Study of the structural and luminescent properties of ZnO nanorod arrays
with the hydrogen peroxide treament
Wen-Yan Su
aand Ching-Fuh Lin
ab*a
Graduate Institute of Photonics and Optoelectronics, National Taiwan University, Taipei, 10617
Taiwan, Republic of China;
b
Graduate Institute of Electronics Engineering, and Department of Electrical Engineering, National
Taiwan University, Taipei, 10617 Taiwan, Republic of China
ABSTRACTOne-dimensional nanostructures, such as nanowires, nanoneedles, nanobelts and nanotubes, have been extensively studied in recent years. These fascinating structures have the excellent physical properties owing to their geometry with high aspect ratio and modify the light-matter interaction. However, the defects of these structures are the obstacles for the practical applications. We report the influence of the hydrogen peroxide (H2O2) treatment on the point defects and
structural defects of ZnO nanorods grown on n-type silicon. The ZnO nanorod arrays are prepared by low-cost hydrothermal method and the H2O2 treatments are investigated in two different approaches. One is to immerse ZnO
nanorod samples into H2O2 solution. The other is a pre-treatment of spin-coating H2O2 solution on the seed layer before
the growth of the ZnO nanorods. In the first approach, we found that the ultraviolet (UV) emission peak of the ZnO nanorods photoluminescence (PL) spectra was strongly dependent on the immersion time. In the second approach, the H2O2 solution not only influences the quality of the seed layer, but also the amount of the oxygen interstitial defects in
the ZnO nanorods grown thereon. As a result, the UV emission intensity from the ZnO nanorods is enhanced almost five times. These effects are attributed to oxygen desorption through oxidation-reduction reactions of hydrogen peroxide on the ZnO surface. The ZnO nanorod arrays with few oxygen interstitial defects are prepared by cost and low-temperature hydrogen peroxide treatments, which are compatible with glass and polymer substrates and expected to enable the fabrication of optoelectronic device with excellent performance.
Keywords: Defects, Nanostructures, Hydrothermal method, ZnO,
* cflin@cc.ee.ntu.edu.tw; phone +886-2-33663540; fax +886-2-23642603;
Nanoengineering: Fabrication, Properties, Optics, and Devices V, edited by Elizabeth A. Dobisz, Louay A. Eldada, Proc. of SPIE Vol. 7039, 70391D, (2008) · 0277-786X/08/$18 · doi: 10.1117/12.794445
1. INTRODUCTION
Zinc oxide (ZnO) is an II-VI semiconductor, which has the direct band gap energy of 3.37 eV [1]. It has strong UV emission due to its large exciton binding energy of 60 meV at room temperature, which is much lager than that of gallium nitride (25 meV) [2], and demonstrates great potentials for applications to blue-ultraviolet (UV) semiconductor lasers [3], light emitting diodes [4-6], and other optoelectronic devices . In addition, ZnO is not toxic and exhibits excellent transparency in the visible spectral range. The amount of material sources for ZnO fabrication is abundant and inexpensive. The PL spectra of ZnO single crystals, powders, nanostructures, and thin film measured at room temperature usually exhibit two bands. One occurs at 380nm, which is related the recombination of free-exciton. The other band, which occurs at 500 ~ 600 nm, is called green-yellow emission. In most studies, researchers have attributed the emission at 500 ~ 600 nm to the recombination of electron-hole pairs through intrinsic defects of ZnO (e.g. zinc interstitials or oxygen vacancies) [2, 7, 8]. In addition, the oxygen interstitial defects of ZnO would induce a depletion layer at the surface [9]. The electrical field inside the depletion layer will obstruct the formations of exciton and decrease the photoluminescence efficiency. In some studies, certain treatments were used to modify this phenomenon through removing the depletion layer, like UV-illumination [10], exposure to ethanol gas [9] and heat treatments [8].
In this work, we have investigated the influence of hydrogen peroxide treatment on the point defects and structural defects of ZnO nanorod arrays grown on n-type silicon wafer by hydrothermal process [11-13]. The photoluminescence of ZnO samples was applied to analysis and discuss in order to gain further insight into the surface characteristics of ZnO nanorod arrays. Our investigation shows that the properties of the hydrogen peroxide treated ZnO nanorod arrays is improved through removing the oxygen interstitial defects by low-cost and low-temperature hydrogen peroxide treatments, which involve processes of either immersing ZnO nanorod arrays into H2O2 solution or a pre-treatment of
spin-coating H2O2 solution on the seed layer before the growth of ZnO nanorod arrays. We discover that
photoluminescence intensity varies as a function of the immersion time. These effects are attributed to oxygen desorption through oxidation-reduction reactions of hydrogen peroxide on the ZnO surface. The result of our research could provide a method of improvement for ZnO nanorod arrays in fabricating optoelectronic devices.
