Numerous studies have indicated that male schizo
phrenic patients have an earlier age at onset than
females (Lewine, 1981; Eaton, 1985; Häfner,1987;
Angermeyer & Kuhn, 1988; Lewine, 1988; Goldstein
et al, 1989). This observation, along with otherfmdings, has led some to hypothesise that a biological
factor relating to gender may play a role in the onset
mechanism of schizophrenia (Seeman, 1983; DeLisi
eta!, 1989, 1991; Castle& Murray, 1991; Hafneret
a!, 1991). Although gender differences in age at onset
are among the most robust fmdings in schizophreniaresearch (Wyatt et a!, 1988), the evidence from
previous studies must be interpreted cautiously due
to differences between the age distributions of male
and female patients.
The ages at onset observed in a series of patients
is biased by the underlying age distribution of the population (Crowe & Smouse, 1977; Heimbuch
et a!, 1980; Chen et a!, 1992b). If the age
distributions of males and females differs, then observed differences in age at onset could be artefactual. Since, in the general population, females tend to live longer than males, they are more likelyto surviveto - and hence suffer onset of disease at - a
laterage. Furthermore,the greatermortality for male
schizophrenic
patients exacerbates
this problem
(Goldstein et a!, 1992; Chen et a!, 1993). No
previous study of gender differences in age at onset
of schizophrenia have corrected this problem. So we
tested whether gender differences in the onset pattern
of schizophrenia would remain after applying
available methods of statistical correction (Chen et
a!, 1993).
Method
The subjects for this study came from two retrospective
cohort family studies. Details of the sample selection and
follow-upproceduresare describedelsewhere(Morrisonet
a!, 1972;Tsuang & Winokur, 1975). Briefly, probands
were selected from a blind review of index admission andfollow-up records and interviewsof 510 consecutive
admissionsto the Iowa PsychopathicHospital from 1934
to 1944witha chartdiagnosisof schizophrenia.Probands
wererediagnosedusing DSM-III (AmericanPsychiatric
Association, 1980) criteria by two expert psychiatrist diagnosticians (Kendler eta!, 1985). However, the criterionlimiting age at onset to 45 years was eliminated, as in
DSM-III—R
(AmericanPsychiatricAssociation, 1987).
Thestructured
interviewdevelopedbyTsuangci a!(1980)
collecteddiagnosticinformation about the probands. We
determined age at onset from all sources of information, including direct interviews, interviews with informants, and
medical charts (Kendler et a!, 1987). Age at onset was
defined as the age when psychoticsymptomsfirst became
evident. The reliability of this defmition was high, as
indicated by an intraclass correlation of 0.95 (Kendilerci
a!, 1987).
Outof orIginal510probands,therewere332DSM-III
schizophrenics. Among these schizophrenic probands, reliable data on age at onset were available for 319 patients.The samplewasequallydividedbetweenmen (50.8%)and
women(49.2°!.).
For males,the durationbetweenage at
onset and age at admission ranged from 0 to 15 years witha meanof 1.1and a standarddeviationof 2.4. For females,
these values ranged from 0 to 19 years with a mean of 1.4
and a standard deviation of 3.1. The difference between
males and females was not significant. Statistical procedures
To correctthe distributionof observedage at onset, we
applied the non-parametric method proposed by Baronet al (1983), with modifications to allow for increasedmortality after onset, which is the case for schizophrenia.
Our simulations indicate that the modified method
works effectively for this situation (Chen eta!, 1993). Four components of this methodology are essential for understanding our application to schizophrenia: (1) the relationship between age at onset and current age (or
625
Gender Differences in Age at Onset of Schizophrenia
STEPHENV. FARAONE,WEt J. CHEN, JILL M. GOLDSTEINand MING T. TSUANG
Numerousstudieshavefoundthat maleschizophrenicpatientshaveearlieragesat onsetthan females.However, noneof thesestudieshavecorrectedthe observedagesfor knowngender
differences
intheagedistribution
ofthepopulation.
Usinga pre-existing
dataset,we applied
a non-parametric
methodto correctthemaleandfemaledistributions
of observed
ageatonset
forsex-specific
agedistributions.
Thedistributions
of observedageat onsetindicatedearlier
onsetamongmales.Aftercorrection,
theage-at-onset
distributions
shiftedtowardolderages,
but the differencebetweenmalesandfemalesremainedstatisticallysignificant.Thus, genderageat ascertainment)whenascertainingfrom a seriesof prevalent cases; (2) the derivation of the age distribution for the susceptible population from which schizophrenic patients come; (3) the procedure for correcting an observed age at onset distribution; and (4) the method for comparing two corrected distributions.
The distribution of the observed age at onset is a function of two random variables: age at onset, X, and current age,
Y(Heimbuch eta!, 1980). We denote particular realisations
of these two random variables by x and y, respectively. When prevalent cases are sampled, the observed age at onset distribution is conditional on X being less than V. Assume
that F(x) =p(Xx) is the true age at onset distribution
which we are trying to estimate, C(y) =p( Yy) is the age distribution of the susceptible population, and
H(x) =p(Xx@X Y) is the observed age at onset distribution. The corresponding density functions of these
three distributions are f(x), c(y), and h(x), respectively.
