Androgen-Receptor Gene
CAG Repeats, Plasma
Testosterone Levels, and
Risk of Hepatitis B-Related
Hepatocellular Carcinoma
Ming-Whei Yu, Shu-Wen Cheng,
Ming-Wei Lin, Shi-Yi Yang, Yun-Fan
Liaw, Hung-Chuen Chang, Tun-Jen
Hsiao, Shi-Ming Lin, Shou-Dong
Lee, Pei-Jer Chen, Chun-Jen Liu,
Chien-Jen Chen
Background: Worldwide,
hepatocellu-lar carcinoma (HCC) is more prevalent
in men than in women, suggesting that
sex hormones and/or
X-chromosome-linked genes may be involved in
hepa-tocarcinogenesis. We investigated the
association of a trinucleotide (CAG)
re-peat in the androgen receptor (AR)
gene (located on the X chromosome)
termed “AR-CAG repeats,” levels of
plasma testosterone, and the risk of
HCC in Taiwanese men. Chronic
hepa-titis B virus (HBV) infection, which is
associated with risk of HCC, is
hyper-endemic in Taiwan. Methods: We
com-pared the number of AR-CAG repeats
in 285 HBV carriers with HCC and in
349 HBV carriers without HCC. We
also conducted a nested case–control
study on participants in a cohort study.
Blood was collected prospectively from
110 case patients and 239 control
sub-jects and was used to determine the
number of AR-CAG repeats and
plasma testosterone level. All statistical
tests were two-sided. Results: The
over-all odds ratio (OR) for HCC was 1.72
(95% confidence interval [CI] = 1.03–
2.89) for HBV carriers with 20 or fewer
AR-CAG repeats compared with those
with more than 24 repeats. This
asso-ciation was observed only in patients
with late-onset HCC (OR = 2.37; 95%
CI = 1.28–4.38). In the nested case–
control study, HBV carriers in the
highest tertile of testosterone levels had
a statistically significantly increased
risk of HCC (OR = 2.06; 95% CI =
1.14–3.70) compared with those in the
lowest tertile. Elevated testosterone was
more strongly associated with
early-onset (OR = 4.67; 95% CI = 1.41–15.38)
than late-onset disease. HBV carriers
with 20 or fewer AR-CAG repeats and
higher testosterone levels had a
four-fold increase in HCC risk compared
with those with more than 24 repeats
and testosterone levels in the lowest
ter-tile. Conclusions: Higher levels of
an-drogen signaling, reflected by higher
testosterone levels and 20 or fewer
AR-CAG repeats, may be associated with
an increased risk of HBV-related HCC
in men. [J Natl Cancer Inst 2000;92:
2023–8]
Hepatocellular carcinoma (HCC) is
one of the most common cancers in the
world. Chronic infection with hepatitis B
virus (HBV) or hepatitis C virus (HCV)
has a major role in the development of the
disease (1), and HCC is more prevalent in
men than in women throughout the world
(1). In Taiwan, where chronic HBV
infec-tion is hyperendemic, the HCC incidence
rate for men is approximately three times
that for women, despite similar rates of
chronic HBV infection (2). This
differ-ence between the sexes may be, at least in
part, attributable to differences in
expo-sure to risk factors for HCC, such as
al-cohol consumption and cigarette smoking
(3,4). However, sex hormone and
X-chromosome-linked genetic factors may
also be important. Abundant data show
the association of testosterone levels and
hepatocarcinogenesis in animal
experi-ments (5–11). In humans, although
nu-merous case reports have suggested that
therapeutic use of androgenic steroids
may induce HCC (12,13), data on the role
of endogenous male hormones in
hepato-carcinogenesis are limited, and an
asso-Affiliations of authors: M.-W. Yu, S.-W. Cheng, S.-Y. Yang, H.-C. Chang, C.-J. Chen (Graduate In-stitute of Epidemiology, College of Public Health), P.-J. Chen, C.-J Liu (Department of Internal Medi-cine, College of Medicine), National Taiwan Uni-versity, Taipei; M.-W. Lin, Laboratory of Epidemi-ology and Biostatistics, Department of Medical Research and Education, Veterans General Hospital, Taipei; Y.-F. Liaw, S.-M. Lin, Liver Research Unit, Chang-Gung Memorial Hospital, Chang-Gung Uni-versity, Taipei; T.-J. Hsiao, Department of Internal Medicine, Tao-Yuan General Hospital, Department of Health, The Executive Yuan, Tao-Yuan, Taiwan; S.-D. Lee, Department of Medicine, Veterans Gen-eral Hospital and School of Medicine, National Yang-Ming University, Taipei.
Correspondence to: Ming-Whei Yu, Ph.D., Graduate Institute of Epidemiology, College of Pub-lic Health, National Taiwan University, No. 1 Jen-Ai Rd., Sec. 1, Rm. 1550, Taipei 100, Taiwan (e-mail: mingwhei@ha.mc.ntu.edu.tw).
See “Notes” following “References.” © Oxford University Press
ciation between the risk of HCC and
pre-diagnostic levels of serum testosterone
remains controversial (14,15).