2. EXPERIMENTAL DETAILS
2.1 Preparation of ZnO nanorod arrays
ZnO nanorod arrays were synthesized from zinc nitrate in an aqueous solution by hydrothermal method [11, 13]. The procedure consisted of two steps: (1) The ZnO thin films served as the seed layers were prepared by a sol-gel method [14, 15]. The coating solution was zinc acetate dehydrate [Zn(CH3COO)2•H2O, Merck, 99.5% purity] dissolved in the
mixed solution of monoethanolamine (MEA) [CH2(OH)CH2(NH2), Merck, 99.5% purity] and 2-methoxyethanol
(2MOE) [CH3O(CH2)2OH, Merck, 99.5% purity]. The concentration of zinc acetate and MEA in 2MOE was chosen to
be 0.5M. The mixture solution was then stirred at 60 ºC for 2 hours to form a homogeneous and stable colloid solution. The ZnO thin film was deposited on silicon substrate by spin-coating method at the rate of 1000 rpm for 20 s and then 3000 rpm for 20 s. Subsequently, the substrates were annealed at 300 ºC for 10 min to ensure ZnO films adhesion to the substrate and remove the residual organic compositions.
(2) After uniformly coating silicon substrates with the seed layer, hydrothermal ZnO nanowires growth was carried out by suspending these samples with ZnO seed layers upside-down in the aqueous solution (50 mM) of zinc nitrate hexahydrate [Zn(NO3)2•6H2O, Sigma Aldrich, 98% purity] and hexamethylenetetramine (HMT) [C6H12N4, Sigma
Aldrich, 99.5% purity] at 90 ºC. The reaction time was four hours. Finally, the samples were removed from the solution, rinsed with deionized water to remove the residual ions and dried in air.
2.2 Hydrogen peroxide treatment
Two sets of ZnO nanorod samples, labeled as Group I and Group II, were carried out hydrogen peroxide treatment through different approaches. Samples in Group I were immersed in a 30% H2O2 solution and heated at 75
o
C for different immersion times, 15 min, 30 min, 45 min, 60min and 90 min. After this procedure, they were cleaned with deionized water to remove the residual H2O2 solution and dried in air at 75
o
C.
For group II, pre-treatments on the seed layer of ZnO nanorod arrays were performed with spin coating method. 30% H2O2 solution was spun coated on the seed layer before the growth of ZnO nanorod arrays at the speed of 1000 r.p.m. for
=
2
=
1200 - 1000800 600 400 200-
0-(c)(a)
(b)
(d)
(f) (e) I I I I I I 300 400 500 600 700 800wavelength (nm)
20s and then 3000 r.p.m. for 30s. Then they were heated at 75 oC for 10 min. Afterwards, ZnO nanorods were grown on the pre-treated seed layer.
The crystallinity and growth direction of ZnO nanorod arrays were analyzed by X-ray diffraction (XRD) pattern using Cu Kα radiation. The photoluminescence (PL) spectra were measured at room temperature by using a Nd:YAG laser at 266nm as the excitation source to examine the properties of the ZnO seed layer and nanorods.