If the illness has negligible effects on mortality and fertility, then the age distribution of the susceptible
population, C(y), is the same as that of the general population. The relationship between true and observed age
at onset distributions is as follows (Baron et a!, 1983):
h(x) = -@-f(x)[I —¿C(x) J. (I)
K is a normalising constant to make the integral of h
equal to one. A key feature of equation (1) is that 1(x) is multiplied by the age distribution beyond age at onset x,
[I —¿C(x)], while a subject's current age, y, is not directly
reflected in the formula. Thus, the cumulative age distribution, C(y), is evaluated at the age of onset, x. A common way to obtain C(y) is to assume that the population is in equilibrium (Cavalli-Sforza & Bodmer, 1971). Assuming !(y) is the survival distribution function
up to agey (i.e., the probability that an individual will not die before reaching age y) and r is the intrinsic rate of
natural growth in the population, then the density function for the age distribution C(y) is:
c(y) = n1(y)e@
where n is a normalising constant to make the integral of c equal to one. Both 1(y) and r can be obtained from vital statistics.
However, because the mortality rate of schizophrenic patients is increased after onset, the age distribution of the susceptible population is no longer the same as that of the general population. Instead, the current age, Y, becomes dependent on the age at onset, X. We denote the conditional age distribution as D(y(x) =p( Yy@X=x) and its density function as d(ylx). Then the density function of the observed age at onset distribution becomes:
h(x)=j@/(x) [l—D(xlx)] (3)
For convenience, we divide both age at onset and age into 18 discrete intervals denoted x, and y., respectively. We then assume that the age distribution of subjects in each onset interval is in equilibrium with its specific survivorship function.Modified from equation (2),the conditionalage distribution for subjects whose onset occurred in the ith
interval, x,, is:
d(yIx1)=nm(yIx,)e@
where n is a normalising constant to make the integral of
d equal to one, and m(y@x,)=p(Yy@X=x1) is the
survivorship function for subjects whose age at onset is x1. Having derived equations (3) and (4), the remaining information we must provide to obtain a corrected distribution of age at onset is the conditional survivorship
function for schizophrenic patients, m(y@x1). As we have
demonstrated elsewhere (Chen et a!, l992a; Chen et a!,
1993), we can apply the standardised mortality ratio of
schizophrenic patients to model their conditional mortality rates as follows. Starting with data from the life table for the general population, we first multiply the number of deaths belonging to intervals that are greater than the age at onset x1 by the standardised mortality ratio, denoted as a. We then derive the new survivorship functions in each interval by subtracting the newly calculated number of deaths from the number alive in the previous interval. For
age y1, j= 1 to 18, the new survivorship function is
calculated as follows:
=I(y,)—(a— 1)(k@.tyk/1OO0OO) for x1<y@and the
- item0
(5)
=0 otherwise
where t@.is the number of deaths in the age interval y@. Thus, based on equation (5), we can compute the
conditional survivorship function for schizophrenic patients
if we specify the standardised mortality ratio, a, and the (2@ survivorship function of the general population, 1(y1).
Comparing the mortality rates of the schizophrenic
probands used in this study to that of the general
population, Tsuang & Woolsen (1977) reported that the
standardised mortality ratios in the first, second, third, and
fourth decades after admission were 4.69, 3.40, 0.86 and 1.44 for males and 3.30, 1.30, 2.06 and 1.80 for females. Because the mean (s.d.) duration between age at onset and age at admission (1.1 (2.4) years for males and 1.4 (3.1) years for females) is less than the length of a single 5-year interval, we use these four values as the standardised mortality ratio, a, to represent the increased mortality rates for four different lengths of time after onset. After the
conditional survivorship function for schizophrenic patients
is calculated according to equation (5), we then use equation (4) to compute their conditional age distribution. Because we did not have the intrinsic rate of natural increase, r, for the Iowa population in 1940, we chose three values based on national data (0.001 for all races, 0.0001 for white, and 0.0074 for all other).
m(y1lx,)=I(y@) for
To estimate the underlying true age at onset distribution,
Age@at-onset
distributionsMale (n=162)Means.d.Female
In =157) Mean s.d.Two-distribution x2 test x2 (d.f.=6)' PObserved24.3 6.128.0 8.326.2 <0.0001Correctedr=0.000126.2 7.131.1 9.928.3 <0.0001r=0.001026.2 7.131.1 9.928.4 <0.0001r=0.007426.4 7.131.5 10.129.2 <0.0001GENDER DIFFERENCES IN AGE AT ONSET OF SCHIZOPHRENIA
Afterbeingadjustedfor the underlyingage distributions,
the corrected distributions move toward older ages as
compared with the observed ones. The means and standard
deviationsof the observedand correctedage at onset
distributions
aresummarised
in TableI. Beforecorrection,
the male distributionis significantlyyounger than the
female one, accordingto the two-distributionx1 test.