Hormonal signals are transmitted
through hormone receptors. The androgen
receptor (AR) gene, located on the long
arm of the X chromosome, encodes AR
protein, which acts as a nuclear
transcrip-tional activator when bound to androgens
(16). An association between the number
of polymorphic exon 1 CAG repeats in
the transactivation domain of the AR gene
and a predisposition for cancer has been
reported. AR genes with fewer CAG
re-peats were associated with an increased
risk of prostate cancer in men (16–18) but
a decreased risk of BRCA1-associated
breast cancer in women (19). The number
of CAG repeats is reflected in the number
of glutamine residues [poly(Q)] in the
amino-terminal domain of the AR protein.
In vitro studies have demonstrated an
in-verse relationship between AR
transacti-vation activity and the number of CAG
repeats (20–22), which is related to the
increased ability of longer poly(Q)
re-gions to inhibit the interaction between
AR and coactivators (22).
Both HCC and nontumorous liver
tis-sue contain ARs (23). In mice, functional
ARs are required for testosterone to
pro-mote hepatocarcinogenesis (11).
In-creased hepatic AR expression was found
in female rats during development of
chemically induced HCC (24). These
findings suggest that the effect of
testos-terone on the development of human
HCC may be AR dependent. Therefore, it
is possible that polymorphisms in the
number of AR-CAG repeats may have a
role in modifying the pathogenesis of
HCC.
In this study, we tested this hypothesis
on the data from our large cohort study,
conducted among men chronically
in-fected with HBV, and also a series of
male patients with HBV-related HCC,
re-cruited on a comprehensive basis in
Tai-wan. We simultaneously evaluated
whether there was an association of the
risk of HCC, prediagnostic plasma levels
of testosterone, and the number of CAG
repeats in the AR gene.
S
UBJECTS ANDM
ETHODSThe Cohort
The cohort consisted of 4841 male HBV carriers aged 30 years or older who attended the specific clinic for asymptomatic HBV carriers at the Liver Unit of Chang-Gung Memorial Hospital (Taipei, Taiwan) and the Government Employee Central
Clinics (Taipei, Taiwan) for regular health exami-nations from 1988 through 1992 (3,25). An HBV carrier is defined as an individual who tests positive for the hepatitis B surface antigen (HBsAg). Written informed consent was obtained from all study par-ticipants, and the investigation was approved by the research ethics committee at the College of Public Health, National Taiwan University, Taipei, and the appropriate institutional review board. After an ini-tial baseline examination, an in-person interview was conducted with the use of a structured question-naire to obtain information on demographic charac-teristics, lifetime habits of alcohol and tobacco use, as well as personal and family histories of major chronic diseases. Blood specimens, including white blood cells, serum, and plasma, were also obtained and frozen at −70 °C until subsequent analysis. Par-ticipants were monitored for incident cancer through various channels, including periodic ultrasonogra-phy measurement and conventional liver function tests every 6–12 months, a personal telephone inter-view, abstraction of their medical records, and a data linkage to the national death certification and cancer registry systems. After 11 years of follow-up, ap-proximately 70% of the HBsAg carriers surviving continued to return for follow-up examination. By September 30, 1999, we had confirmed 138 incident cases of HCC and had sufficient DNA samples from 112 of these patients for the AR genetic polymor-phism assay. HCC was diagnosed on the basis of histologic findings or an elevated level of serum ␣-fetoprotein (艌400 ng/mL) combined with at least one positive image from angiography, sonography, and/or computed tomography. On average, 4.3 years elapsed between blood collection and diagnosis of HCC.
Three hundred forty-nine control subjects were selected for the 112 case patients (control subject/ case patient ratio⳱ one to seven control subjects per case patient), matched to case patients for date of blood collection (within 3 months) and year of birth (within 5 years, except for one elderly case patient who was matched within 10 years). The control sub-jects were randomly selected from cohort subsub-jects with available DNA samples who were alive and remained unaffected with HCC throughout the fol-low-up period.
Recruitment of Hospital-Based Case
Patients
To increase the statistical power for detecting a statistically significant association between the num-ber of AR-CAG repeats and the risk of HCC, we also selected patients with newly diagnosed HCC from male HBV carriers with HCC who participated in our ongoing genetic epidemiology study of HCC. These patients were consecutively recruited from three major hospitals (Chang-Gung Memorial Hos-pital, National Taiwan University HosHos-pital, and pei Veterans General Hospital) in Taipei City, Tai-wan. On average, fewer than 5% of the hospital patients meeting the diagnostic criteria who were approached for interview refused to participate. The first 175 male HBV carriers with newly diagnosed HCC who gave written informed consent for collec-tion of blood samples were included in this study. Two case patients were excluded because the poly-merase chain reaction failed to amplify their DNA, leaving 173 case patients in our analysis.
Thus, we had a total of 285 case patients with
HCC, 112 from the cohort study and 173 from the hospital-based group.
Laboratory Analysis
The status of serum HBsAg was determined by a radioimmunoassay (Abbott Laboratories, Chicago, IL). DNA was purified from peripheral white blood cells. The CAG trinucleotide repeat found in exon 1 of the AR gene was amplified, and the number of repeats was determined as described previously (18). Plasma testosterone levels were measured by a competitive immunoassay with the use of direct che-miluminescent technology (Chiron Diagnostics Corp., East Walpole, MA). Because the onset of HCC may affect the plasma level of testosterone, testosterone was not measured in case patients re-cruited from a hospital after diagnosis of liver can-cer. Because of our limited budget, we tested plasma testosterone in all cohort-based case patients who had sufficient frozen plasma samples (110 case pa-tients) but only a random sample of matched control subjects (239 control subjects). These assays were conducted by laboratory personnel blinded to case– control status.
