005, which is an annual increase of approximately 7%. There are a number of therapeutic options for early breast cancer with a small tumor size, and the success rate of therapy is higher than for advanced stage dis-ease. However, the average detection size of breast
cancer tumors in Taiwan is > 2 cm, which is larger than that detectable with the diagnostic capabilities in Europe and North America. The median age at diagnosis of breast cancer is 45–49 years in Taiwan, and this age group is more likely to present with a dense breast pa-renchyma pattern (DBPP). This median age is signifi-cantly lower than that of Caucasian women in Western countries, where breast cancer peaks between the ages of 70 and 74 years,1 and this older age group is
more likely to present with a non-dense parenchyma pattern (NDBPP).
Background/Purpose: The incidence of breast cancer in Taiwan is increasing, and this form
of cancer has already become the number one malignancy. Taiwanese women share simi-lar characteristics of breast cancer with other developed Asian countries such as a younger age at diagnosis. In addition, a large proportion of Asian women have dense glandular breast tissue, which lowers the sensitivity of mammography for cancer detection. In this study, we compared possible differences in image interpretation of digital mammography and a dedicated breast magnetic resonance imaging system (using spiral RODEO).
Methods: From March 2008 to April 2009, we retrospectively collected data on 106
Taiwanese women who received both digital mammography and dedicated breast mag-netic resonance imaging examinations in the same period. We divided these cases into non-dense and dense groups according to breast density, and compared the image inter-pretations based on the different modalities.
Results: There were statistically significant differences (p < 0.05) in the “no match”, “partial
match”, and “exact match” image interpretations categories between the non-dense and dense groups.
Conclusion: Mammography may be useful for screening in non-dense breast tissue, but
breast magnetic resonance imaging should be an additional screening tool in populations with dense breast tissue.
Received: Nov 18, 2009 Revised: Mar 18, 2010 Accepted: Jun 12, 2010
KEY WORDS: Asian breast cancer; breast MRI;
dense breast parenchyma pattern;
Taiwanese breast cancer
Is Breast MRI Screening More Effective Than
Digital Mammography in Asian Women?
Ting-Kai Leung
1,2,3, Pai-Jung Huang
2,3, Ching-Shyang Chen
2,3, Yi-Hsiang Lin
1,2,
Chih-Hsiung Wu
2,3, Chi-Ming Lee
1,2,3*
1Department of Diagnostic Radiology, Taipei Medical University Hospital, Taipei, Taiwan
2Breast Health Center, Taipei Medical University Hospital, Taipei, Taiwan
3Department of Medicine, Taipei Medical University, Taipei, Taiwan
*Corresponding author. Department of Diagnostic Radiology, Taipei Medical University Hospital, 252, Wu Hsing Street, Taipei 110, Taiwan. E-mail: hk8648@tmu.edu.tw
Thus, it is controversial whether mammographic breast cancer screening is appropriate for Asian women. Increased breast parenchyma density is associated with breast cancer risk, and obscures the detection of early-stage, small-sized breast tumors with mammography.2 It is well-known that Asian women have relatively smaller breasts and are affected by breast cancer at a younger age; both factors that are associated with a DBPP.3
Recently, digital mammography and a dedicated breast magnetic resonance imaging (BRMRI) system have become the major screening and diagnostic tools for breast cancer detection and staging in our hospital. In this study, we compared the diagnostic capabilities of digital mammography with the BRMRI system. Faced with a relatively high percentage of DBPP in Taiwanese women, we divided the studied cases into NDBPP and DBPP groups to compare possible differences in image interpretations.
2. Methods
2.1. Study sampleIn total, data on 106 ethnic Chinese (Taiwanese) women were retrospectively collected between March 2008 and April 2009 from the hospital database. Cases were included if the patient had received both digital mam-mography and BRMRI examinations in the same pe-riod. Women were between 28 and 75 years old, and none had undergone major breast surgery in the pe-riod between the two examinations.
2.2. Image processing by the two modalities Digital mammograms consisted of the original stan-dard mediolateral oblique and craniocaudal views obtained using a Senography 2000D mammography platform (GE Healthcare, Waukesha, WI, USA). Cases also underwent an examination with a 1.5T dedicated
spiral breast MRI system (Aurora System; Aurora Imaging Technology Inc., North Andover, MA, USA) using the Spiral RODEO pulse sequence. Pre- and post-enhanced subtracted images were obtained, and an enhanced curve analysis was performed.
