國 立 交 通 大 學
生 物 科 技 系 暨 研 究 所
碩士論文
同時轉染 IL-6 轉殖基因可提升樹突狀細胞與
分泌 TGF-β1 細胞融合疫苗的免疫力
Superior immunogenic effects of IL-6 gene-simultaneously-transfected dendritic cell/TGF-β1-expressed cell fusion vaccine
研 究 生:溫立筠
指導教授:廖光文 教授
同時轉染 IL-6 轉殖基因可提升樹突狀細胞與
分泌 TGF-β1 細胞融合疫苗的免疫力
Superior immunogenic effects of IL-6 gene-simultaneously- transfected dendritic cell/TGF-β1-expressed cell fusion vaccine
cytokines 研 究 生:溫立筠 Student:Li-Yuan Wen 指導教授:廖光文 Advisor:Kuang-Wen Liao 國 立 交 通 大 學 生 物 科 技 系 碩 士 論 文 A Thesis
Submitted to Department of Biological Science and Technology College of Biological Science and Technology
National Chiao Tung University in partial Fulfillment of the Requirements
for the Degree of Master
in
Biological Science and Technology
July 2009
i
同時轉染 IL-6 轉殖基因可提升樹突狀細胞與
分泌 TGF-β1 細胞融合疫苗的免疫力
研究生:溫立筠 指導教授:廖光文 博士 國立交通大學生物科技系暨研究所中文摘要
在近年來,樹突細胞相關的抗癌疫苗(Dendritic cell (DC)-based antitumor vaccine)被視為很具發展潛力的癌症免疫治療方式。然而,在臨床的 實行上只有某部分的病人在經過樹突細胞疫苗治療後有出現明顯腫瘤消退的現 象。其中一個可能的解釋是很多惡性腫瘤可能會透過分泌免疫抑制的因子,像 TGF-β1,的方式透過抑制宿主的免疫反應來限制樹突細胞疫苗的效力。在此研 究中,我們展現了同時地將免疫調節相關的基因傳遞進細胞中並融合細胞可以提 升樹突細胞融合疫苗的效力。 在本研究中,IL-6 因為具有拮抗 TGF-β1 的免疫抑制活性的能力,被拿來 作為實驗中的轉殖基因。此外,LPPC (Lipo-PEI-PEG Complex),我們實驗室所 開發的創新帶正電微脂體,在同時融合與轉染的過程中扮演轉染劑的角色。結果 顯示,LPPC 的加入不會影響 PEG 的融合效率而且同時地 IL-6 轉殖基因也成功地 被 LPPC 帶入細胞。一致地,接種 LPPC/IL-6/PEG 樹突細胞融合疫苗的動物引發 出較佳的免疫反應。因此,我們認為此製作疫苗的策略具有在臨床應用上提升基 因改良的樹突細胞融合疫苗的應用潛能。
ii
Superior immunogenic effects of IL-6 gene-simultaneously
-transfected dendritic cell/TGF-β1-expressed cell fusion vaccine
Student: Li-Yuan Wen Advisor: Dr. Kuang Wen Liao Institute of Biological Science and Technology
National Chiao Tung University
ABSTRACT
Dendritic cell (DC)-based antitumor vaccine is a promising immunotherapy for cancer in recent years. However, the clinical results in the clinical trails showed that the treatments of DC vaccines benefit only a subset of patients to result in tumor regression. One possible explanation has been suggested that many malignant tumors would secrete immunosuppressive factors such as TGF-β1, which is possible to limit the efficacy of the DC-based vaccine by suppressing the host immune responses. In this study, we demonstrated that delivering an immuno-modulated gene into cells and fusing cells simultaneously could improve the efficacy of DC fusion vaccine.
IL-6, an antagonist against the immunosuppressive activity of TGF-β1, was used as the transgene in this study. In addition, LPPC, a novel cationic liposome, plays the role in the co-transfection during fusion process.The results showed that the addition of LPPC didn’t interfere with the fusion efficiency of PEG and it indeed transfected simultaneously the IL-6 gene into the cells during the fusion process. Consistently, animals vaccinated with LPPC/IL-6/PEG DC vaccines elicit superior immunogenic effects. Therefore, this strategy may have the potential to be a promising strategy for gene-modified DC fusion vaccine in the clinical use.
iii
Acknowledgement
轉眼間,想不到這已是我生活在新竹的第六個年頭了。想到兩年前,因為 認為興趣比什麼都重要的想法,讓我決定從應用化學系轉換跑道來生科學院。謝 謝廖光文老師願意收一個完全門外漢的學生(而且還是那多出來的一個…);謝謝 廖光文老師兩年來不論是學術或是做人做事上的指導,甚至在已經是畢業前夕了, 依然不忘給我做最後的叮嚀,我真的很感謝老師,我會一直銘記在心,時時提醒 自己的。也要謝謝這兩年來所有給過我幫助的人;中研院生醫所的陶秘華老師 和 Butter 學長,謝謝您們給我機會學習取出和培養 BMDCs 的技術以及提供 GM-CSF,那是我論文的基石;黃兆祺老師和逸禪,謝謝老師願意借我螢光顯微鏡 來使用,而且還很有耐心地解決我使用以及處理影像的問題,我真的學到很多, 你們家的螢光顯微鏡真的超棒的!另外也要謝謝交大材料所的維琳幫我前處理 要照電子顯微鏡的樣品還有交大應化系的廖奕翰老師和 David 願意借我 AFM 並幫 我照樣品,真是超級感謝您們的。還有我的大學同學兼室友茹夢,謝謝妳嘗試幫 我照 SEM,還幫我找朋友測表面電位;也是大學同學的子正,謝謝你幫我測 DLS。 我知道我寫在這裡的感謝,大部分的您們可能都沒辦法看到,但我真的由衷地感 謝各位老師、學長和朋友們,是您們讓我知道學術是沒有界限的,只要有學術問 題,總會有各種領堿的人熱心地伸出援手的,這讓我覺得格外溫暖。 當然,每天朝夕相處的實驗室夥伴們也提供了我好多好多的幫助,不論是生 活上的或是工作上的。我們組的 leader,彥谷學長和于鈴學姐,你們帶著毫無 經驗的我入門,教我各種實驗操作流程、陪我設計實驗和討論實驗結果,這對一 開始懵懂的我幫助真的很大,謝謝你們;尤其是于鈴學姐,你總是不厭其煩地聆 聽我的煩惱,不論是實驗上的或是生活裡的,真的很感謝妳!做事有效率又很熱 心、細心的阿伯,這兩年真的麻煩你很多,很感謝你,我相信你一定會是個很罩 的大學長 XD!陳小莉妳一直是我的好夥伴,很開心能進第一天進 lab 就認識妳, 相信妳這麼有毅力一定能實現自己的理想的,加油哦!何珮和啾咪,雖然我們 在不同組,共事的機會不多,不過一起玩樂的次數倒不少 XD,大家一起有過的 美好回憶會永遠刻劃在腦袋中的,祝妳們都一切順利,心享事成哦!還有靜敏、 馬馬、筱葳和維瞳,你們真的都是很棒的夥伴,不論是在實驗上給我的幫忙或是 平時給我的加油打氣,我真的都是既感謝又感動,謝謝你們哦,我相信你們一定 也都會實驗順利的,大家加油!最後,也要感謝我的家人們:爸爸、媽媽、哥哥、 書寧和鄭棋文,你們幫了我好大好大好大的忙,在我最無助、最谷底的時候,拉 著我往樂觀處走,不讓我喪失信心;尤其是鄭棋文,謝謝你每天陪在我身邊,不 管是半夜或是假日,只要我上工,你總是不缺席。也因為有你無微不至的照顧和 照三餐不間斷的鼓勵,我才能撐過最後這段艱難的日子,真的真的很謝謝你,很 開心有你這個家人。
iv
Contents
Abstract in Chinese ... i
Abstract ... ii
Acknowledgement ... iii
Contents ... iv
List of Figures ... vi
List of Table ... vii
Abbreviations ... viii
Chapter 1. Introduction ... 1
Chapter 2. Materials and methods ... 4
2.1 Materials ... 4 2.1.1 Plasmids ... 4 2.1.2 Chemicals ... 4 2.1.3 Antibodies ... 6 2.1.4 Kits ... 6 2.1.5 Buffers ... 6 2.1.6 Cell lines ... 8
2.1.7 Media and cytokines ... 8
2.1.8 Mice ... 9
2.2 Methods ... 9
2.2.1 Preparation of Lipo-PEI-PEG Complex (LPPC) ... 9
2.2.2 Nanoparticle characterization by Scanning Electron Microscopy ... 10
v
2.2.4 Evaluation of particle size and charge ... 10
2.2.5 Plasmids DNA extraction ... 11
2.2.6 In vitro transfection ... 12
2.2.7 Generation of red fluorescent protein-secreting BALB/3T3 and green fluorescent protein-secreting B16-F10 to build up fusion model ... 12
2.2.8 Generation of bone marrow-derived DCs and phenotype of cell surface ... 13
2.2.9 Co-transfection-fusion protocol ... 13
2.2.10 TGF-β secretion of different cell lines detected by ELISA ... 14
2.2.11 TGF-β derived from immortalized cells on BMDC’s phenotypes ... 14 2.2.12 Splenocyte isolation ... 15 2.2.13 Animal immunization ... 16 2.2.14 Statistical analysis ... 16 Chapter 3. Results... 18 3.1 Liposome characterization ... 18
3.2 In vitro transfection efficiency of LPPC/DNA complex ... 18
3.3 The effect of LPPC on the co-transfection-fusion protocol ... 20
3.4 The effect of TGF-β derived from immortalized cells on bone marrow- derived dendritic cells (BMDC) ... 21
3.5 Generation of DC/TGF-β-expressed cell fusion vaccines using the co- transfection-fusion protocol ... 22 3.6 Evaluation of the efficacy of co-transfection-fusion DC vaccine
vi
... 23
Chapter 4. Discussion ... 25
References ... 47
vii
List of Figures
Fig. 1 Micrograph of Lipo-PEI-PEG Complex (LPPC) nanoparticles .... 30
Fig. 2 DNA retarding assay for LPPC-DNA complexes ... 31
Fig. 3 Particle size and zeta-potential analysis of LPPC-DNA complexes
... 32
Fig. 4 In vitro transfection efficiency of LPPC/DNA complexes ... 33
Fig. 5 Addition of LPPC has no effect on fusion efficiency caused by
PEG ... 34
Fig. 6 Cytokine production by fusion cells after the co-transfection-fusion
protocol ... 36
Fig. 7 Generation of bone marrow-derived dendritic cells ... 37
Fig. 8 TGF-β secretion of different cell lines ... 38
Fig. 9 Effect of TGF-β derived from immortalized cells on BMDC’s
phenotypes ... 39
Fig. 10 Fusion efficiencies of LPPC/IL-6/PEG DC vaccine and PEG DC
vaccine ... 40
Fig. 11 Cytokine production by LPPC/IL-6/PEG DC fusion cells and
PEG DC fusion cells ... 42
Fig. 12 TGF-β secretion by DC fusion cells ... 43