Statistical Analysis
Since there is no a priori point at which cutoffs may be applied to identify allele-specific risk groups, the numbers of AR-CAG repeats were origi-nally categorized as deciles based on the distribution among control subjects. Cut points were chosen af-ter combining categories with similar risks. Uncon-ditional logistic regression was used to compute the odds ratios (ORs) and their 95% confidence inter-vals (CIs). Because the AR gene is inherited mater-nally and the case patients and control subjects were not matched on maternal ethnicity, the ORs of HCC associated with various numbers of AR-CAG re-peats were adjusted for year of birth (continuous variable) and maternal ethnicity (Fukien Taiwanese, Hakka Taiwanese, and Mainland Chinese). In the case–control study nested within the cohort study, subjects were divided into tertiles of plasma testos-terone levels based on the distribution among the control group. Tertiles of testosterone values were chosen as cut points to have a sufficient number of case patients and control subjects in each cell so that both the independent effect of testosterone and its interactive effect with the number of AR-CAG re-peats could be determined. Age, alcohol consump-tion, chronic liver disease, and cigarette smoking are HCC risk factors that might be associated with blood testosterone levels (26,27). Multivariate-adjusted ORs of HCC associated with testosterone levels were thus computed after adjustment for matching variables (e.g., year of birth and the time that blood was drawn) and potential confounders, including al-cohol drinking, cigarette smoking, a history of chronic liver disease, and educational level (senior high school and above, junior high school, or pri-mary school and below). For univariate analyses, Mantel’s2test for a trend was used to assess the
dose–response relationship. For multivariate analy-ses, tests for linear trends were performed in logistic regression by assigning the medians of each cat-egory as scores. In addition to total HCC, we con-ducted analyses stratified by age at diagnosis of HCC. The cut point between early- and late-onset HCC, 50 years of age, was as defined in our
previ-ous study of familial aggregation. In this study, the cumulative HCC risk to first-degree relatives was greater for case patients diagnosed at less than 50 years of age than for those diagnosed at 50 years of age or older (28). The effect modifying the relation-ship of testosterone and HCC by the AR genotype was examined by adding interaction terms (genotype and testosterone) to a logistic regression model and computing the likelihood ratio statistic. All P values were from two-tailed tests.
R
ESULTSThe mean age at diagnosis of cancer
was 55.3 ± 9.3 years (± standard
devia-tion; range
⳱ 36–79 years) for
cohort-based case patients and 53.3 ± 9.9 years
(range
⳱ 36–83 years) for hospital-based
case patients (P
⳱ .091). The
cohort-based case patients and hospital-cohort-based
case patients were similar with respect to
the distribution of the number of
AR-CAG repeats (P
⳱ .399) (Table 1). To
gain statistical power, we thereafter
com-bined the two groups of case patients in
the analyses performed to assess the
inde-pendent effect of the AR polymorphism
on HCC risk.
The number of CAG repeats ranged
from 14 to 31 among case patients
(me-dian
⳱ 22 repeats) and from 15 to 35
among control subjects (median
⳱ 23
re-peats). Compared with male HBsAg
car-riers who had more than 24 CAG repeats,
those with 20 repeats or fewer had an
overall OR of 1.72 (95% CI
⳱ 1.03–2.89;
P
⳱ .040) for HCC after adjustment for
year of birth and maternal ethnicity
(Table 2). AR genes with more than 24
CAG repeats were found in 40.2% (39 of
97) of case patients diagnosed with HCC
before age 50 years but in only 21.8% (41
of 188) of case patients diagnosed at age
50 years or older. The difference in the
proportion of subjects with more than 24
CAG repeats between the two groups of
patients was statistically significant, even
after adjusting for maternal ethnicity (P
⳱ .001). Subsequent stratification
ac-cording to age at diagnosis of cancer
showed that AR genes with 20 CAG
re-peats or fewer were a statistically
signifi-cant risk factor for late-onset HCC
diag-n o s e d a t a g e 5 0 y e a r s o r o l d e r
(multivariate-adjusted OR
⳱ 2.37; 95%
CI
⳱ 1.28–4.38; P ⳱ .006) but not for
early-onset HCC.