2.3. Breast density pattern classification
Mammographic breast density patterns were mea-sured using the standards of the American College of Radiology (ACR) for overall breast composition. In brief, four different categories (fatty to dense tissue) are de-scribed as: (1) almost entirely fat; (2) predominantly fat with scattered fibroglandular densities; (3) heteroge-neously dense; and (4) extremely dense. The method of classification is explained in Table 1.
2.4. Evaluation of imaging results
Breast density was assessed by radiologists who had at least 5 years of experience with mammographic and BRMRI image examination and interpretation. The breast density pattern classifications and breast imag-ing reportimag-ing and data system (BIRADS) were used in mammographic interpretation. BIRADS is the standard adopted by the ACR, and is commonly used world-wide.4,5 The interpretation of BRMRI also uses stan-dards of the ACR BIRADS-MRI lexicon.6
Two radiologists participated in the BRMRI and mammography analysis. To analyze the results of the radiologists’ interpretations between digital mammog-raphy and BRMRI, we created three other categories of radiological results. These categories reflected the re-sults of both examinations interpreted by the radiolo-gists as an exact match (identical suggestions; the same category), a partial match (similar suggestions; the same group but not the same category), or no match (different suggestions; different group) for inter-pretations of negative for malignancy, positive for ma-lignancy, and request for further follow-up (Table 1).
Table 1 The three different categories of radiologists’ interpretations for digital mammography and breast magnetic
resonance imaging (BRMRI)
Three different categories of radiological results The standard applied to categorize these results
An exact match between digital mammography Results of both imaging modalities indicated identical suggestions of: and BRMRI interpretations (1) negative malignancy; (2) positive malignancy; or (3) request for
further follow-up.
A partial match between digital mammography Results of both imaging modalities indicated similar but not identical and BRMRI interpretations suggestions of: (1) negative malignancy coupled with probably benign
and request for further follow-up; (2) positive malignancy coupled with probable malignancy; or (3) request for a short-interval follow-up. No match between digital mammography and Results of both imaging modalities indicated totally different BRMRI interpretations suggestions of: (1) negative malignancy; (2) positive malignancy;
Figure 1 (A) Mammogram of a case example in the non-dense parenchyma pattern group shows a large tumor and cluster of
microcalcifications (thin white arrow) at the superior left breast with enlarged lymph nodes (thick white arrow), which was diag-nosed as advanced infiltrative ductal carcinoma and lymph node metastasis. (B) The corresponding breast magnetic resonance imaging subtracted image (white arrow) matched the mammographic interpretation; and (C) shows a characteristic “wash-out” enhancing curve pattern, which is more likely to appear in malignancy.
B 00:00 −100 −70 −40 −10 20 80 110 140 170 200 50 01:30 04:35 07:40 10% 135% 185% C A Enhancement (%)
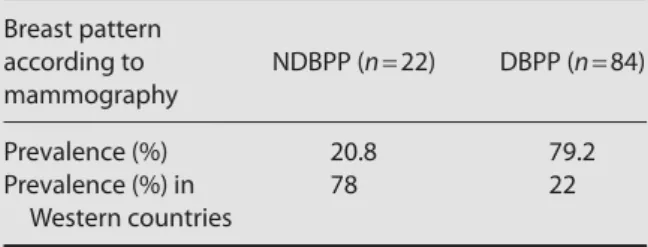
Table 2 Analysis of the prevalence of breast patterns
Breast pattern according to NDBPP (n = 22) DBPP (n = 84) mammography Prevalence (%) 20.8 79.2 Prevalence (%) in 78 22 Western countries
NDBPP = non-dense breast parenchyma pattern; DBPP = dense breast parenchyma pattern.
Biopsy was performed on some of the cases as sug-gested by both of the radiologists or surgeons, and speci-mens taken by core-needle, needle-localized or excisional biopsy were sent for pathology.
2.5. Statistical analysis
We combined the four ACR breast density types into two simple categories by combining types 1 and 2 into the NDBPP category and types 3 and 4 into the DBPP category. For statistical evaluation of the radiological results between the NDBPP and DBPP groups, data were evaluated by the χ2 test using Microsoft Excel, and expressed as a count and percentage. Values of p < 0.05 were considered statistically significant.