Fig. 13 Cytokines profiles produced by splenocytes from mice
immunized with LPPC/IL-6/PEG DC vaccine or PEG DC vaccine ... 44
Fig. 14 The cell proliferation of Ag-specific splenocytes from
LPPC/IL-6/PEG DC vaccine-immunized mice ... 45
viii
List of Table
ix
Abbreviations
APCs antigen-presenting cells CTLs cytotoxic T lymphocytes DCs dendritic cells IFN-γ interferon-γ
IL-6 interleukin-6 IL-10 interleukin-10 TNF- tumor necrosis factor-
MHC major histocompatibility complex PBS phosphate buffer saline
LPPC Lipo-PEI-PEG-Complex PEG polyethylene glycols
LPS lipopolysaccharide TAA tumor-associated antigens TGF- transforming growth factor beta
GM-CSF Granulocyte-macrophage colony-stimulating factor
1
Chapter 1. Introduction
Dendritic cells (DCs) are specialized APCs that can activate specific CTLs and Th cells through Ag presentation via MHC class I and class II molecules, respectively (Porgador and Gilboa 1995; Ohshima and Delespesse 1997; Shen, Reznikoff et al. 1997). The characters of DCs have resulted in the development of DC-based tumor vaccines, which have been proven to induce strong antitumor effect in animal studies (Gong, Apostolopoulos et al. 2000; Homma, Toda et al. 2001) and are used clinically already (Kugler, Stuhler et al. 2000; Kikuchi, Akasaki et al. 2001; Kugler, Stuhler et al. 2003).
As the expected emergence of Ag-loss variants and the fact that few TAAs has been defined, DC-based vaccines that generated using whole tumor-derived material, such as live tumors or tumor lysates, are favored recently. DC fusion vaccine has been reported to give protects against the lethal challenge of tumor cells and eradicate established tumors effectively (Gong, Chen et al. 1997; Lespagnard, Mettens et al. 1998; Gong, Koido et al. 2002). However, in the clinical trials, although the vaccination with DC/tumor hybrids stimulated antitumor immune responses in a majority of patients, only a subset demonstrated a significantly tumor regressions (Avigan, Vasir et al. 2004; Berzofsky, Terabe et al. 2004).
One possible explanation for the limited clinical outcome is that whole-tumor substrate may comprise immunosuppressive factors, such as TGF-β, IL-10 or PGE-E2 that weaken the vaccine efficacy (Kuppner, Sawamura
2
Furthermore, there have been demonstrated that tumor-derived TGF-β inhibits the efficacy of the DC/tumor fusion vaccine, and this effect appears to occur in the microenvironment where DCs and T cells interact (Kao, Gong et al. 2003). These findings suggest that tumor-derived TGF-β respresent a critical target for improving the efficacy of DC/tumor fusion vaccine.
According to literatures, interleukin (IL)-6 almost completely antagonizes the immunosuppressive effects of TGF-β on T cell proliferation in eyes with endotoxin-induced uveitis (Ohta, Yamagami et al. 2000). Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-β and restores the NK killing activity (Hsiao, Liao et al. 2004). Therefore, the IL-6’s anti-TGF-β activities may be used to relieve the inhibitory effect of tumor-derived TGF-β on the efficacy of DC fusion vaccine to generate a superior DC/tumor fusion vaccine.
On the other hand, genetically-engineered cell-based vaccines have also been reported to elicit the potent anticancer immune responses (Hsieh, Chen et al. 2000; Berzofsky, Terabe et al. 2004; Frankenberger, Regn et al. 2005; Naruishi, Timme et al. 2006). The B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic IL-2 has been proven to induce immunological and clinical responses in some of the patients with metastatic renal cell carcinoma (Antonia, Seigne et al. 2002). The cytokine-secreting tumor vaccine, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), also induces strong tumor-specific immunogenic effects in clinical trials (Nelson, Simons et al. 2000).
Consequently, combined the concepts of DC/tumor hybrids and genetically-engineered tumor cells, we designed a strategy to antagonize the effect of tumor-derived TGF-β in the microenvironment by simultaneously
3
delivering the IL-6 gene into DCs, tumor cells, or fused cells during the fusion process. In this study, we used LPPC, a novel cationic liposome developed by our laboratory, as the transfection reagent to perform the co-transfection-fusion strategy. LPPC could not only deliver IL-6 gene into the cell mixture successfully but also has no effect on the fusion efficiencies of vaccine during fusion process. Although the DC vaccines made by co-transfection-fusion strategy still continue to secrete TGF-β, these vaccines could elicit superior immunogenic effects in the short period of immunization than the DC vaccine made by PEG alone, which proposed the gene transfection and cell fusion can be performed simultaneously.
4
Chapter 2. Materials and Methods
2.1 Materials
2.1.1 Plasmids
Plasmid Description Source pAAV-MCS pCMV promoter for MCS Stratagene,
CA, USA pAsRed2-N1 Encodes AsRed2, a variant of
Anemonia sulcata red fluorescent
protein
BD
Biosciences Clontech pAAV-MCS-IL-6 SalI-HindIII fragment, containing
human IL-6 fragment
Liao’s lab
pAAV-MCS-hrGFP BamHI-XhoI fragment, a reporter gene
Liao’s lab
2.1.2 Chemicals
Chemical Source Application 100bp DNA ladder Protech DNA
electrophoresis 1Kb DNA ladder Protech DNA
electrophoresis 3,3’-dioctadecyloxacarbocyanine
(DiOC18)
5
Agarose MDBio DNA electrophoresis Ampicillin AMRESCO Antibiotic DLPC Avanti LPPC
prepration
DMEM GIBCO cell culture medium
DOPC Avanti LPPC prepration
EDTA Tedia Cell passage EtBr AMRESCO DNA staining Fetal Bovine Serum GIBCO Cell culture G418 SIGMA Antibiotic for
cell selection Isopropanol C-Echo DNA extraction LB agar AMRESCO Transform
Propium iodide (PI) SIGMA Cell live/dead staining
Penicillin-Streptomycin-Amphotericin-B mix solution (PSA)
Biological Industries
Cell culture
Poly(ethylene) glycol (PEG) SIGMA Cell fusion RPMI GIBCO Cell culture
medium
Trypan blue stain GIBCO Cell staining Trypsin GIBCO Cell passage
6
Tween 20 MP Detergent for ELISA washing
buffer
2.1.3 Antibodies
Antibodies Source Catalog number Mouse anti-mouse MHC class I Serotec MCA2189
PE anti-mouse CD11c BioLegend 117307 FITC rat anti-mouse IgG BioLegend 406001 FITC anti-mouse I-A/I-E BioLegend 107605 FITC anti-mouse CD86 BioLegend 105005
2.1.4 Kits
Kit Source Catalog number Application NucleoBond
PC100
Macherey-Nagel 740573 DNA extraction
Mouse IL-2 R&D DY402 ELISA Mouse IL-4 R&D DY404 ELISA Human IL-6 R&D DY206 ELISA Mouse IL-10 R&D DY417 ELISA Mouse IFN-γ R&D DY485 ELISA Mouse TGF-β1 R&D DY1679 ELISA
2.1.5 Buffers
7
0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA in ddH2O
1X PBS
137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4
2% Blocking buffer
1 g nonfat powdered milk dissolved in 50 ml 1X PBS buffer 50X TAE buffer
242 g Tris base, 0.5 M EDTA (pH8.0) 100 ml, 57.1 ml acetic acid, then add ddH2O to 1 L.