3. RESULTS AND DISCUSSION
The optical properties of the untreated ZnO nanorods and H2O2-treated samples in group I were characterized by the PL
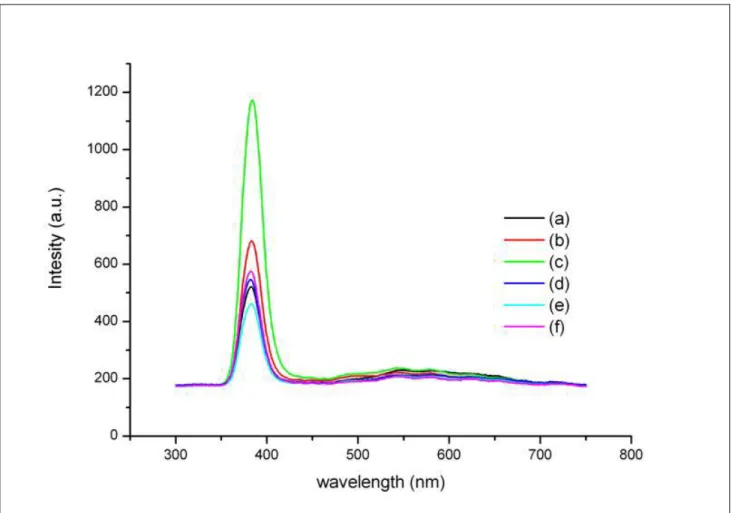
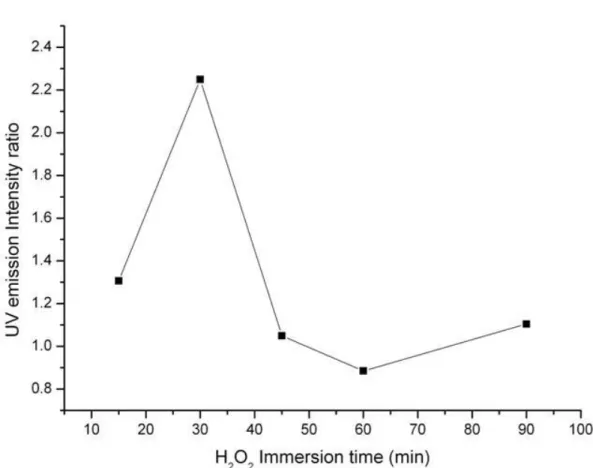
spectral measurement at room temperature. As shown in Fig. 1, the sharp UV emission peaks at 383nm, which originate from the excitonic recombination corresponding to the band edge emission of ZnO, are dominative. Furthermore, these UV peaks vary with the immersion time of samples in group I. In order to discuss the tendency of the time-dependent UV emission in detail, we plot the UV intensity ratio of samples in group I to untreated ZnO nanorods versus the immersion time. The result is shown as Fig. 2. From this figure, the ratio increases with the immersion time up to 30min and then starts to decrease as the immersion process continues.
Fig. 1. PL spectra of ZnO nanorods immersed in H2O2 for (a) 0 min, (b) 15 min, (c) 30 min, (d) 45 min,
(e) 60 min and (f) 90 min.
2.4 -2.2
2
2.0-
1.8-C ct6-C0
1.4-EC)
1.2-I • I • I I•:p
• 10 20 30 40 50 60 70 80 90 100H202 Immersion time (mm)
Fig. 2. the UV emission intensity ratio of H2O2-treated samples in group I to untreated ZnO nanorods
depending on the immersion time
For t = 0 ~ 30 min, the increase in PL intensity implies that more electron-hole pairs recombine through radiative process than through nonradiative means. The enhancement of PL intensity is attributed to the oxygen desorption on the surface of ZnO nanorods and we infer that the reactions are Oi⎯ / Oi2⎯ + H2O2 → H2O + O2(g) + e⎯ / 2e⎯. It is believed that
photogenerated holes are trapped by the surface defect states of the interstitial oxygen ions (Oi⎯ and Oi2⎯), and thus the
electron-hole pairs tend to recombine nonradiatively. As the oxygen is removed from the ZnO surface, fewer centers of nonradiative recombination lead to an increase in PL intensity. In addition, Oi⎯and Oi2⎯induce the depletion layer under
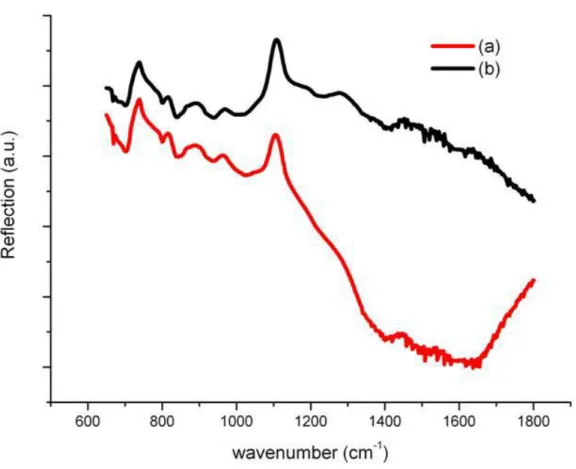
the surface of ZnO. Because the electrons and holes move to opposite direction under an electric field induced in the depletion layer, the probability of forming excitons is decreased. The increase in PL intensity can be also viewed as a result of the reduction in depletion width. Furthermore, the surface area to volume ratio of ZnO nanorods is very large and the influence of above mechanisms on surface of ZnO nanorods is more obvious. An additional experimental evidence for the effect of hydrogen peroxide treatment is given in Fig. 3. This figure shows the Fourier Transform Infrared Reflection (FTIR) spectra of the untreated ZnO nanorods and the ZnO nanorods immersed in H2O2 solution for
15min in the range of 600~1800 cm-1. The peaks at 1416 cm-1 and 1330 cm-1 are associated with bending of OH [16, 17].