3. Results
Among the 106 cases, 22 cases were included in the NDBPP group (ACR types 1 and 2), while 84 cases were
included in the DBPP group (ACR types 3 and 4) (Table 2). There was only one mismatch in the interpretation of digital mammography and BRMRI in the NDBPP group, which reflects a good correlation between these two modalities in the NDBPP group. A representative case in the NDBPP group is presented in Figure 1. However, 19.0% (16/84) of the cases in the DBPP group did not have matching results in the interpretation between digital mammography and BRMRI. In the NDBPP group, 9.1% (2/22) of cases exhibited partial matches between digital mammography and BRMRI interpretation ver-sus 23.8% (20/84) of cases in the DBPP group. A repre-sentative case from the DBPP group is shown in Figure 2. In the NDBPP group, 86.4% (19/22) of cases showed exact matches between digital mammography and BRMRI interpretation versus 57.2% (48/84) of cases in the DBPP group. Using the χ2 test, significant differences in results were detected in all of the groups (p < 0.05; Table 3).
Our analysis of the ability of these two imaging modalities to detect breast cancer was also based on the pathological results after core-needle, needlocalized or excisional biopsy. In the DBPP group, le-sions in 9.5% (8/84) of cases could not be detected or precisely described by digital mammography, but the position could be determined by BRMRI. In contrast, in the NDBPP group, no cases were missed with digital mammography.
4. Discussion
Our findings indicate that it is difficult to detect breast lesions by digital mammography alone in women with
DBPP. The “no match” category for digital mammogra-phy and BRMRI interpretations was 4.5% in the NDBPP group and up to 19.0% in the DBPP group (Table 3). According to the results of the present study, we believe
that digital mammography is reliable as a screening or diagnostic tool for Asian and Chinese women with NDBPP. Mammo graphy can reliably image microcalcifi-cations and solid tumors with good contrast from the fatty background tissue of the breast. The aim of image production during mammography is to separate fatty tissue from breast glandular tissues of low contrast density on the basis of different X-ray absorption. Mam-mographic density estimation is based on a single two-dimensional projection of the breast. In contrast, BRMRI distinguishes different tissue types on the basis of their signal production after radiofrequency stimulation within a strong magnetic field. MRI evaluation of the breast is three-dimensional and the image analysis is assisted by a post-enhanced kinetic curve and subtraction tech-niques only allow contrast-enhanced lesions to be de-picted. Because the image is processed by subtraction of all of the background tissue, a possible lesion can only be identified in the presence of extremely dense glandular tissue, different types of implantation, or fi-brotic changes after chemotherapy with BRMRI.7,8
Previous studies have indicated that MRI is signifi-cantly more sensitive than mammography, ultrasound, and a combination of both.9 MRI and mammography B A C 00:00 −100 −70 −40 −10 20 50 80 110 170 140 200 01:30 49% 113% −60% 04:36 07:42 D Enhancement (%)
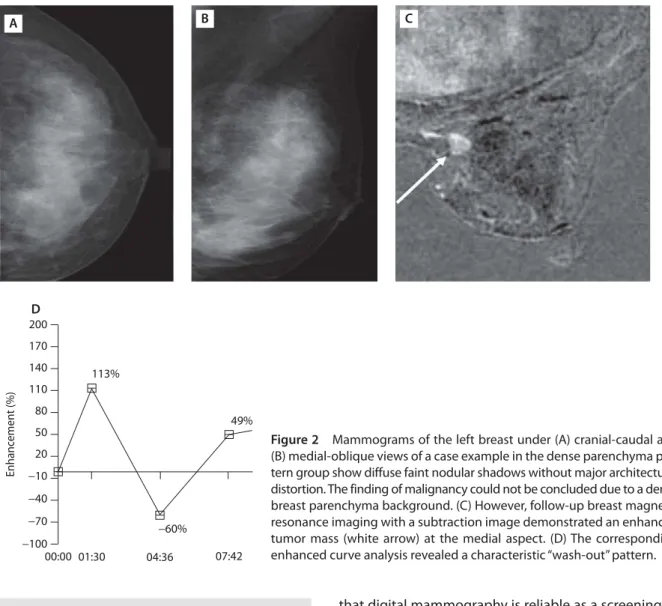
Figure 2 Mammograms of the left breast under (A) cranial-caudal and
(B) medial-oblique views of a case example in the dense parenchyma pat-tern group show diffuse faint nodular shadows without major architectural distortion. The finding of malignancy could not be concluded due to a dense breast parenchyma background. (C) However, follow-up breast magnetic resonance imaging with a subtraction image demonstrated an enhanced tumor mass (white arrow) at the medial aspect. (D) The corresponding enhanced curve analysis revealed a characteristic “wash-out” pattern.