Buffer S1 (for DNA extraction)
50 mM Tris-HCl, 10 mM EDTA, 100 μg/ml RNase A, pH 8.0 Buffer S2 (for DNA extraction)
200 mM NaOH, 1% SDS Buffer S3 (for DNA extraction)
2.8 M KAc, pH 5.1
Buffer N2 (for DNA extraction)
100 mM Tris, 15% ethanol, 900 mM KCl, 0.15% Triton X-100, adjusted to pH 6.3 with H3PO4
Buffer N3 (for DNA extraction)
100 mM Tris, 15% ethanol, 1.15 M KCl, adjusted to pH 6.3 with H3PO4
Buffer N5 (for DNA extraction)
100 mM Tris, 15% ethanol, 1 M KCl, adjusted to pH 8.5 with H3PO4
EDTA-trypsin
2.5 g trypsin, 0.1 M EDTA (pH8.0) in 1L 1X PBS, pH 7.4, 0.2 μm filtered. LB medium
8
Reagent Diluent (for ELISA)
1% BSA in PBS, pH7.2-7.4, 0.2 μm filtered Staining buffer (for surface marker expression)
0.5% nonfat powdered milk in 1X PBS buffer Stop solution (for ELISA)
1 N HCl PBST 0.5% Tween 20 in 1X PBS buffer Versene 0.2 g EDTA in 1 L 1X PBS 2.1.6 Cell lines
Murine BALB/3T3 fibroblasts and murine B16-F10 melanoma cells were obtained from American Type Culture Collection (ATCC) and grown in CM. Additional culture supernatants used for the study were from other tumor cell lines: JC (murine mammary adenocarcinoma), CT-26 (murine colon cancer cell line), K-BALB (murine Ki-MSV transformed fibrosarcoma) and control 3T3 (fibroblasts).
2.1.7 Media and cytokines
Complete medium (CM) for BALB/3T3_pAsRed and B16.F10_pLEGFP consisted of DMEM with 10% heat-inactivated FBS (GIBCO) and 1% Penicillin-Streptomycin-Amphotericin-B mix solution (Biological Industries).
9
heat-inactivated FBS, 1% PSA and 2-ME (50ϻM). Recombinant mouse GM-CSF was a gift from Dr. Tao, Mi-Hua of Institute of Bio-Medical Sciences, Academia Sinica, Taipei, Taiwan.
2.1.8 Mice
Female BALB/c mice aged 3-5 wk were purchased from The National Laboratory Animal Center and housed at the Animal Maintenance Facility at The National Chiao Tung University. Experiments were conducted at age 10-14 wk.
2.2 Methods
2.2.1 Preparation of Lipo-PEI-PEG Complex (LPPC)
DOPC and DLPC (50mg/ml) were added into the round bottom flask, and 1 ml methanol was as well. Then the container of lipid mixture was placed to the rotary evaporator (37℃, without vacuum treatment, minimum rotary speed) until dry. After about 2 days, the dry lipid film was hydrated by steam (about 37℃) for 2~3 hours. And 5ml aqueous medium (containing 0.675g PEI and 0.22g PEG) was added gently to the container of lipid. Then the container was kept agitating violently for 10 minutes. After vortex, place the LPPC at RT overnight. And the turbid medium of LPPC was extruded through 200 nm mesh nine times. Then the product could be used for the following experiment.
10
2.2.2 Nanoparticle characterization by Scanning Electron Microscopy
The morphology of nanoparticles was observed by scanning electron microscopy (SEM). The nanoparticle formulations were prepared as described above and diluted in demineralized water. Then the sample was directly transferred and dried onto a silicon chip by vacuum freeze-drying system. The SEM picture was taken at ×10,000, 10 kV.
2.2.3 DNA retarding assay
The degree of complexation between different amount of LPPC with constant amount of DNA was observed by the DNA retarding assay. 1μg DNA was pre-incubated with SYBR Green I nucleic acid gel stain (Molecular Probes, Invitrogen), and the stained DNA was then incubated with different amount of LPPC to form liposome-DNA complex (lipoplex) for 30 minutes at room temperature. The liposome-DNA complexes were electrophoresed in a 0.8% agarose gel; the gel-running time was 30 minutes at 100V and 500 mA. The liposome-DNA complexes were treated with heparin (1 μg) as the control groups to see if heparin could release DNA from the liposome-DNA complexes. The gel was photographed over a UV box.
2.2.4 Evaluation of particle size and charge
The particle size of the LPPC-DNA complexes was measured by the dynamic light scattering. Complexes used in the measurement were of 18 μg DNA content and formed in 1 ml ddH2O that was pre-filtered with a 0.22 μm
11
The surface charge of the LPPC-DNA complexes was measured as zeta potential by a ZetaPlus Z-potential analyzer.
2.2.5 Plasmids DNA extraction
Plasmids pAsRed2-N1 and pLEGFP were two reporter genes who encoded red fluorescent protein and green fluorescent protein, respectively. Besides, the pIL-6 plasmid was created by inserting a human IL-6 gene into a pAAV-MCS vector. And these plasmids were purified from transformed Escherichia coli using a NucleoBond PC 100 kit (Macherey-Nagel, Duran, Germany) according to the manufacturer’s instructions. At first, a single colony of E. coli was inoculated in 100 ml of LB broth contained antibiotics and at 37℃ at agitation for 16 hours. The bacteria were recovered by centrifugation at 8000 rpm for 15 minutes at 4℃. The pellet was collected, and 4 ml buffer S1 with RNase (Macherey-Nagel, Duran, Germany) was added to dispense the pellet. Then 4 ml buffer S2 (Macherey-Nagel, Duran, Germany) was added to the suspension. The lysate was mixed gently and incubated at room temperature for 3 minutes (no more than 5 minutes). The pre-cooled 4 ml buffer S3 (Macherey-Nagel, Duran, Germany) was then added to the solution and mixed gently until a homogeneous suspension containing and off-white flocculate as formed. The mixture was incubated on ice for 5 minutes and then spun at 13000 rpm for 25 minutes at 4℃. The supernatant was loaded onto the NucleoBond AX 100 Midi column which was equilibrated with 2.5 ml buffer N2 (Macherey-Nagel, Duran, Germany). The flow-through was emptied by gravity flow and discarded. 10 ml buffer N3 (Macherey-Nagel, Duran, Germany) was added to wash the column twice. The DNA was eluted by 5 ml buffer N5 (Macherey-Nagel, Duran, Germany). Then 3.5 ml isopropanol was added to precipitate the DNA. The mixture was
12
incubated on ice for 10 minutes and recovered by centrifugation at 13000 rpm for 30 minutes at 4℃. 6 ml 70% ethanol was added to the pellet and the solution was spun at 13000 rpm for 5 minutes. Finally, the pellet was dissolved in appropriate amount of ddH2O and stored at -20 .℃
2.2.6 In vitro transfection
Cells were seeded in the 6-well plate at a density of 2.5×105 cells/well and cultured with 2 ml growth medium for 24 hours respectively. Cells were transfected plasmid DNA encoding GFP with different amounts of LPPC. Briefly, 3 μg plasmid DNA and 50μg LPPC were each diluted into 250μl Opti-MEM I medium (Gibco, Grand Island, NY) and vortexed. The DNA solution was added into LPPC solution after 5 minutes (Notice: do not reverse the order), and then vortexed. After 25 minutes, the cells were rinsed and supplemented with 200μl Opti-MEM I medium. The LPPC/DNA mixture was gently added to each well. Finally, 600μl Opti-MEM I medium were added into each well. After 12 hours incubation, 2 ml fresh growth medium were added into each well. After 48 hours the gene expressions were measured by FACScan flow cytometry (Becton Dickinson, San Jose, CA).
2.2.7 Generation of red fluorescent protein-secreting BALB/3T3 and green fluorescent protein-secreting B16-F10 to build up fusion model
BALB/3T3 was transfected with pAsRed2-N1 (BD Biosciences Clontech ) and B16-F10 was transfected adenovirusly with plasmids DNA encoding GFP green fluorescent protein, respectively. To select the fluorescent-permanent cell lines, limit dilution and usage of antibiotics, G418 (500 μg/ml), were performed to BALB/3T3_pAsRed cells. Besides, only G418 selection (1.5 mg/ml) was
13
used to B16-F10_pLEGFP cells. The intensities of fluorescence expression were analysis by FACS analysis. These two fluorescent proteins-expressed cell lines were used to measure the effect of LPPC addition on fusion efficiencies as the model.
2.2.8 Generation of bone marrow-derived DCs and phenotype of cell surface
Erythrocyte-depleted murine bone marrow cells obtained from BALB/c mouse femurs and tibias were cultured in CM supplemented with 200U/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (Lutz, Kukutsch et al. 1999). At day 0 bone marrow precursors were seeded at 2.5 × 106 per 100mm bacteriological dish in 10 ml CM containing rmGM-CSF. At day 3 another 10 ml CM containing 200U/ml rmGM-CSF were added to the plates. At day 6 half of the culture supernatant was collected, centrifuged, and the cell pellet resuspended in 10 ml fresh CM containing 200 U/ml rmGM-CSF, and given back into the original plate. At day 8 non-adherent cells were collected by gentle pipetting, centrifuged at 300g for 5 min at RT, and resuspended in 10 ml CM into a fresh 100 mm tissue culture plastic dish containing 200U/ml rmGM-CSF and 0.5 ϻg/ml LPS for 2 days for complete maturation. Then the non-adherent cells with the typical morphologic features of DCs were used for the experiment. For phenotypic analysis, immature DCs at day 8 and mature DCs at day 10 were stained with a panel of antibodies, including MHC class I and II, CD86 and CD11c (BioLegend, San Diego, CA) and quantified by flow cytometry (Becton Dickinson, San Jose, CA).