Because the samples were immersed in the water during the growth of ZnO nanorods, the oxygen adsorbed to the surface would rather become O-H. The intensity of the peaks about O-H for ZnO nanorods immersed in H2O2 solution is smaller
than that of untreated ZnO nanorods. Consequently, we can conclude that the oxygen adsorption to the surface of samples is removed by hydrogen peroxide.
(a)
(b)
I
CO C0
0
0)C
0) I I I I • P • P • P 600 800 1000 1200 1400 1600 1800wavenumber (cm)
Fig. 3. FTIR reflection spectra of the ZnO nanorods (a) immersed in H2O2 for 15min and (b) without H2O2 treatment
For t = 30 ~ 60 min, most oxygen interstitial defects on the surface have been removed but the hydrogen peroxide still continue to release oxygen. The reaction Oi⎯ /Oi2⎯ + H2O2 → H2O+O2(g)+ e⎯ /2e⎯ cannot proceed because of lacking Oi⎯
and Oi2⎯. The oxygen resulting from the decomposition of hydrogen peroxide will adsorb on the ZnO surface and ionize
to become Oi⎯ and Oi2⎯ by extracting electrons from ZnO conduction band; O2(g) + 2e⎯→ 2Oi⎯ , O2(g) + 4e⎯→ 2Oi2⎯ [18],
and Oi⎯ is believed to be dominant [19]. Consequently, the depletion layer is formed in the surface and in the
grain-boundary of ZnO and reduces the UV emission again.
For t = 60~90 min, the oxygen ions accumulate enough to react with hydrogen peroxide. As oxygen adsorption is removed again, the UV emission will start to increase. The amounts of Oi⎯ and Oi2⎯ affect the behavior of H2O2.
However, H2O2 can only react with the oxygen near the ZnO surface. In other words, the Oi⎯ and Oi2⎯ inside of ZnO
cannot be removed by H2O2, so we try to restrain the production of Oi⎯ and Oi2⎯ by applying H2O2 treatment on the seed
layer of ZnO nanorods during the formation of ZnO nanorods.
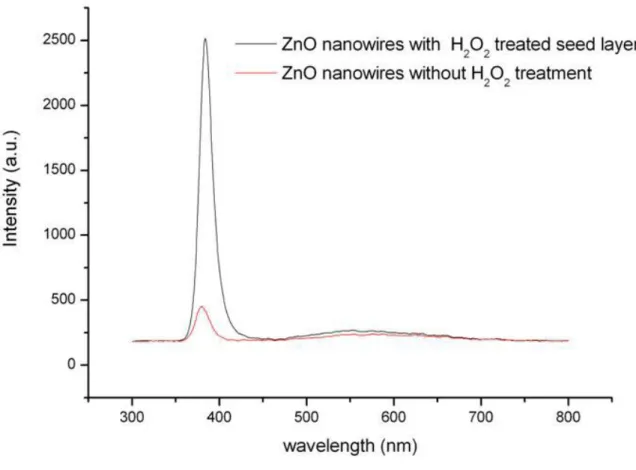
Fig. 4 shows a series of PL spectra of samples in group II. H2O2 solution had been spin-coated onto the seed layer before
the growth of ZnO nanorods. From this figure, a strong UV emission peak, which is almost five times the intensity of untreated ZnO nanorods, is observed. The increase in PL intensity can be viewed as a result of few oxygen interstitial defects. As H2O2 solution is spin-coated on the seed layer of ZnO nanorods, it removes Oi⎯ and Oi2⎯ of the seed layer. It
is suggested that the seed layer likely reduce the Oi⎯ and Oi2⎯ density and less lattice distortion stress exist during the
growth of ZnO nanorods. Therefore, the structure of ZnO nanorods has fewer defects and the probability of forming excitons increases. The pre-treatment of seed layer has excellent performance on the reduction of oxygen interstitial defects for ZnO structure.