Table 3 Analysis of interpretations of results between
digital mammography and breast magnetic resonance imaging (BRMRI)*
Breast pattern NDBPP DBPP
according to group group p (by χ2)
mammography (n) (n = 22) (n = 84)
Exact match between 19 (86.4) 48 (57.2) < 0.05 digital mammography and
BRMRI interpretations Partial match between
digital mammography and 2 (9.1) 20 (23.8) < 0.05 BRMRI interpretations
No match between 1 (4.5) 16 (19.0) < 0.05 digital mammography
and BRMRI interpretations
*Data are presented as n (%). NDBPP = non-dense breast paren-chyma pattern; DBPP = dense breast parenchyma pattern.
are more specific than ultrasound alone or in combina-tion with mammography. MRI underestimates the tumor extent in 12.5% of cases, and has been shown to have the highest sensitivity for tumor detection, while mam-mography and sonography underestimate the tumor extent in 37% and 40% of cases, respectively.10 More-over, MRI has been found to be more accurate in assess-ing tumor extent and multifocality in patients with dense breasts. In patients with multifocal or multicentric car-cinoma, mammography detected lesions in 35% of cases, ultrasound detected 30%, and MRI detected 100%.10
van Gils et al11 demonstrated that the prevalence of NDBPP (ACR types 1 and 2) could be as high as 78%, com-pared with 22% for DBPP (ACR types 3 and 4), which is representative of most Western countries (Table 2). The ratio of NDBPP to DBPP in our study sample is almost reversed compared with their previous results. Although the case number is small, we believe that the results are representative of developed Asian countries such as Taiwan, Hong Kong, North Korea, Singapore, and Japan. van Gils et al11 also found that women with high mammographic breast densities are at higher risk of breast cancer; the incidence of breast cancer in NDBPP was 26.4% versus 73.4% in DBPP. Their study investi-gated whether these women should receive more fre-quent screening or screening with alternative techniques that increase the length of the preclinical detectable phase to reduce breast cancer mortality.
Breast density is a major factor influencing the incidence of breast malignancy, and has been dis-cussed extensively in the past two decades. In a normal woman, mammographic densities correspond to dif-ferent amounts of fat and connective and epithelial tis-sue. Fat appears radiographically dark on film-screen mammograms, and radiographically opaque areas rep-resent epithelial and connective tissues.12 Most cases of high mammographic density are not abnormalities, but varied distributions in healthy breast tissue. Similar to van Gils et al,11 Boyd et al13 found that high mammo-graphic density may be related to a fourfold increased risk of developing breast cancer. Kolb et al14 found that the diagnostic sensitivity of mammography in women with a fatty breast pattern is 98%. Mammography alone detected 45% of all cancers and 30% of all invasive can-cers; however, in patients with DBPP, the sensitivity of mammography was as low as 48%. It has been shown that mammography detected 20.5% of all cancers and 9.4% of all invasive cancers in patients with DBPP.14 Asian women have higher breast densities relative to the smaller breast size compared with Caucasian women. Maskarineca et al15 found that the percentage of dense tissue to the whole breast volume of both Chinese and Japanese women appeared to be higher than that in Caucasian women. In spite of the considerably smaller proportion of the non-dense areas, the overall propor-tion of dense breast tissue in breasts of Chinese and Japanese women is 20% higher than in Caucasian women
in the same age group.15 Maskarineca et al also showed that regardless of race, women with lower mammo-graphic densities have a lower risk of breast cancer. It is unclear whether the presence of many dense areas in the breast corresponds to a higher cancer risk.15–17 In fact, mammographic density usually reflects the opac-ity of epithelial and stromal tissue in the breast within the lucent background of non-dense fatty tissue. Ductal carcinoma in situ and infiltrative ductal carcinomas origi-nate in epithelial cells, and therefore, greater areas of fibroglandular tissue with greater numbers of cells are at a higher risk during increased epithelial prolifera-tion.18 Stromal fibrosis also contributes to high mammo-graphic densities. It is possible that there is a relationship between stromal fibrosis and the risk of breast cancer. This can be explained by the variety of growth factors that are thought to play a role in both breast develop-ment and cancer developdevelop-ment.19–21 The masking hy-pothesis proposed by Egan and Mosteller may also explain why radiographically dense patterns are associ-ated with an increased risk of breast cancer.22 They found that breast cancer was not difficult to detect by mam-mography in breasts with non-dense glandular paren-chyma, but was unreliable in detecting cancer in dense glandular parenchyma. Cases of missed cancer detec-tion during a first mammographic examinadetec-tion due to the masking effect of dense glandular tissue of the breast may be detected in subsequent mammographic exami-nations. The apparent excess of cancers detected in this specific group with initial masking of the tumor in dense breasts can cause the group to appear to be at a higher risk than those with non-dense breast tissue.