2.2.9 Co-transfection-fusion protocol
14
2:1 ratio using LPPC and polyethylene glycol (PEG; m.w. 1450) solution (Sigma-Aldrich), as described (Wang, Saffold et al. 1998). In brief, BALB/3T3 and DCs were mixed and washed twice with serum-free medium prewarmed to 37℃. LPPC/IL-6 complex was then added to the mixed cells for 2 hr at 37℃, 5% CO2. The cells were centrifuged at 300 × g for 5 min. After removing the
medium, 1 ml of PEG was added to the cell pellet over 1 min and the suspension was stirred gently for 2 min. An additional 10 ml of serum-free medium was added to the cell suspension over the next 3 min with continued stirring. The resultant cell mixture was pelleted at 400 × g, 5 min and grown for 48hr in CM with GM-CSF. Then the fusion cells were prepared for further experiment, and the supernatant of fusion cells was collected to detect the production of IL-6 protein or TGF-β1 protein used ELISA kits (Human IL-6 and Mouse TGF-β1, R&D Systems). The fusion model of BALB/3T3_pAsRed and B16-F10_pLEGFP were transfected and fused at 1:1 ratio using the same protocol.
2.2.10 TGF-β secretion of different cell lines detected by ELISA
Supernatants of different cell lines were collected at 12 hours, 24 hours and 36 hours and stored immediately at -20℃ until further use respectively. Bioactive and total TGF-β secretions were determined by ELISA (R&D Systems). The supernatants were acidified according to the manufacturer’s instructions.
15
Bone marrow cells derived from BALB/c mouse femurs and tibias were cultured in CM supplemented with 200 U/ml rmGM-CSF as described above. On day 6 of culture, DCs were collected and cultured at 106 cells/ml in CM supplemented with rmGM-CSF and with or without the addition of conditioned medium from BALB/3T3 cells for 6 days. Cytokine and conditioned medium were replenished every 2 days. DCs were matured with 0.5 g/ml LPS (SIGMA-ALDRICH) for 2 days. The expressions of MHC II and CD86 molecules were analyzed by FACScan flow cytometry (Becton Dickinson, San Jose, CA).
2.2.12 Splenocyte isolation
Mice were sacrificed by dislocation and their spleens were quickly harvested in a laminar flow hood. Spleens were placed in a 280 μm-pored mesh and chopped by scissors. 10 ml of RPMI 1640 (Invitrogen Co., USA) supplemented with 10% FBS, 0.2% NaHCO3 and 1% PSA were slowly added
onto the mesh while spleens were being ground until the spleen tissue became white. Single cell suspension was collected in a Petri dish and recovered by centrifugation at 1200 rpm at 4℃ for 5 min. Supernatant was discarded and 10 ml 1× ACK lysis buffer was added for 5 min at room temperature. 1× ACK lysis buffer can lyse the red blood cells while leaving the rest of the lymphocytes and leucocytes. The mixture was then diluted by 10 ml of RPMI 1640 and cells were recovered by centrifugation at 1200 rpm at 4℃ for 5 min. After the supernatant was discarded, the cells were rinsed by 10 ml PBS once again. Finally, cells were resuspended in RPMI 1640 and underwent cell calculation by trypan blue exclusion. For the cell proliferation of splenocytes, cells were seeded in a
16
96-well at 2.5 × 10 5 per well. For the cytokine profiles for splenocytes, cells were seeded in a 24-well plate at 1 × 10 6 per well.
2.2.13 Animal immunization
BALB/c mice were vaccinated once (i.p.) on day 0 with 1 × 106 DC fusion vaccine made by LPPC with IL-6 gene and PEG treatments (LPPC/IL-6/PEG DC vaccine) or DC fusion vaccine made by PEG treatment alone (PEG DC vaccine). To test the efficacy of the vaccines, each group of the animals were sacrificed and the splenocytes of the immunized mice were seeded in 96-well plates (2.5 × 105 cells/well) or 24-well plates (1 × 106 cells/well) with Ags to measure the proliferation or cytokines expressions. For cell proliferation, MTT assay was used to estimate at 72 hrs. The cell proliferation rate was calculated as O.D. value of sample divide into O.D. value of splenocytes alone. For cytokines expressions, supernatants of each sample were collected at 48 hrs and frozen at -80℃. Supernatants concentrations of TNF-α, IL-2, IFN-γ, IL-10 and IL-4 were measured by ELISA.
17
All figures are expressed as mean ± SD. All data were computed by student-test. All statistical significant was set up at p < 0.05.
18
Chapter 3. Results
3.1 Liposome characterization
Lipo-PEI-PEG Complex (LPPC) was composed of two neutral lipids: DOPC and DLPC, and two hydrophilic polymers: polyethyleneimine (PEI) and polyethylene glycol (PEG). After the preparation by following the protocol described in “Materials and Methods”, LPPCs were made and appeared as milky, turbid solution. Based on our lab’s unpublished results, it has been shown that LPPC could be centrifuged to the bottom of tube at 8000 rpm. Sequentially, dynamic light scattering (DLS) revealed the sizes of LPPC were in a range of 160-to-260 nm, with the average size of about 218.35±12.94 nm. Moreover, Zeta-potential value of the liposome showed a cationic property for LPPC (Table 1; 40.25±11.24 mV), which might be use to adsorb proteins or DNA.
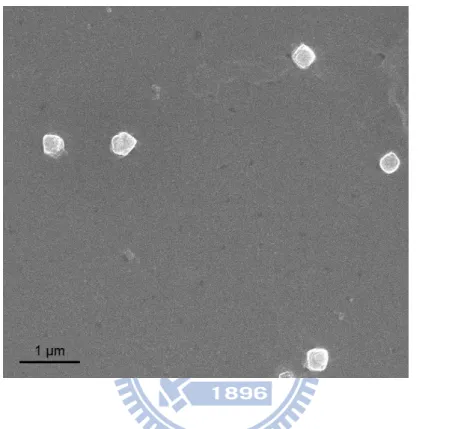
The structures and sizes of LPPC were also observed by micrography and the SEM image confirmed that liposomes were spherical shape. The measured values for the sizes of LPPC accorded with the values calculated from detection of DLS, about 200 nm (Fig. 1).
3.2 In vitro transfection efficiency of LPPC/DNA complex
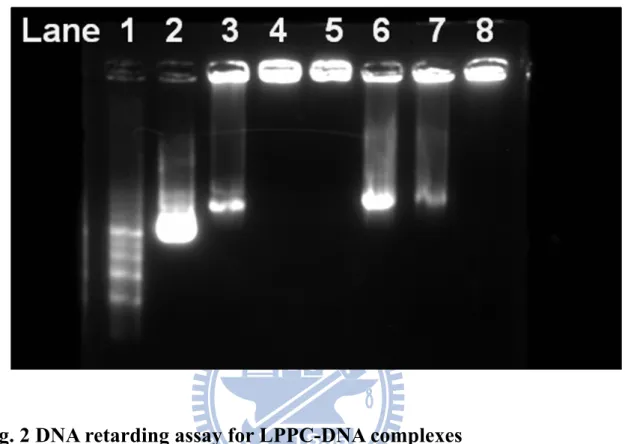
The anionic DNA might bind to cationic LPPC through electrostatic force. Thus, retard assay were used to verify whether LPPC could adsorb the DNA. The results showed the retards for migration of DNA on agarose gel-electrophoresis were proportional to the additional amounts of LPPC (Fig. 2; lane 2-5). Heparin is a kind of anionic mucopolysaccharides and it was used to
19
compete with the electrostatic force between LPPC and DNA to release DNA from LPPC. The results indicated that the abilities of heparin to release DNA were depend on the additional amounts of LPPC (Fig. 2 Lane 6-8). It revealed that LPPC indeed captured DNA and the bound DNA could be released by the addition of heparin, which is dose-dependent.
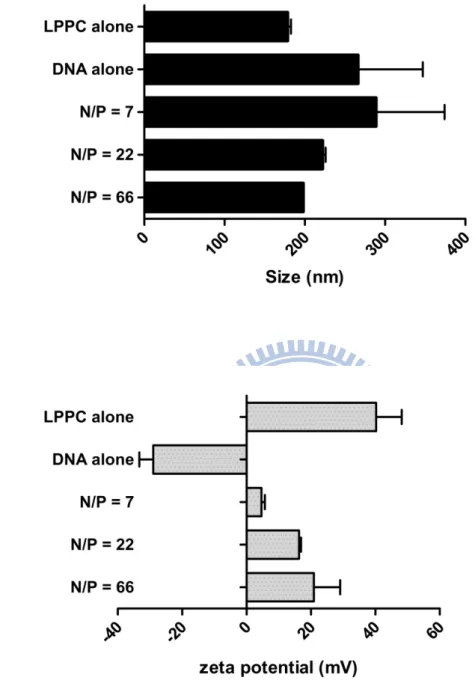
The degrees of LPPC/DNA complexation with different N/P ratios were also observed their effects on particle size and zeta-potential via DLS and ZetaPlus Z-potential analyzer respectively. Thus, different amounts of LPPC were interacted with 18 μg DNA to measure the changes in their particle size and surface charge. The results showed that the sizes of LPPC/DNA complexes at N/P=7 were increased comparing to LPPC alone (Fig. 3a). However, the sizes of LPPC/DNA complexes were decreased as higher as the N/P ratio, indicating LPPC could cause the condensation of the adsorbed DNA (Fig. 3a). Sequentially, the surface charges of particles were measured and the results showed that the average values for DNA or LPPC was -28.95 mV and 40.25 mV respectively. As the N/P ratio was raised, the surface charges of LPPC/DNA complexes ranged from 4.64 mV to 20.9 mV (Fig. 3b). Therefore, together results indicated that LPPC did interact and compact DNA to form complex with less positively charged.