—
I • I •
I • I I
ZnO nanowires with H,02 treated seed layer
ZnO nanowires without H2 treatment
Co
=
2
=
2500 -2000. 1 500 laUD 500-
0-300 400 500 600 700 600wavelength (nni)
Fig. 4. PL spectra of ZnO nanorods with H2O2 treated seed layer and without H2O2 treatment
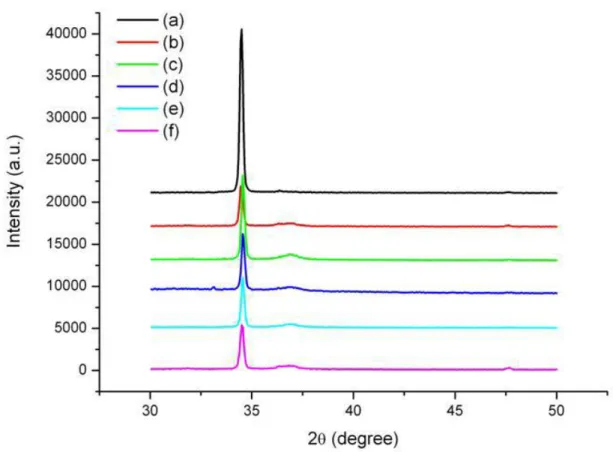
Fig. 5 shows the XRD patterns for samples in group I before and after immersion in 30% H2O2 solution. It is found that
no other characteristic peaks corresponding to the precursors such as zinc nitrate and zinc hydroxide are observed in Fig. 5. The dominant (002) peak of XRD patterns shows that the samples are all of high c-axis orientations. The intensity of (002) peak for samples in group I gradually decreases with the immersion time up to 90min. At the same time, these samples have a very weak (101) peak as the immersion time increases. The study of Yufeng Chen et al. [19] has suggested that the thicker ZnO film has the better crystalline quality during the growth of the film. This means that the ZnO crystalline property of the lower layer is worse than that of the upper layer. Because the growth mechanism of ZnO films and ZnO nanorods is similar, the ZnO nanorods have better crystalline quality at the external portion. This tendency is similar to that of ZnO films. From the result of XRD measurement, we infer that there are more dislocations of Zn2+ or O
i2⎯ related (002) and (101) plane at the internal portion of ZnO than at the external portion. As the external
portion was etched off by 30% H2O2 solution with a pH of 3-4, the uncovered dislocations caused the change of XRD
pattern in samples in group I. This tendency can explain the decrease of the intensity in (002) peak and the increase of the intensity in (101) peak for samples in group Ias the immersion time increases. In comparison with the weak (101) peak, the sharp (002) peak is relatively strong, suggesting that the hydrogen peroxide treatment does not noticeably reduce the crystal quality of ZnO nanorods. This result indeed reflects that the PL spectra is mainly caused by the desorption of surface oxygen instead of the change of the structure property
(a)
40000-(b)
35000 -30000 -(e)
(U 25000 20000 - 15000-10000-________ft
5000--
0-30 35 40 45 5028 (degree)
Fig. 5. XRD pattern of ZnO nanorods immersed in H2O2 for (a) 0 min, (b) 15 min, (c) 30 min, (d) 45 min,
(e) 60 min and (f) 90 min.
4. CONCLUSIONS
In conclusion, we report here a simple and inexpensive method for improving ZnO-nanorod structure quality by removing oxygen interstitial defects. We observe a UV emission enhanced PL in the samples immersed in hydrogen peroxide. This change of luminescent properties is mainly related to the desorption of the surface oxygen and the reduction of the depletion layer width. From the result of FTIR measurement, we confirm that H2O2 can remove the
oxygen adsorbed to ZnO surface. The change of crystalline properties of the samples in group I is due to the different properties of external and internal portions in ZnO nanorods. In addition, the UV emission at 383 is enhanced almost five times by pre-treatment on the seed layer with spin-coating H2O2 solution. Therefore, this could be an excellent technique
providing a route to fabrication of low-cost and high quality ZnO nanorods with only few oxygen interstitial defects for applications.
REFERENCES
[1] R. E. Service, "Materials science - Will UV lasers beat the blues," Science, 276, 895-895 (1997).
[2] C. M. Jin, A. Tiwari, and R. J. Narayan, "Ultraviolet-illumination-enhanced photoluminescence effect in zinc oxide thin films," J. Appl. Phys., 98, 083707 (2005).
[3] M. H. Huang, S. Mao, H. Feick, H. Q. Yan, Y. Y. Wu, H. Kind, E. Weber, R. Russo, and P. D. Yang, "Room-temperature ultraviolet nanowire nanolasers," Science, 292, 1897-1899 (2001).