In Taiwan, a national project of 2-year interval screen-ing mammograms for 50- to 70-year-old women has detected significantly more early breast cancers.23 The role of mammographic screening in decreasing the mor-tality rate is a derived benefit in this specific age group, as they are more predisposed to NDBPP. However, sta-tistics collected from the Hong Kong Cancer Registry24 show a correspondingly high incidence of breast can-cer in women younger than 40 years old in a popula-tion mainly from southern China. Unfortunately, it has also been reported that breast cancer in this age group may have more aggressive biological characteristics.25 This pathological pattern is also commonly seen in our clinical practice in Taiwan. In studies of Japanese women, Uchida et al26 reported that mammography missed 16% of breast cancers. Nagata et al27 also confirmed that breast density is an important determinant of breast cancer risk in Japanese women. They quantitatively mea-sured the mammographic density, and found that a higher risk was associated with a larger breast size and with a higher proportion of glandular density, espe-cially for the extremely dense pattern.27 A study by Jakes et al28 in Singapore showed an increased risk of breast cancer associated with a higher-density pattern with characteristics of extensive nodular and linear
densities, with a nodular size larger than normal lobules. In South Korea, although breast cancer is already the most common female cancer, its early detection rate is low compared with Western developed countries.29 In South Korea, there is a continually increasing incidence of breast cancer, with a recorded historic high incidence of 31.4 cases per 100,000 women in 2002, according to data from the Korean Breast Cancer Society, which is only slightly lower than that seen in Japan with an inci-dence of 42.3 per 100,000 women. Son et al30 found that the clinical characteristics of Korean breast cancer pa-tients showed a pattern of a younger age (< 50 years old) and increasing early stage and asymptomatic cases. This finding reflects the need for more effective breast screening programs for young Korean women.30
However, our data suggest that there are significant limitations to the use of mammography in women with dense breast tissue. We propose that it is difficult for dig-ital mammography to serve as a screening and diagnostic tool for Asian and Chinese women with DBPP. There are some limitations of this study, including a small patient number, not considering both positive and false-negative results of BRMRI and mammography, and breast sonography was not included in this study. Although sonography has been shown to have advantages for screening in patients with DBPP, the overall tumor detec-tion rate is not satisfactory, and thus its reliability is limited. In conclusion, digital mammography is useful due to its accurate detection in patients with NDBPP. However, since Asian woman frequently have dense breasts, es-pecially in the premenopausal population, some breast cancers might be missed if screening involves only mammography. Unless its use is limited for financial reasons, BRMRI should be considered for a wider appli-cation for Asian women and in other populations with dense breasts.
References
1. Shen YC, Chang CJ, Hsu C, Cheng CC, Chiu CF, Cheng AL. Significant difference in the trends of female breast cancer inci-dence between Taiwanese and Caucasian Americans: implica-tions from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev 2005;14:1986–90.
2. Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 1976;37:2486–92. 3. El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mam-mographic breast density by race. Ann Epidemiol 2001;4:257–63. 4. Kopans DB, D’Orsi CJ, Adler DD. Breast Imaging Reporting and
Data System, 2nd ed. Reston, Virginia: American College of Radiology, 1995.