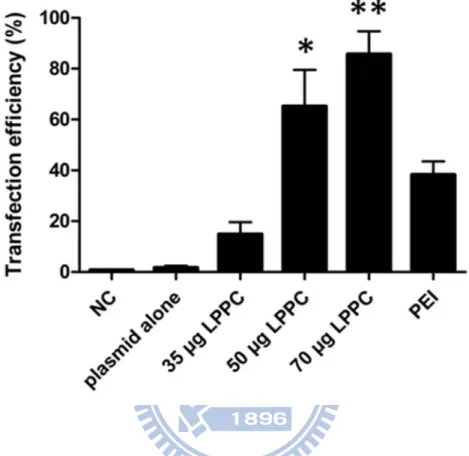
Furthermore, plasmid pAAV-MCS-hrGFP encoded green fluorescent proteins as a reporter gene was used to complex with LPPC and transfected into BALB/3T3 cells to measure the abilities of gene transfection for LPPC. Here, PEI was served as a positive control (38.3 ± 5.3% ) and plasmid alone was served as a negative control (1.7 ± 0.8%) in this experiment. As Figure 4 shown, following more additional amounts of LPPC, the transfection efficiencies of
20
constant amount of DNA (3 g) were increased (14.9 ± 4.7% at 35 μg LPPC with DNA, 65.2 ± 14.3% at 50μg LPPC with DNA and 85.7 ± 9.0% at 70μg LPPC with DNA). Hence, according to the results, LPPC possessed the abilities to bind DNA and deliver genes into cells in vitro.
3.3 The effect of LPPC on the co-transfection-fusion protocol
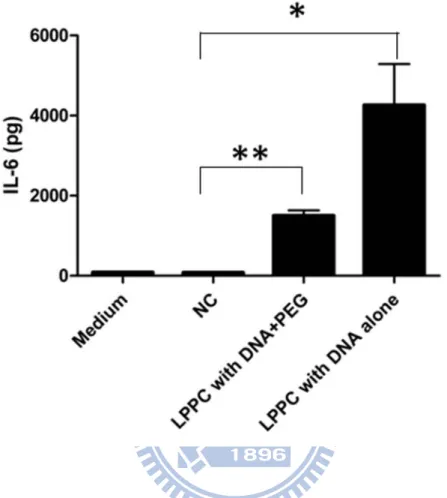
To verify the possibility of the co-transfection-fusion strategy, the effect of LPPC addition on the fusion efficiency was determined firstly. Thus, the red fluorescent protein-expressed BALB/3T3 cells (BALB/3T3_pAsRed) and green fluorescent protein-expressed B16-F10 cells (B16-F10_pLEGFP) was used to measure the effect of LPPC. The two fluorescent cells were fused by PEG with or without LPPC addition and the results were showed as dual fluorescent dot plot (Fig. 5a). To calculate the data from 3 independent experiments, Figure 5b showed the mean fusion efficiencies were 9.8 ± 2.0% using PEG treatment alone and 9.5 ± 2.3% using LPPC and PEG treatments simultaneously, which were both significantly higher than LPPC treatment alone (5.1 ± 0.7%; p < 0.05). Therefore, the addition of LPPC didn’t affect the fusion efficiency by PEG. Besides, the simultaneous addition of DNA to LPPC had no effect on the fusion efficiency (Fig. 5b; column 3). Next, to examine whether LPPC could deliver DNA into cells successfully during the PEG fusion process, the IL-6 plasmid was used as a reporter gene to monitor the effect of fusion procedure on gene expression. The IL-6 in supernatants derived from cells with different treatments were analyzed and figure 6 showed that LPPC alone could transfect IL-6 gene into the cells and result in IL-6 secretion (4264.5 ± 1024.8 pg). Although the fusion reagent PEG could not completely abolish the expression of IL-6, it
21
lowered the secretion of IL-6 into supernatant (1504.1 ± 130.5 pg). In addition, the IL-6 amount of the group of two cells mixture (NC) was as low as the medium alone group. These results clearly demonstrated that the co-transfection fusion strategy is workable.
3.4 The effect of TGF-β derived from immortalized cells on bone
marrow-derived dendritic cells (BMDC)
In advance of performing the co-transfection-fusion strategy on DC hybrids, the DCs should be generated from bone marrow of mouse firstly. At day 8, the immature BMDC cells (iBMDC) were morphologically homologous, which were in irregularly round shape with small protrusions (Fig. 7a). After LPS stimulation, iBMDCs proceeded toward maturation and mBMDCs with large dendrites were observed predominantly by phase contrast microscopy (Fig. 7b). Moreover, the surface markers expressions between the iBMDCs and mBMDCs were analyzed by flow cytometry (Fig. 7c). The results showed that iBMDCs and mBMDCs had almost the same expression levels of MHCI and CD11c. In contrast to these two molecules, the expressions of MHCII and CD86 on mBMDCs were higher than the expressions on iBMDCs (p < 0.01), which indicated that mBMDCs had better antigen presentation activities.
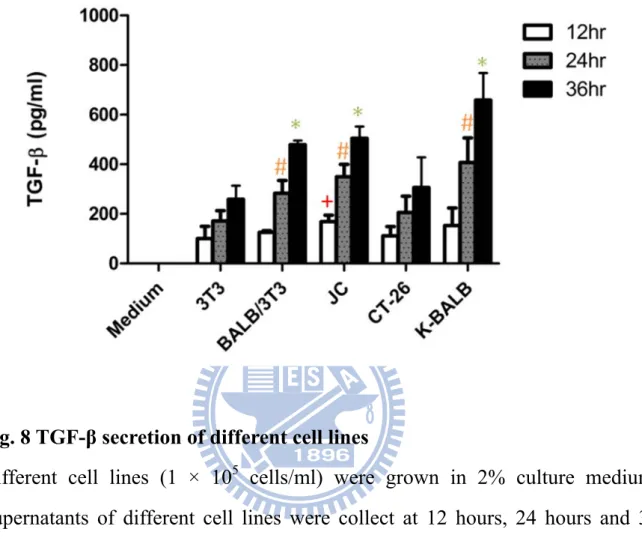
According to literatures, one possible mechanism of immunosuppression for tumor is resulted from the effect of tumor-derived TGF-β on host immune system. Thus, the abilities of different cell lines for TGF-β secretions were determined. As shown in Figure 8, JC (a murine mammary adenocarcinoma), CT-26 (a murine colon carcinoma) and K-BALB (a murine Ki-MSV transformed fibrosarcoma) secreted similar or higher levels of TGF-β compared
22
with 3T3 cells (a murine fibroblast; p < 0.01). BALB/3T3 (a murine fibroblast), one of the cell lines used in the previous fusion protocol, also secreted significantly higher amount of TGF-β than the 3T3 cells (p < 0.01). Thus, we chose BALB/3T3 as the fusion partner with DCs in this study.
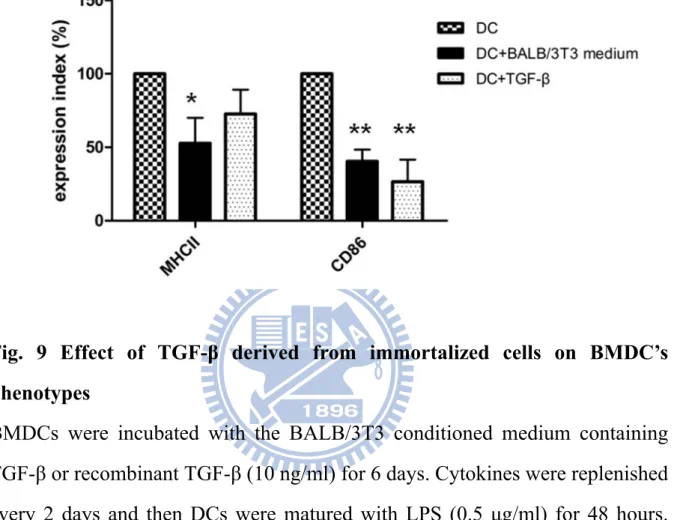
Furthermore, the effects of cultured medium containing TGF-β on surface expressions of DCs were monitored. As the result shown, the recombinant TGF-β or the BALB/3T3 cultured medium both caused significant decreases in the levels of CD86 (p < 0.01) (Fig. 9). Moreover, the BALB/3T3 cultured medium also made a significant decrease of the MHCII expression (p < 0.05) but the recombinant TGF-β (10 ng) had no effect on the MHCII expression (Fig. 9). It indicated that the BALB/3T3 cultured medium could affect DCs’ abilities of antigen presentation by reducing the expressions of MHCII and CD86.