Using data from the prospectively
col-lected plasma samples from 110 case
pa-tients and 239 control subjects within the
cohort study of 4841 male HBsAg
carri-ers, we examined the relation between
plasma testosterone levels and HCC risk
(Table 3). A strong trend of increasing
risk of HCC was observed with increasing
levels of plasma testosterone
(multivari-ate-adjusted ORs by tertile
⳱ 1.00
[ref-erent]; 1.09 [95% CI
⳱ 0.57–2.07], and
2.06 [95% CI
⳱ 1.14–3.70]; P
for trend⳱
.009). To investigate further the
associa-tion between plasma testosterone level
Table 2. Frequency distribution of the number of CAG repeats in the androgen receptor (AR) gene among early- and late-onset hepatitis B surface antigen (HBsAg)-positive case patients with hepatocellular carcinoma (HCC) compared with HBsAg-positive control subjects*
Characteristic
No. of AR-CAG repeats
Pfor trend†
>24 23–24 21–22 艋20
No. of control subjects 111 88 112 38
Total No. of case patients 80 70 88 47
Univariate OR (95% CI) 1.00‡ 1.10 (0.72–1.69) 1.09 (0.73–1.63) 1.72 (1.03–2.87) .097 Adjusted OR§,㛳 (95% CI) 1.00‡ 1.11 (0.72–1.70) 1.08 (0.72–1.61) 1.72 (1.03–2.89) .104
No. of early-onset case patients¶ 39 16 29 13
Univariate OR (95% CI) 1.00‡ 0.52 (0.27–0.99) 0.74 (0.43–1.27) 0.97 (0.47–2.02) .639 Adjusted OR§,㛳 (95% CI) 1.00‡ 0.56 (0.27–1.17) 0.86 (0.46–1.63) 1.13 (0.49–2.63) .978
No. of late-onset case patients 41 54 59 34
Univariate OR (95% CI) 1.00‡ 1.66 (1.01–2.72) 1.43 (0.88–2.30) 2.42 (1.35–4.35) .011 Adjusted OR§ (95% CI) 1.00‡ 1.63 (0.97–2.73) 1.27 (0.77–2.10) 2.37 (1.28–4.38) .029
*OR⳱ odds ratio; CI ⳱ confidence interval. †All P values are from two-sided tests. ‡Referent.
§Adjusted for year of birth (continuous variable) and maternal ethnicity (Fukien Taiwanese, Hakka Taiwanese, and Mainland Chinese). 㛳One case patient was excluded from analysis because of missing data on maternal ethnicity.
¶Case patients who were diagnosed at younger than 50 years.
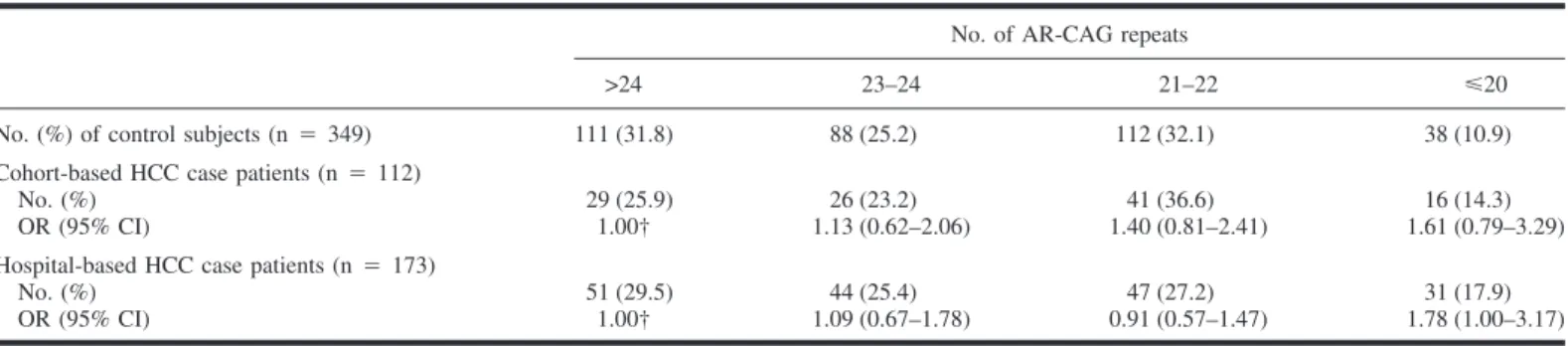
Table 1. Distribution of the number of CAG repeats in the androgen receptor (AR) gene in hepatitis B surface antigen (HBsAg)-positive case patients and control subjects*
No. of AR-CAG repeats
>24 23–24 21–22 艋20
No. (%) of control subjects (n⳱ 349) 111 (31.8) 88 (25.2) 112 (32.1) 38 (10.9) Cohort-based HCC case patients (n⳱ 112)
No. (%) 29 (25.9) 26 (23.2) 41 (36.6) 16 (14.3)
OR (95% CI) 1.00† 1.13 (0.62–2.06) 1.40 (0.81–2.41) 1.61 (0.79–3.29) Hospital-based HCC case patients (n⳱ 173)
No. (%) 51 (29.5) 44 (25.4) 47 (27.2) 31 (17.9)
OR (95% CI) 1.00† 1.09 (0.67–1.78) 0.91 (0.57–1.47) 1.78 (1.00–3.17)
*HCC⳱ hepatocellular carcinoma; OR ⳱ odds ratio; CI ⳱ confidence interval. †Referent.
and HCC risk, we repeated the basic
analyses including only those case–
control matched sets in which the case
patients were diagnosed 4 years or more
after the start of follow-up. With the
re-maining 62 case patients and 140 control
subjects, we observed results similar to
those from previous analyses with all of
the case patients and control subjects
(multivariate-adjusted OR
⳱ 2.53; 95%
CI
⳱ 1.13–5.66 for the highest versus the
lowest tertile; P
for trend⳱ .016). Elevated
plasma testosterone levels were more
strongly associated with early-onset
dis-ease than with late-onset disdis-ease.
Data in Table 4 show the effect of the
combined contributions of the number of
AR-CAG repeats and the level of
testos-terone to the risk of developing HCC.