5. Marilyn AR, Judith S, Kent AG, Barbara S, Paul N, Jeff S. Relation-ship of mammographic parenchymal patterns to breast cancer risk factors and smoking in Alaska native women. Cancer Epidemiol Biomarkers Prev 2003;12:1081–6.
6. Erguvan-Dogan B, Whitman GJ, Kushwaha AC, Phelps MJ, Dempsey PJ. BI-RADS-MRI: a primer. AJR Am J Roentgenol 2006;187:W152–60. 7. Thompson J, Leach MO, Kwan-Lim G, Gayther SA, Ramus SJ,
Warsi I, Lennard F, et al. Assessing the usefulness of a novel MRI-based breast density estimation algorithm in a cohort of women at high genetic risk of breast cancer: the UK MARIBS study. Breast Cancer Res 2009;11:R80.
8. Leung TK, Chu JS, Huang PJ, Lin YH, Lee CM, Chen CS, Tai CJ, et al. Breast MRI for monitoring images of an adenomyoepithelioma with malignant features, before, during, and after chemother-apy. Breast J 2010, in press.
9. Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23:8469–76. 10. Van Goethem M, Schelfout K, Dijckmans L, Van Der Auwera JC,
Weyler J, Verslegers I, Biltjes I, et al. MR mammography in the pre-operative staging of breast cancer in patients with dense breast tissue: comparison with mammography and ultrasound. Eur Radiol 2004;14:809–16.
11. van Gils CH, Otten JD, Hendriks JH, Holland R, Straatman H, Verbeek. High mammographic breast density and its implications for the early detection of breast cancer. J Med Screen 1999;6:200–4. 12. Gram IT, Funkhouser E, Tabar L. The Tabar classification of
mam-mographic parenchymal patterns. Eur J Radiol 1997;24:131–6. 13. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ.
Mam-mographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1998;7:1133–44.
14. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002;225:165–75. 15. Maskarineca G, Menga L, Ursinb G. Ethnic differences in
mam-mographic densities. Int J Epidemiol 2001;30:959–65.
16. Tseng M, Byrne C, Evers KA, London WT, Daly MB. Acculturation and breast density in foreign-born, U.S. Chinese women. Cancer Epidemiol Biomarkers Prev 2006;15:1301–5.
17. Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Peterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 2005;6:798–808. 18. McCormack VA, dos Santos Silva. Breast density and
parenchy-mal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159–69.
19. Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, White E. Breast density as a predictor of mammo-graphic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000;92:1081–7.
20. Boyd NF. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst 1992;84:1170–9. 21. Oza AM, Boyd NF. Mammographic parenchymal patterns: a
marker of breast cancer risk. Epidemiol Rev 1993;15:196–208. 22. Egan RL, Mosteller RC. Breast cancer mammography patterns.
Cancer 1977;40:2087–90.
23. Chen CY, Tzeng WS, Tsai CC, Mak CW, Chen CH, Chou MC. Adjusting mammography-audit recommendations in a lower-incidence Taiwanese population. J Am Coll Radiol 2008;5:978–85.
24. Hong Kong Cancer Registry. Cancer Registry Annual Report. Hong Kong SAR: Hong Kong Hospital Authority, 2004.
25. Kwong A, Cheung P, Chan S, Lau S. Breast cancer in Chinese women younger than age 40: are they different from their older counterparts? World J Surg 2008;32:2554–61.
26. Uchida K, Yamashita A, Kawase K, Kamiya K. Screening ultrasonog-raphy revealed 15% of mammographically occult breast cancers. Breast Cancer 2008;15:165–8.
27. Nagata C, Matsubara T, Fujita H, Nagao Y, Shibuya C, Kashiki Y, Shimizu H. Mammographic density and the risk of breast cancer in Japanese women. Br J Cancer 2005;92:2102–6.
28. Jakes RW, Duffy SW, Ng FC, Gao F, Ng EN. Mammographic paren-chymal patterns and risk of breast cancer at and after a prevalence screen in Singaporean women. Int J Epidemiol 2000;29:11–9. 29. Ryu E, Ahn O, Baek SS, Jeon MS, Han SE, Park YR, Ham MY.
Predictors of mammography uptake in Korean women aged 40 years and over. J Adv Nurs 2008;64:168–75.
30. Son BH, Kwak BS, Kim JK, Kim HJ, Hong SJ, Lee JS, Hwang UK, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg 2006;141:155–60.