[4] Y. R. Ryu, T. S. Lee, J. A. Lubguban, H. W. White, B. J. Kim, Y. S. Park, and C. J. Youn, "Next generation of oxide photonic devices: ZnO-based ultraviolet light emitting diodes," Appl. Phys. Lett., 88, 241108 (2006).
[5] S. H. Park, S. H. Kim, and S. W. Han, "Growth of homoepitaxial ZnO film on ZnO nanorods and light emitting diode applications," Nanotechnology, 18, 055608 (2007).
[6] Z. P. Wei, Y. M. Lu, D. Z. Shen, Z. Z. Zhang, B. Yao, B. H. Li, J. Y. Zhang, D. X. Zhao, X. W. Fan, and Z. K. Tang, "Room temperature p-n ZnO blue-violet light-emitting diodes," Appl. Phys. Lett., 90, 042113 (2007).
[7] Y. Y. Peng, T. E. Hsieh, and C. H. Hsu, "White-light emitting ZnO-SiO2 nanocomposite thin films prepared by the target-attached sputtering method," Nanotechnology, 17, 174-180 (2006).
[8] Y. Sun, N. G. Ndifor-Angwafor, D. J. Riley, and M. N. R. Ashfold, "Synthesis and photoluminescence of ultra-thin ZnO nanowire/nanotube arrays formed by hydrothermal growth," Chem. Phys. Lett., 431, 352-357 (2006).
[9] T. Gao and T. H. Wang, "Synthesis and properties of multipod-shaped ZnO nanorods for gas-sensor applications," Appl. Phys. A, 80, 1451-1454 (2005).
[10] M. Tokumura, A. Ohta, H. T. Znad, and Y. Kawase, "UV light assisted decolorization of dark brown colored coffee effluent by photo-Fenton reaction," Water Res., 40, 3775-3784 (2006).
[11] Quanchang Li, Vageesh Kumar, Yan Li, Haitao Zhang, Tobin J. Marks, and R. P. H. Chang, "Fabrication of ZnO Nanorods and Nanotubes in Aqueous Solutions," Chem. Mater., 17, 1001-1006 (2005).
[12] Changsong Liu, Yoshitake Masuda, Yunying Wu, and O. Takai, "A simple route for growing thin films of uniform ZnO nanorod arrays on functionalized Si surfaces," Thin Soild Films, 503, 110-114 (2006).
[13] Min Guo, Peng Diao, Xindong Wang, and S. Cai, "The effect of hydrothermal growth temperature on preparation and photoelectrochemical performance of ZnO nanorod array films," J. Solid State Chemistry, 178, 3210-3215 (2005).
[14] M. R. Wang, J. Wang, W. Chen, Y. Cui, and L. D. Wang, "Effect of preheating and annealing temperatures on quality characteristics of ZnO thin film prepared by sol-gel method," Meter. Chem. Phys., 97, 219-225 (2006). [15] M. Ohyama, H. Kozuka, and T. Yoko, "Sol-gel preparation of ZnO films with extremely preferred orientation
along (002) plane from zinc acetate solution," Thin Solid Films, 306, 78-85 (1997).
[16] B. M. Keyes, L. M. Gedvilas, X. Li, and T. J. Coutts, "Infrared spectroscopy of polycrystalline ZnO and ZnO : N thin films," J. Crystal Growth, 281, 297 (2005).
[17] X. N. Li, S. E. Asher, S. Limpijumnong, B. M. Keyes, C. L. Perkins, T. M. Barnes, H. R. Moutinho, J. M. Luther, S. B. Zhang, S. H. Wei, and T. J. Coutts, "Impurity effects in ZnO and nitrogen-doped ZnO thin films fabricated by MOCVD," J. Crystal Growth, 287, 94 (2006).
[18] H. Windischmann and P. Mark, "MODEL FOR THE OPERATION OF A THIN-FILM SNOX CONDUCTANCE-MODULATION CARBON-MONOXIDE SENSOR," J. Electrochem. Soc., 126, 627 (1979).
[19] Y. F. Chen, L. Wang, C. L. Mo, Y. Pu, W. Q. Fang, and F. Y. Jiang, "Study of structural and luminescent properties of high-quality ZnO thin films treatment with hydrogen peroxide solution," Mater. Sci. Semicond. Process., 8, 569-575 (2005).