Al-though we observed no statistically
sig-nificant interaction between testosterone
and AR-CAG repeats (P
⳱ .24), the
as-sociation between the level of
testoster-one and HCC risk appeared stronger for
male HBsAg carriers with 20 CAG
re-peats or fewer, although not statistically
significantly so (P
⳱ .24 for the
interac-tion). Male HBsAg carriers with 20 CAG
repeats or fewer in the highest tertile of
testosterone had a multivariate-adjusted
OR of 8.32 (95% CI
⳱ 0.86–80.81; P ⳱
.068) compared with those who had a
similar number of CAG repeats in the
lowest tertile of testosterone. The
compa-rable multivariate-adjusted OR among
male HBsAg carriers with more than 24
Table 3. Risk of hepatocellular carcinoma (HCC) by tertile of baseline plasma testosterone levels among 110 hepatitis B surface antigen (HBsAg)-positive case patients and 239 HBsAg-positive control subjects nested within the Taiwan HCC cohort study*
Tertile testosterone, ng/mL
Pfor trend†
Lowest (0.87–4.73) Middle (4.74–6.38) Highest (6.39–13.99)
All case patients versus all control subjects
No. of case patients/No. of control subjects 25/79 27/80 58/80
Univariate OR (95% CI) 1.00‡ 1.07 (0.57–2.00) 2.29 (1.31–4.02) .002 Adjusted OR§,㛳 (95% CI) 1.00‡ 1.09 (0.57–2.07) 2.06 (1.14–3.70) .009 Early-onset case patients ¶ versus their matched control subjects
No. of case patients/No. of control subjects 5/28 6/27 24/30
Univariate OR (95% CI) 1.00‡ 1.24 (0.34–4.56) 4.48 (1.50–13.36) .002 Adjusted OR§,㛳 (95% CI) 1.00‡ 1.83 (0.45–7.35) 4.67 (1.41–15.38) .007 Late-onset case patients versus their matched control subjects
No. of case patients/No. of control subjects 20/51 21/53 34/50
Univariate OR (95% CI) 1.00‡ 1.01 (0.49–2.08) 1.73 (0.88–3.41) .095
Adjusted OR§ (95% CI) 1.00‡ 1.02 (0.49–2.15) 1.64 (0.81–3.32) .132
*OR⳱ odds ratio; CI ⳱ confidence interval. †All P values are from two-sided tests. ‡Referent.
§Adjusted for year of birth (continuous variable), the time of blood draw (continuous variable), alcohol consumption, cigarette smoking, history of chronic liver disease, and educational levels (senior high school and above, junior high school, or primary school and below).
㛳One case patient was excluded from analysis because of missing data on educational levels, habits of cigarette smoking and alcohol consumption, and history of chronic liver disease.
¶Case patients diagnosed at younger than 50 years.
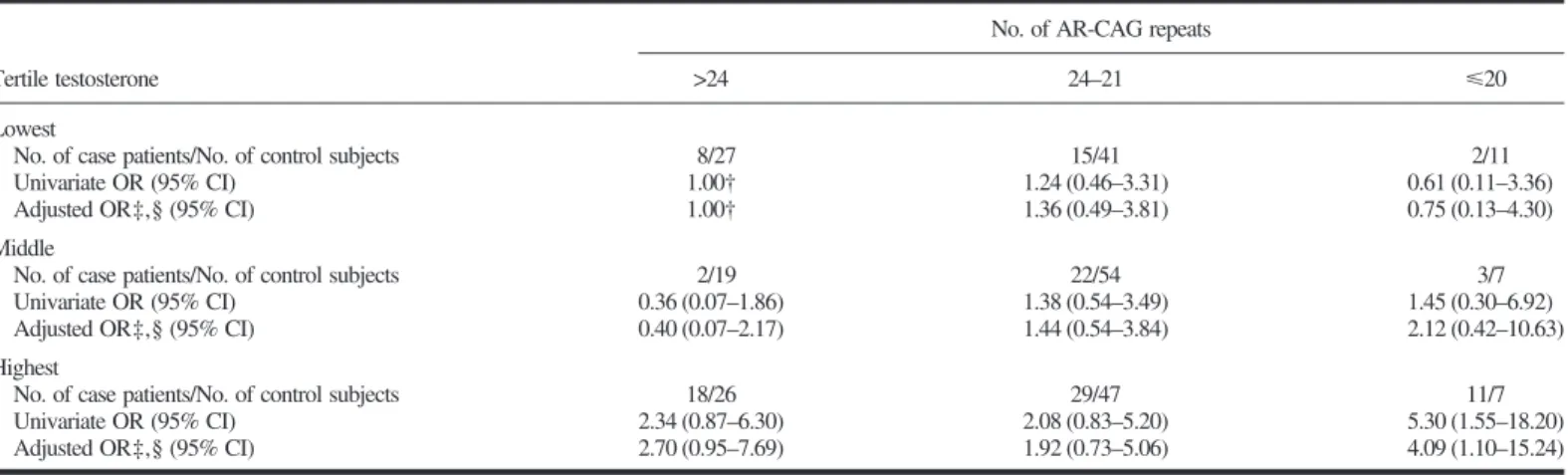
Table 4. Risk of hepatocellular carcinoma (HCC) by the number of CAG repeats in the androgen receptor (AR) gene and tertile of baseline plasma testosterone levels among 110 hepatitis B surface antigen (HBsAg)-positive case patients and 239 HBsAg-positive control subjects nested within the Taiwan HCC cohort study*
Tertile testosterone
No. of AR-CAG repeats
>24 24–21 艋20
Lowest
No. of case patients/No. of control subjects 8/27 15/41 2/11
Univariate OR (95% CI) 1.00† 1.24 (0.46–3.31) 0.61 (0.11–3.36)
Adjusted OR‡,§ (95% CI) 1.00† 1.36 (0.49–3.81) 0.75 (0.13–4.30)
Middle
No. of case patients/No. of control subjects 2/19 22/54 3/7
Univariate OR (95% CI) 0.36 (0.07–1.86) 1.38 (0.54–3.49) 1.45 (0.30–6.92) Adjusted OR‡,§ (95% CI) 0.40 (0.07–2.17) 1.44 (0.54–3.84) 2.12 (0.42–10.63) Highest
No. of case patients/No. of control subjects 18/26 29/47 11/7
Univariate OR (95% CI) 2.34 (0.87–6.30) 2.08 (0.83–5.20) 5.30 (1.55–18.20) Adjusted OR‡,§ (95% CI) 2.70 (0.95–7.69) 1.92 (0.73–5.06) 4.09 (1.10–15.24)
*OR⳱ odds ratio; CI ⳱ confidence interval. †Referent.