3.5 Generation of DC/TGF-β-expressed cell fusion vaccines using
the co-transfection-fusion protocol
To generate DC/TGF-β-expressed cell fusion vaccines, BALB/3T3 cells were fused with DCs. For detection of the fusion efficiency, DCs and BALB/3T3 were labeled with DiO and DiI lipophilic fluorescent dyes, respectively. The fusion efficiencies were determined by the percent of double-stained cells using flow cytometer. The dual fluorescent dot plot showed that LPPC could not significantly affect the abilities of PEG to fuse cells (Fig. 10a). After calculation of data from 3 independent experiments, the results revealed that PEG treatment without or with LPPC both caused about 50% of double-stained cells (Fig. 10b). There was no significant difference in fusion efficiency between PEG treatment and LPPC/DNA with PEG treatments, which
23
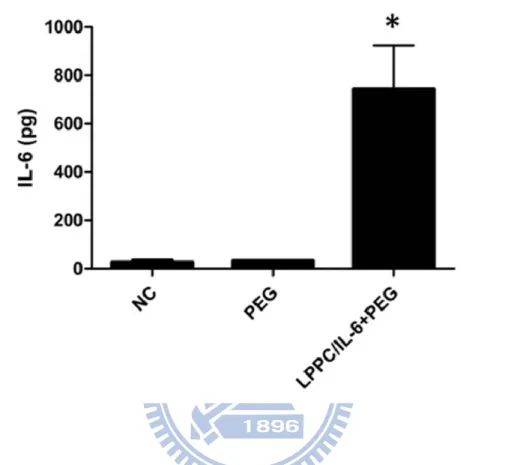
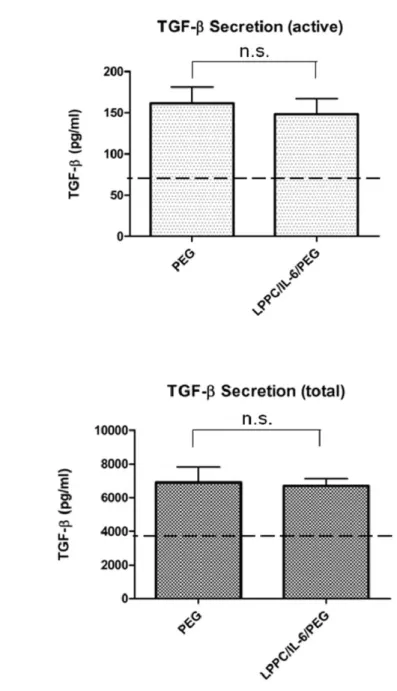
coincided with the results in the BALB/3T3_pAsRed and B16-F10_pLEGFP fusion experiments. Sequentially, the results showed LPPC could also simultaneously deliver IL-6 gene into the DCs and BALB/3T3 cells during fusion process (Fig. 11; 743.6 ± 179.7 pg). While the PEG treatment only induced the expression of IL-6 (33.0 ± 2.4 pg) and it was as similar as the amounts in the cultured medium (27.4 ± 9.9 pg; Fig. 11). Sequentially, whether the simultenous transfection of IL-6 gene by LPPC could affect the expression of TGF-β was verified. Figure 12 showed that the simultaneous transfection of IL-6 gene by LPPC could not affect the secretion of TGF-β comparing to PEG treatment without LPPC addition.
3.6 Evaluation of the efficacy of co-transfection-fusion DC vaccine
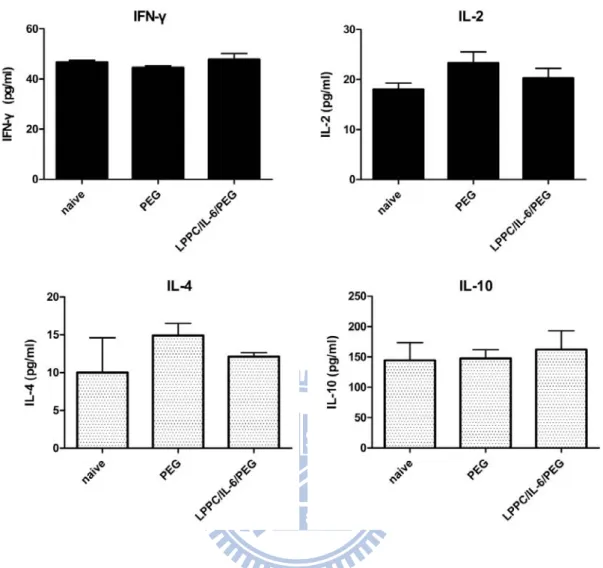
To evaluate the efficacies of co-transfection-fusion DC vaccines for initiating the host immune system, each kind of vaccines were respectively administered to mice by i.p. on day 0 and the splenocytes from naïve mice or immunized mice were harvested at 7th day after immunization to monitor the cytokines profiles and cell proliferation with stimulation of antigens derived from BALB/3T3 cells. The results showed that there were no significantly differences between PEG, LPPC/IL-6 with PEG and naïve groups among the productions of IFN-γ, IL-2, IL-4 and IL-10 (Fig. 13), which might be due to the period of immunization was too short to trigger enough immune response. However, the LPPC/IL-6 with PEG group induced a significant proliferative response comparing to the naïve group with the stimulation of antigen (p < 0.05) (Fig. 14). In contrast, PEG alone group could not induce any significant proliferative response comparing to the naïve group with the stimulation of
24
antigen. Besides, without the stimulation of antigens, there were no significant differences among all groups, which indicated that the LPPC/IL-6 with PEG group could trigger an antigen-specific immune response during a short period of immunization than PEG group.
25
Chapter 4. Discussion
In this study, we firstly proved that the co-transfection-fusion strategy of using LPPC as a transfection reagent to deliver a transgene simultaneously during the PEG fusion process is workable. According to this strategy, the LPPC/IL-6/PEG DC vaccines were made by this co-transfection-fusion protocol, they could produce interleukin (IL)-6, a pleiotropic cytokine that was known to increase host immune responses and antagonize immunosuppressive effects of TGF-β (Hsiao, Liao et al. 2004). They seemingly had the abilities to initiate the specific host immunity faster than the PEG DC vaccine in mice.
DC-based vaccines represent a promising approach to stimulating specific-antitumor immunity (Banchereau, Palucka et al. 2001; Reichardt, Brossart et al. 2004). Thus, a large number of strategies have been developed to deliver Ags to DC including the defined peptides, specific tumor-associated Ags (TAA) or whole tumor cell material by using viral or non-viral technique in recent years (Mayordomo, Zorina et al. 1995; Boczkowski, Nair et al. 1996; Paglia, Chiodoni et al. 1996; Schmidt, Ziske et al. 2003; Tian, Wang et al. 2008). However, strategies in which single TAA is loaded onto DCs are limited by few defined TAAs or the possibilities of immunologic escape through down-regulation of the target Ag in tumor cells (Wang and Rosenberg 1996).In contrast, strategies that DCs loaded with whole tumor cells or tumor lysate have the advantage to induce multiple antitumor immunities against a broad tumor Ags, even unidentified tumor Ags (Trefzer, Herberth et al. 2000; Walden 2000; Tian, Wang et al. 2008). Furthermore, the comparison between the efficacies of
26
DC/tumor fusion vaccines and tumor lysate-pulsed DC vaccines demonstrated that DC/fusion vaccines had superior efficacy, because DC/tumor fusion vaccines have the longer interaction with tumor antigens in vivo (Kao, Zhang et al. 2005). However, in a clinical trial for patients with metastatic breast carcinoma, immunizations with DC/tumor hybrids induced antitumor immunity in a majority of patients while tumors regressed on only a subset of patients (Avigan, Vasir et al. 2004). The possibilities for the clinical failures may result from the weakness of patients to have a compromised immune system, or the maturation state or subsets of DCs to cause immune tolerance or Treg expansion (de Vries, Lesterhuis et al. 2003; McIlroy and Gregoire 2003; Tuyaerts, Aerts et al. 2007). Moreover, soluble immunosuppressive factors (TGF-β, VEGF, IL-10, PGE-E2) secreted by malignant tumors are present in the microenvironment and
interfere with effective T-cell function (Kuppner, Sawamura et al. 1990; Torre-Amione, Beauchamp et al. 1990; Bomstein, Ophir et al. 1993)
Consistently, the inhibitory effect of tumor-derived TGF-β on the efficacy of DC/tumor fusion vaccine has been demonstrated in mouse model (Kao, Gong et al. 2003). TGF-β is a potent immunosuppressive cytokine that often secreted by tumor cells (Pasche 2001). It has been reported that TGF-β could inhibit CTL generation and allow tumors to escape immune surveillance (Kehrl, Wakefield et al. 1986; Rook, Kehrl et al. 1986). Moreover, TGF-β has a direct effect on DC which would interfere with the abilities of DCs to present antigen, stimulate the IFN- expression of tumor-specific CTL, and migrate to draining lymph nodes (Kobie, Wu et al. 2003). Similarly, in our study, most tumor cell lines did secrete higher amounts of TGF-β (Fig. 8), and the immortalized cells-derived TGF-β had the inhibitory effect on the Ag presentation abilities of DCs (Fig. 9). Therefore, several TGF-β neutralizing strategies to boosting antitumor immunity
27
have been reported to resolve the inhibitory effect of TGF-β. Transgenic expression of a dominant-negative TGF-β receptor (TGF-β-R) in DCs can enhance the ability of host immune response against tumors (Muraoka, Dumont et al. 2002; Shah, Tabayoyong et al. 2002). In addition, neutralization of TGF-β by using an adenovirus encoding TGF-β-R to infect tumor cells and then fusing with DCs has been proven to enhance the efficacy of DC fusion vaccine (Zhang, Berndt et al. 2008). On the other hand, IL-6 has the ability to antagonize the immunosuppressive effects of TGF-β (Ohta, Yamagami et al. 2000; Hsiao, Liao et al. 2004), which implied that the IL-6’s anti-TGF-β activities might be used in gene-modified DC fusion vaccine as an alternative approach.
We found that LPPC could not only deliver IL-6 gene into the cell mixture but also has no effect on the fusion efficiencies of vaccine during fusion process (Fig. 10). As expect, the LPPC/IL-6/PEG DC vaccine showed a superior efficacy in triggering antigen-specific immunogenic effects than the PEG DC vaccine (Fig. 14), which may result from the strong antagonism of IL-6 against TGF-β in the microenvironment.