‡Adjusted for year of birth (continuous variable), the time of blood draw (continuous variable), alcohol consumption, cigarette smoking, history of chronic liver disease, educational levels (senior high school and above, junior high school, or primary school and below), and maternal ethnicity (Fukien Taiwanese, Hakka Taiwanese, and Mainland Chinese).
§One case patient was excluded from analysis because of missing data on maternal ethnicity, habits of cigarette smoking and alcohol consumption, history of chronic liver disease, and educational levels.
repeats was 2.70 (95% CI
⳱ 0.95–7.69; P
⳱ .063). Male HBV carriers in the
high-est thigh-estosterone tertile and 20 AR-CAG
repeats or fewer had an increased risk of
HCC that was approximately fourfold
higher than male HBV carriers in the
low-est tlow-estosterone tertile and more than 24
AR-CAG repeats.
D
ISCUSSIONMost HBV carriers in Taiwan are
in-fected by the virus during their early
childhood through vertical transmission
but are usually not affected with HCC
un-til several decades after infection (2).
Al-though there has been a great deal of
progress in elucidating the risk factors
associated with the development of HCC
(1,3,4,25,28,29), our understanding of the
molecular mechanisms of HCC remains
rudimentary. The predominance of males
with HCC has long been observed in
humans and various animal models,
in-cluding HBV-transgenic mice (1,2,5–
8,10,11,30,31). Castration of male mice
decreased the incidence of chemically
in-duced HCC compared with that of intact
males, whereas chronic testosterone
ad-ministration to female or castrated male
animals increased the risk of spontaneous
or chemically induced HCC (5–11).
These findings raise the possibility that
testosterone may promote the
develop-ment of HCC in humans. In our initial
nested case–control study carried out
within a cohort of 9691 adult males
re-cruited from six townships of Taiwan, an
elevated prediagnostic blood level of
tes-tosterone was associated with the risk of
HCC after we controlled for confounding
by the HBsAg carrier status and other
potential HCC risk factors (14). However,
in a nested case–control study conducted
in a high-incidence area of China, no
sta-tistically significant difference in the
ter-tile distribution of blood testosterone
be-tween HBsAg-positive male patients with
HCC and control subjects was noted,
al-though there was a 50% greater risk for
male HBsAg carriers in the highest
tes-tosterone tertile relative to those in the
lowest testosterone tertile (15). Because
these two studies are limited by the small
number of case patients studied, a more
detailed analysis is required to re-examine
the androgen hypothesis involved in the
development of HCC.
The action of testosterone is ultimately
mediated through the AR, which has been
found in HCC and nontumorous liver
tis-sue, but tumor tissues have a higher
con-tent of AR (23). In a rat model for HCC
induction, increased hepatic AR
expres-sion was observed in female rats during
prolonged oral administration of a
chemi-cal carcinogen (24). It has also been
shown that the growth rate of chemically
induced liver tumors in normal male mice
is 20-fold higher than the rate in male
mice with testicular feminization, which
lack functional ARs (11). To our
knowl-edge, this study presents the most direct
evidence that AR contributes to the risk of
developing HCC in humans by
identify-ing a statistically significant association
between the number of AR-CAG repeats
and the risk of HCC. The number of
AR-CAG repeats is apparently inversely
asso-ciated with transactivation capabilities of
the AR in vitro (20–22).
The increased risk of HCC associated
with AR genes that have 20 CAG repeats
or fewer appears to be relatively modest
(OR
≈2). In particular, we observed that
the length of the poly(Q) sequence in the
AR protein had a statistically significant
influence on the risk of developing
late-onset HCC only. Furthermore, older male
HBV carriers who developed HCC had
statistically significantly fewer CAG
re-peats than the younger patients. Because
the grouping of AR-CAG repeats was
based on the analysis of the odds of
de-veloping HCC, these findings require
confirmation. However, fewer AR-CAG
repeats (i.e., <19 or 20 repeats) have also
been reported to be associated with an
in-creased risk of prostate cancer, which
usually occurs in men older than 60 years
of age (16–18). A 5%–10% increase in in
vitro transcriptional activation for each
decrement of 10 CAG repeats has been
observed (20). Thus, it is reasonable to
speculate that length variation within the
normal range of repeats, typically 14–35
repeats, observed in this study population
cannot have a strong influence on the
transcriptional activity in vivo. However,
subtle differences in AR transactivation
activity over a lifetime may have a
sub-stantial impact on the transition from the
HBV carrier state to HCC.
As we have reported previously (14),
men with higher baseline levels of
testos-terone were more likely to be
subse-quently diagnosed with HCC than men
with lower testosterone levels. It is
un-likely that this association can be
ex-plained by an effect of prevalent but
un-diagnosed cancer on plasma testosterone
levels. The effect of elevated testosterone
levels on the development of HCC was
essentially unchanged when we excluded
case patients diagnosed within 4 years of
the time blood was drawn. Also, we were
not able to explain our results on the basis
of confounding. The association with
tes-tosterone level remained statistically
sig-nificant after we incorporated many
known HCC risk factors that may be
as-sociated with circulatory levels of
testos-terone into the analysis, such as age,
al-cohol consumption, chronic liver disease,
and cigarette smoking (26,27). Although
our study was limited by only a single
testosterone measurement for each study
subject, testosterone levels in men are
relatively stable over time, and thus a
single determination of plasma
testoster-one level should sufficiently represent the
long-term hormonal milieu in men.
Tes-tosterone levels begin to decline in men at
about 40 years of age and decrease
roughly 10% per decade thereafter
throughout the remainder of life (26). In
this study, higher plasma testosterone
lev-els were associated with an increased risk
of HCC in younger and older male HBV
carriers. However, the association
be-tween testosterone level and risk of HCC
appears to be stronger in relatively
younger men, when levels of this
hor-mone are naturally higher.
The effect of testosterone levels on the
risk of HCC may depend on the number
of AR-CAG repeats carried by an
indi-vidual, although we found that the
inter-action between the two factors was not
statistically significant. Male HBV
carri-ers in the highest tertile of testosterone
levels and with 20 AR-CAG repeats or
fewer had an increased risk of HCC that
was approximately fourfold higher than
male HBV carriers in the lowest tertile of
testosterone levels and with more than 24
AR-CAG repeats (Table 4). This finding
is compatible with the results from the
mouse model system for testicular
femi-nization, demonstrating that androgens
contribute to the development of HCC
through AR-mediated mechanisms (11).
However, modification of the testosterone
effect by the number of AR-CAG repeats
may differ between early- and late-onset
HCC. Future studies with a substantially
larger study population than in this study
are required to explore this issue.
In summary, our results suggest that
the number of AR-CAG repeats is
asso-ciated with the risk of HCC among male
HBV carriers. There may be an additive
effect on HCC risk of elevated
testoster-one levels in individuals with low
num-bers of AR-CAG repeats, but the exact
nature of this relationship remains to be
elucidated. Chronic infection with HBV
or HCV has been shown to be associated
with an increased risk of HCC and
ac-count for the vast majority of HCC cases
worldwide (1). In Taiwan, HCV seems to
play a relatively minor role in the
devel-opment of HCC. We were not able to
ex-amine the androgen hypothesis for
HCV-related HCC in the cohort study because
of the low prevalence (i.e., <2%) of HCV
infection in the general Taiwanese
popu-lation (1). On the other hand, AR was
detected in the liver regardless of sex
(23). In light of the animal study
indicat-ing increased hepatic AR expression
dur-ing the development of HCC in female
rats (24), it is reasonable to hypothesize
that AR may also be involved in the
he-patocarcinogenesis among women.
Al-though both male and female HBV
carri-ers have a higher incidence of HCC than
noncarriers, the development of HCC
oc-curs with much greater frequency in men
than in women (2). We are presently
con-ducting a multicenter case–control study
to recruit a sufficient number of female
patients with HCC to explore the
associa-tion of HCC with the number of AR-CAG
repeats among women.
R
EFERENCES(1) Yu MW, Chen CJ. Hepatitis B and C viruses in the development of hepatocellular carcinoma. Crit Rev Oncol Hematol 1994;17:71–91. (2) Yu MW, Tsai SF, Hsu KH, You SL, Lee SS,
Lin TM, et al. Epidemiologic characteristics of malignant neoplasms in Taiwan. II. Liver can-cer. J Natl Public Health Assoc 1988;8:125– 38.
(3) Yu MW, Gladek-Yarborough A, Chiamprasert S, Santella RM, Liaw YF, Chen CJ. Cyto-chrome P450 2E1 and glutathione S-transferase M1 polymorphisms and susceptibility to hepa-tocellular carcinoma. Gastroenterology 1995; 109:1266–73.
(4) Yu MW, Chiu YH, Chiang YC, Chen CH, Lee TH, Santella RM, et al. Plasma carotenoids, glutathione S-transferase M1 and T1 genetic polymorphisms, and risk of hepatocellular car-cinoma: independent and interactive effects. Am J Epidemiol 1999;149:621–9.