To elevate the efficacy of DC fusion vaccine, several types of gene-modified DC fusion vaccines have been established. In addition to the TGF-β-R-secreting DC/tumor fusion vaccines (Zhang, Berndt et al. 2008), Suzuki et al. showed IL-12 gene-transgeic murine colon-26 adenocarcinoma cells were fused with DCs markedly enhanced antitumor effect in vivo therapeutic model (Suzuki, Fukuhara et al. 2005). Iinuma et al. also reported that IL-12-modified DCs and IL-18-modified murine neuroblastoma cells were fused together to induce protective and therapeutic effects for liver-metastasis neuroblastoma (Iinuma, Okinaga et al. 2006). The essential need for previous strategies is the tumor cells have to be cultured. However, the clinical isolated
28
tumor cells may not be cultured and maintained until completion of transfection and fusion procedural. Therefore, the co-transfection/fusion strategy may be a more suitable choice to prepare the DC/autologous tumor cell vaccine in the clinical setting.
Our strategy showed that immuno-modulated gene transfection can be simultaneous with fusion protocol. We proposed that IL-6 gene should be delivered into BALB/3T3 cells, DCs or hybrids. If the IL-6 gene was delivered to DCs or hybrids, the immuno-stimulating effects of IL-6 may affect directly on DCs or hybrids as other gene-modified DC fusion cells (Suzuki, Fukuhara et al. 2005; Iinuma, Okinaga et al. 2006; Zhang, Berndt et al. 2008). Alternatively, if the IL-6 gene was delivered to BALB/3T3 cells, there have been reported that the use of genetically-modified tumor cells as antitumor vaccines could elicit potent immunogenic effects (Hsieh, Chen et al. 2000; Antonia, Seigne et al. 2002; Berzofsky, Terabe et al. 2004; Frankenberger, Regn et al. 2005; Naruishi, Timme et al. 2006). Based on these ideas, the preparation of gene-modified DC vaccine has no needs to be performed sequentially. Besides, the use of LPPC as the transfection reagent in the co-transfect-fusion strategy may be replaced with other viral vectors to perform higher transfection efficiency during the PEG fusion process.
Furthermore, effective adjuvants for enhancing the abilities of DC/tumor cell fusion vaccines have also been reported, including exogenous IL-12, IL-18, or CpG oligodeoxynucleotides (CpG ODNs) to activate tumor-specific T cells (Gong, Koido et al. 2002; Vasir, Wu et al. 2008). In addition, pre-treatment of DCs and tumor cells with TLR agonist penicillin-killed Streptococcus pyogense (OK-432) and heat shock respectively induce stronger induction of antigen-specific CTLs (Koido, Hara et al. 2007). Moreover, we speculate that
29
the addition of these adjuvants in combination with the co-transfection-fusion strategy may further enhance the effectiveness of DC fusion vaccines. Besides, the combination of other immuno-stimulating genes, such as IL-15 with IL-6 may activate NK cell cytotoxicity in presence of tumor-derived TGF-β (Lin, Chuang et al. 2008). Thus, the co-transfection of IL-6 and IL-15 into DC/tumor fusion cells by our strategy may result in extremely stronger antitumor immunity.
In conclusion, our study demonstrated that the co-transfection-fusion strategy is feasible. We generated IL-6-secreting DC fusion vaccines made by LPPC/IL-6/PEG and proved this vaccine could elicit superior immunogenic effects in the short period of immunization, which proposed the gene transfection and cell fusion can be performed simultaneously. This alternative approach is simple and has the potential to be a promising strategy for gene-modified DC fusion vaccine in the clinical use.
30
Figures
Fig. 1 Micrograph of Lipo-PEI-PEG Complex (LPPC) nanoparticles
The SEM photo of LPPC; the sizes of LPPC nanoparticles were ranged from 200 nm to 300 nm. Length of scale bar = 1 μm.
31
Fig. 2 DNA retarding assay for LPPC-DNA complexes
LPPC at different amounts were incubated with a fixed amount (18 μg) of DNA for 30 min at room temperature, and the different DNA complexes were run on an agarose gel. Lane 1 was DNA marker and lane 2 was naked DNA, lane 3 to lane 5 were 100μg LPPC, 200μg LPPC and 300μg LPPC respectively. The replacement of DNA from complexes by competition of heparin was shown on lane 6 to lane 8. These DNA complexes were run in a 0.8% agarose gel.
32
a.
b.
Fig. 3 Particle size and zeta-potential analysis of LPPC-DNA complexes
100μg, 300μg and 900μg LPPC were mixed with fixed amount (18 μg) of DNA and gave rise to DNA complexes at different N/P ratios. a. and b. The particle sizes and zeta-potential of LPPC, DNA and LPPC-DNA complexes at different N/P ratios from 7,22 and 66 measured by DLS and ZetaPlus Z-potential analyzer respectively.
33
Fig. 4 In vitro transfection efficiency of LPPC/DNA complexes
3 μg DNA were complexed with different amounts of LPPC and then transfected into BALB/3T3 cells. After 48 hours, the transfection efficiencies of each group were measured by flow cytometer. NC: cells alone; PEI: 5mM PEI, 18 μl; data represents the mean ± S.D. of three independent experiments. * represents p < 0.01, ** represents p < 0.001 v.s. PEI.
34
a.
35
Fig. 5 Addition of LPPC has no effect on fusion efficiency caused by PEG
BALB/3T3_pAsRed cells and B16-F10_pLEGFP cells were fused at 1:1 ratio by PEG treatment alone, LPPC+PEG treatments or LPPC treatment alone. The fusion efficiencies of each treatment were examined by FACS analysis. a. The characteristic diagram of one experiment for measuring the fusion efficiency; b. Statistical analysis of fusion efficiencies of each treatment. Data represents the mean ± S.D. of three independent experiments. * represents p < 0.05 v.s. LPPC alone.
36
Fig. 6 Cytokine production by fusion cells after the co-transfection-fusion protocol
18 g IL-6 plasmid was complexed with 300 g LPPC firstly and then added into cells during the PEG fusion process. After 48 hours, the supernatants of fused cells made by LPPC/DNA complex and PEG treatments or LPPC/DNA treatment alone were collected and examined by ELISA to measure the expression of IL-6 protein level. NC: cells mixture alone; data represents the mean ± S.D. of two independent experiments.* represents p < 0.05, ** represents p < 0.01 v.s. NC.
37
a. b.
c.
CD11c MHCI MHCII CD86
Fig. 7 Generation of bone marrow-derived dendritic cells
Bone marrow derived dendritic cells were generated using medium containing 200 U/ml GM-CSF; iBMDC and mBMDC were harvested at Day 8 and 10. a. Phase contrast microscope revealed the morphology of iBMDC. b. Phase contrast microscope revealed the morphology of mBMDC. Original magnification, × 320. c. Surface phenotypes were determined using FACS cytometer . The antibodies CD11c, MHCI, MHCII and CD86 were used to stain the iBMDC (the upper raw) and mBMDC (the lower raw), respectively. Data represents the mean ± S.D. of three independent experiments.
mBMDC iBMDC
209.0±60.13 47.6±29.24 129.6±45.00 13.2±3.80
38
Fig. 8 TGF-β secretion of different cell lines
Different cell lines (1 × 105 cells/ml) were grown in 2% culture medium. Supernatants of different cell lines were collect at 12 hours, 24 hours and 36 hours after seeding, respectively. Supernatants and medium alone were each assessed for TGF-β by ELISA. All cell lines tested expressed TGF-β. Data represents the mean ± S.D. in duplicate of three independent experiments (n = 6). + represents p < 0.01 v.s. 3T3 of 12 hr; # represents p < 0.01 v.s. 3T3 of 24 hr; * represents p < 0.01 v.s. 3T3 of 36 hr.
39
Fig. 9 Effect of TGF-β derived from immortalized cells on BMDC’s phenotypes
BMDCs were incubated with the BALB/3T3 conditioned medium containing TGF-β or recombinant TGF-β (10 ng/ml) for 6 days. Cytokines were replenished every 2 days and then DCs were matured with LPS (0.5 μg/ml) for 48 hours. DCs of each treatment were stained with anti-MHCII or CD86 antibodies and analyzed by flow cytometer. The results are the mean ± S.D. of two independent experiments. * represents p < 0.05 v.s. DC without any treatments; ** represents p < 0.01 v.s. DC without any treatments.
40
a.
41
Fig. 10 Fusion efficiencies of LPPC/IL-6/PEG DC vaccine and PEG DC vaccine
DiI-labeled BALB/3T3 cells were fused with DiO-labeled DCs at 1:2 ratio by LPPC/IL-6/PEG treatments or PEG treatment alone, and fusion efficiencies were examined by FACS analysis. a. The characteristic diagram of one experiment for measuring the fusion efficiency in upper right panel; b. Statistical analysis of fusion efficiencies of each treatment. Data represents the mean ± S.D. of two independent experiments.
42
Fig. 11 Cytokine production by LPPC/IL-6/PEG DC fusion cells and PEG DC fusion cells
LPPC/IL-6/PEG DC fusion cells and PEG DC fusion cells have been generated. After 48 hours, the supernatants of fused cells were collected and examined by ELISA to measure the expression of IL-6 protein level. NC: cells mixture alone; data represents the mean ± S.D. of two independent experiments.* represents p < 0.05 v.s. NC.
43
a.
b.
Fig. 12 TGF-β secretion by DC fusion cells
DC/BALB/3T3 fusion cells continue to secrete bioactive TGF-β. The supernatants of LPPC/IL-6/PEG DC fusion cells and PEG DC fusion cells were collected 48 hours after the experiment. ELISA was performed on supernatants in acidified or nonacidified procedures to detect total or active forms of cells-derived TGF-β. NC: medium alone; data represents the mean ± S.D. of two or three independent experiments.