(5) Agnew LR, Gardner WU. The incidence of spontaneous hepatomas in C3H, C3H (low milk factor), and CBA mice and the effect of estrogen and androgen on the occurrence of these tumors in C3H mice. Cancer Res 1952; 12:757–61.
(6) Vesselinovitch SD, Mihailovich N. The effect of gonadectomy on the development of hepa-tomas induced by urethan. Cancer Res 1967; 27:1788–91.
(7) Toh YC. Effect of neonatal castration on liver tumor induction by N-2-fluorenylacetamide in suckling BALB/c mice. Carcinogenesis 1981; 2:1219–21.
(8) Firminger HI, Reuber MD. Influence of adre-nocortical, androgenic, and anabolic hormones on the development of carcinoma and cirrhosis of the liver in AXC rats fed N-2-fluorenyl-diacetamide. J Natl Cancer Inst 1961;27: 559–95.
(9) Reuber MD. Influence of hormones on N-2-fluorenyldiacetamide-induced hyperplastic he-patic nodules in rats. J Natl Cancer Inst 1969; 43:445–52.
(10) Vesselinovitch SD, Itze L, Mihailovich N, Rao KV. Modifying role of partial hepatectomy and gonadectomy in ethylnitrosourea-induced he-patocarcinogenesis. Cancer Res 1980;40: 1538–42.
(11) Kemp CJ, Leary CN, Drinkwater NR. Promo-tion of murine hepatocarcinogenesis by testos-terone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci U S A 1989;86:7505–9.
(12) Mokrohisky ST, Ambruso DR, Hathaway WE. Fulminant hepatic neoplasia after androgen therapy [letter]. N Engl J Med 1977;296: 1411–2.
(13) Farrell GC, Joshua DE, Uren RF, Baird PJ, Perkins KW, Kronenberg H. Androgen-induced hepatoma. Lancet 1975;1:430–2. (14) Yu MW, Chen CJ. Elevated serum testosterone
levels and risk of hepatocellular carcinoma. Cancer Res 1993;53:790–4.
(15) Yuan JM, Ross RK, Stanczyk FZ, Govindara-jan S, Gao YT, Henderson BE, et al. A cohort study of serum testosterone and hepatocellular carcinoma in Shanghai, China. Int J Cancer 1995;63:491–3.
(16) Ross RK, Pike MC, Coetzee GA, Reichardt JK, Yu MC, Feigelson H, et al. Androgen me-tabolism and prostate cancer: establishing a model of genetic susceptibility. Cancer Res 1998;58:4497–504.
(17) Ingles SA, Ross RK, Yu MC, Irvine RA, La Pera G, Haile RW, et al. Association of pros-tate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptor. J Natl Cancer Inst 1997;89:166–70.
(18) Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Brufsky A, Talcott J, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A 1997;94:3320–3.
(19) Rebbeck TR, Kantoff PW, Krithivas K, Neu-hausen S, Blackwood MA, Godwin AK, et al. Modification of BRCA1-associated breast can-cer risk by the polymorphic androgen-receptor CAG repeat. Am J Hum Genet 1999;64: 1371–7.
(20) Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal do-main affect transactivation function. Nucleic Acids Res 1994;22:3181–6.
(21) Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen re-ceptor: possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet 1995;4:523–7.
(22) Irvine RA, Ma H, Yu MC, Ross RK, Stallcup MR, Coetzee GA. Inhibition of p160-mediated coactivation with increasing androgen receptor polyglutamine length. Hum Mol Genet 2000; 9:267–74.
(23) Nagasue N, Ito A, Yukaya H, Ogawa Y. An-drogen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterol-ogy 1985;89:643–7.
(24) Ostrowski JL, Ingleton PM, Underwood JC, Parsons MA. Increased hepatic androgen re-ceptor expression in female rats during dieth-ylnitrosamine liver carcinogenesis: a possible correlation with liver tumor development. Gas-troenterology 1988;94:1193–200.
(25) Chen CJ, Yu MW, Liaw YF, Wang LW, Chi-amprasert S, Matin F, et al. Chronic hepatitis B carriers with null genotypes of glutathione S-transferase M1 and T1 polymorphisms who are exposed to aflatoxin are at increased risk of hepatocellular carcinoma. Am J Hum Genet 1996;59:128–34.
(26) Dai WS, Kuller LH, LaPorte RE, Gutai JP, Falvo-Gerard L, Caggiula A. The epidemiol-ogy of plasma testosterone levels in middle-aged men. Am J Epidemiol 1981;114:804–16. (27) Johnson PJ. Sex hormones and the liver. Clin
Sci (Colch) 1984;66:369–76.
(28) Yu MW, Chang HC, Liaw YF, Lin SM, Lee SD, Liu CJ, et al. Familial risk of hepatocellu-lar carcinoma among chronic hepatitis B carri-ers and their relatives. J Natl Cancer Inst 2000; 92:1159–64.
(29) Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 1992;339:943–6.
(30) Dunsford HA, Sell S, Chisari FV. Hepatocar-cinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res 1990;50:3400–7.
(31) Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature 1991;351: 317–20.
N
OTESSupported by grants NSC 88–2318-B-002–002 (Frontier Medical Genomic Program) and NSC 89– 2320-B-002–104 from the National Science Coun-cil, Executive Yuan, Taiwan.
Manuscript received May 10, 2000; revised Sep-tember 26, 2000; accepted October 6, 2000.