44
Fig. 13 Cytokines profiles produced by splenocytes from mice immunized with LPPC/IL-6/PEG DC vaccine or PEG DC vaccine
Mice were immunized with LPPC/IL-6/PEG DC vaccine or PEG DC vaccine (1 × 106 cells/injection) on day 0. After a week, immunized mice were sacrificed and the splenocytes were isolated (1 × 106 cells/well) to incubate with BALB/3T3 lysate (3.3 × 105 cells/well). After 48 hours, the supernatants were collected and detected a broad array of the protein levels by ELISA. There were no significantly difference between LPPC/IL-6/PEG group and PEG group among the cytokines which are related to Th1 or Th2 response.
45
Fig. 14 The cell proliferation of Ag-specific splenocytes from LPPC/IL-6/PEG DC vaccine-immunized mice
Splenocytes from immunized mice of each group were isolated and seeded in 96-well plate (2.5 × 105 cells/well) in the absence or presence of BALB/3T3 lysate (2.5 × 104 cells/well). MTT assay have been performed after 3 days incubation. The result showed that splenocytes from mice vaccinated with LPPC/IL-6/PEG DC vaccine could stimulate Ag-specific immune response (n=2, * represents p < 0.05 v.s. naïve group).
46
Table
Appearance Milky, turbid solution
Components DOPC, DLPC, PEI and PEG
Size (nm) 218.35±12.94
Zeta potential (mV) 40.25±11.24
Character Could be easily purified by
centrifugation
47
References
Antonia, S. J., J. Seigne, et al. (2002). "Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma." J Urol
167(5): 1995-2000.
Avigan, D., B. Vasir, et al. (2004). "Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses." Clin Cancer Res 10(14): 4699-708.
Banchereau, J., A. K. Palucka, et al. (2001). "Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine." Cancer Res 61(17): 6451-8.
Berzofsky, J. A., M. Terabe, et al. (2004). "Progress on new vaccine strategies for the immunotherapy and prevention of cancer." J Clin Invest 113(11): 1515-25.
Boczkowski, D., S. K. Nair, et al. (1996). "Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo." J Exp Med 184(2): 465-72.
Bomstein, Y., R. Ophir, et al. (1993). "Release of immunosuppressive factor(s) by MOPC-315 murine plasmacytoma cells: a possible mechanism of defence." Anticancer Res 13(6A): 2125-9.
de Vries, I. J., W. J. Lesterhuis, et al. (2003). "Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients." Clin Cancer Res 9(14): 5091-100.
Frankenberger, B., S. Regn, et al. (2005). "Cell-based vaccines for renal cell carcinoma: genetically-engineered tumor cells and monocyte-derived
48
dendritic cells." World J Urol 23(3): 166-74.
Gong, J., V. Apostolopoulos, et al. (2000). "Selection and characterization of MUC1-specific CD8+ T cells from MUC1 transgenic mice immunized with dendritic-carcinoma fusion cells." Immunology 101(3): 316-24.
Gong, J., D. Chen, et al. (1997). "Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells." Nat Med
3(5): 558-61.
Gong, J., S. Koido, et al. (2002). "Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12." Blood 99(7): 2512-7.
Homma, S., G. Toda, et al. (2001). "Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice." J Gastroenterol 36(11): 764-71. Hsiao, Y. W., K. W. Liao, et al. (2004). "Tumor-infiltrating lymphocyte secretion
of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity." J Immunol 172(3): 1508-14.
Hsieh, C. L., D. S. Chen, et al. (2000). "Tumor-induced immunosuppression: a barrier to immunotherapy of large tumors by cytokine-secreting tumor vaccine." Hum Gene Ther 11(5): 681-92.
Iinuma, H., K. Okinaga, et al. (2006). "Superior protective and therapeutic effects of IL-12 and IL-18 gene-transduced dendritic neuroblastoma fusion cells on liver metastasis of murine neuroblastoma." J Immunol
176(6): 3461-9.
Kao, J. Y., Y. Gong, et al. (2003). "Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine." J Immunol 170(7): 3806-11.
49
fusion vaccine compared with tumor lysate-pulsed dendritic cell vaccine in colon cancer." Immunol Lett 101(2): 154-9.
Kehrl, J. H., L. M. Wakefield, et al. (1986). "Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth." J Exp Med 163(5): 1037-50.
Kikuchi, T., Y. Akasaki, et al. (2001). "Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells." Cancer Immunol Immunother 50(7): 337-44.
Kobie, J. J., R. S. Wu, et al. (2003). "Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines." Cancer Res 63(8): 1860-4.
Koido, S., E. Hara, et al. (2007). "Synergistic induction of antigen-specific CTL by fusions of TLR-stimulated dendritic cells and heat-stressed tumor cells." J Immunol 179(7): 4874-83.
Kugler, A., G. Stuhler, et al. (2000). "Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids." Nat Med 6(3): 332-6.
Kugler, A., G. Stuhler, et al. (2003). "Retraction: Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids." Nat Med 9(9): 1221.
Kuppner, M. C., Y. Sawamura, et al. (1990). "Influence of PGE2- and cAMP-modulating agents on human glioblastoma cell killing by interleukin-2-activated lymphocytes." J Neurosurg 72(4): 619-25.
Lespagnard, L., P. Mettens, et al. (1998). "Dendritic cells fused with mastocytoma cells elicit therapeutic antitumor immunity." Int J Cancer
50
Lin, C. Y., T. F. Chuang, et al. (2008). "Combined immunogene therapy of IL-6 and IL-15 enhances anti-tumor activity through augmented NK cytotoxicity." Cancer Lett 272(2): 285-95.
Lutz, M. B., N. Kukutsch, et al. (1999). "An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow." J Immunol Methods 223(1): 77-92.
Mayordomo, J. I., T. Zorina, et al. (1995). "Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity." Nat Med 1(12): 1297-302.
McIlroy, D. and M. Gregoire (2003). "Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact." Cancer Immunol Immunother 52(10): 583-91.
Muraoka, R. S., N. Dumont, et al. (2002). "Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases." J Clin Invest
109(12): 1551-9.
Naruishi, K., T. L. Timme, et al. (2006). "Adenoviral vector-mediated RTVP-1 gene-modified tumor cell-based vaccine suppresses the development of experimental prostate cancer." Cancer Gene Ther 13(7): 658-63.
Nelson, W. G., J. W. Simons, et al. (2000). "Cancer cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer as vaccines for the treatment of genitourinary malignancies." Cancer Chemother Pharmacol 46 Suppl: S67-72.
Ohshima, Y. and G. Delespesse (1997). "T cell-derived IL-4 and dendritic cell-derived IL-12 regulate the lymphokine-producing phenotype of alloantigen-primed naive human CD4 T cells." J Immunol 158(2): 629-36. Ohta, K., S. Yamagami, et al. (2000). "IL-6 antagonizes TGF-beta and abolishes
51
immune privilege in eyes with endotoxin-induced uveitis." Invest Ophthalmol Vis Sci 41(9): 2591-9.
Paglia, P., C. Chiodoni, et al. (1996). "Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo." J Exp Med 183(1): 317-22.
Pasche, B. (2001). "Role of transforming growth factor beta in cancer." J Cell Physiol 186(2): 153-68.
Porgador, A. and E. Gilboa (1995). "Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes." J Exp Med 182(1): 255-60.
Reichardt, V. L., P. Brossart, et al. (2004). "Dendritic cells in vaccination therapies of human malignant disease." Blood Rev 18(4): 235-43.
Rook, A. H., J. H. Kehrl, et al. (1986). "Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness." J Immunol 136(10): 3916-20. Schmidt, T., C. Ziske, et al. (2003). "Intratumoral immunization with tumor
RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model." Cancer Res 63(24): 8962-7.
Shah, A. H., W. B. Tabayoyong, et al. (2002). "Suppression of tumor metastasis by blockade of transforming growth factor beta signaling in bone marrow cells through a retroviral-mediated gene therapy in mice." Cancer Res
62(24): 7135-8.
Shen, Z., G. Reznikoff, et al. (1997). "Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules." J Immunol 158(6): 2723-30.
52
interleukin-12-secreting cancer cells augments in vivo antitumor immunity: characteristics of syngeneic and allogeneic antigen-presenting cell cancer hybrid cells." Clin Cancer Res 11(1): 58-66.
Tian, F., L. Wang, et al. (2008). "Vaccination with transforming growth factor-beta insensitive dendritic cells suppresses pulmonary metastases of renal carcinoma in mice." Cancer Lett 271(2): 333-41.
Torre-Amione, G., R. D. Beauchamp, et al. (1990). "A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance." Proc Natl Acad Sci U S A 87(4): 1486-90.
Trefzer, U., G. Herberth, et al. (2000). "The hybrid cell vaccination approach to cancer immunotherapy." Ernst Schering Res Found Workshop(30): 154-66.
Tuyaerts, S., J. L. Aerts, et al. (2007). "Current approaches in dendritic cell generation and future implications for cancer immunotherapy." Cancer Immunol Immunother 56(10): 1513-37.
Vasir, B., Z. Wu, et al. (2008). "Fusions of dendritic cells with breast carcinoma stimulate the expansion of regulatory T cells while concomitant exposure to IL-12, CpG oligodeoxynucleotides, and anti-CD3/CD28 promotes the expansion of activated tumor reactive cells." J Immunol 181(1): 808-21. Walden, P. (2000). "Hybrid cell vaccination for cancer immunotherapy." Adv
Exp Med Biol 465: 347-54.
Wang, J., S. Saffold, et al. (1998). "Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines." J Immunol 161(10): 5516-24.
53
T lymphocytes: implications for cancer therapy." J Leukoc Biol 60(3): 296-309.
Zhang, M., B. E. Berndt, et al. (2008). "Expression of a soluble TGF-beta receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy." J Immunol 181(5): 3690-7.