After correction, the male distributions are still significantly
different from the females, regardlessof which r is used
in the adjustment.In fact, for all threevaluesof r, the
means and standard deviations of the corrected distributions
are similar.
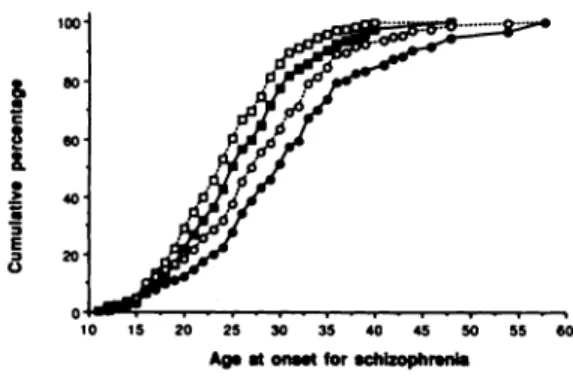
Discussion
As predicted, the distribution of the observed age
at onset of schizophrenia is biased toward younger
ages. As shown in Fig. 1, only 5.6% of males and
18.5% of females have observed ages at onset greater
than 35 years. After correction, these percentages
increase to! 1.8% and 30.1%, respectively. The non negligible downward biases in the observed age at
onset distributions is consistent with the pattern
demonstrated
in simulation studies (Chen et a!,
1993).
However, although the observed ages at onset for
male and female schizophrenics are biased, we also
show that the observed gender differences are not
spurious. Through our non-parametric method, we
controlled for three potential confounders in the
comparison: the age composition of the population
of origin, excess mortality among schizophrenic
patients, and the gender differences in mortality
among these patients.
Nevertheless, our results may be limited by the
assumptions
required
in our derivation
of the
corrected age at onset distribution. First, we assumed
that the ascertainment of patients was random with
respect to their age at the time of sampling. This
seems reasonable given that the Iowa Psychiatric
Hospital was the only in-patient state psychiatric
facility available in Iowa. Of course, our hospitalised
series of patients may not be representative of
prevalent cases in other respects. This will limit the
extent to which our results can be generalised but
does not compromise the validity of our procedure
for correcting the distribution of age at onset. For
example, if cases with early onset have a more severe
form of illness they may be more likely to be
hospitalised than cases with late onset. Thus,
although our observed and corrected age at onset
distributions may not generalise to non-hospitalised
cases, this does not confound our correction
procedure.
We also assumed that the age distribution of the
population is in equilibrium when deriving the
&
C8
&
a 3 E 3 0 50 40 20 10 15 20 25 30 35 40 45 50 55 60Ag, at onuS for schizophrenia
Fig. 1 The cumulative percentagesof observed and correctedage at-onset distributions for male(o )and female (0) schizophrenics (open symbols, observed distribution; closed symbols, corrected distribution). The intrinsic rate of natural growth of the population, r, was set to be 0.001 in the correcting procedure.
After the underlying age distribution is determined, a
corrected age at onset distribution adjusted for the age distribution can be derived by solving equation (3) forf(x).
To determine the value of K, we calculate all 1(x,)
accordingto equation (3) without K and then use the sum
of theseitemsas the K to divideeach item. This makesthe
sum of the re-evaluated1(x,) equal to one as required by
the formula.
Based on the density function, 1(x,), we then calculate
the mean and standard deviation for the corrected age at
onset distribution. To compare the corrected male and
female age at onset distributions, we grouped the ages at
onset into seven intervals (<15, 15—19,
20—24,
25—29,
30-34, 35-40, >40) andcomparedthe distributionswith
a two-distribution
@
(Press et a!, 1986).
Resufts
Figure 1 displays the cumulative distributions for both
observedand correctedage at onset for malesand females
in which r is set to be 0.0010. The corrected distributionsfor r=0.0001 and 0.0074are similar and are not shown.
Table 1
Observed and corrected age-at-onset distributions for male
andfemaleschizophrenics
1. x2 with 6 degrees of freedom (dividingages at onset into seven intervals).
conditional age distributions for schizophrenic patients. Because we did not have the intrinsic rate of natural growth (r) of the Iowa population in 1940, we were unable to test this assumption directly. However, we tried three values of r based on national data. The differences between male and female corrected age at onset distributions did not depend on the value of r.
Our inferences are also limited by the accuracy of the assessment of age at onset based on medical records. We are reassured by the very high level of agreement between raters (intraclass r= 0.95). Nevertheless, the procedure may systematically over- or underestimate age at onset. However, such systematic error would neither invalidate our correction procedure nor bias our comparison of male and female distributions.
In summary, we conclude that the earlier observed age at onset for male than for female schizophrenic patients is not due to demographic confounding. This provides further support for hypotheses about gender differences in the disorder (Angermeyer & KUhn, 1988). For example, gender differences in age at onset may indicate a possible protective effect of female sex hormones (Seeman, 1983; Häfner et a!, 1991). That is, oestrogens have been found to have a small neuroleptic effect (Seeman & Lang, 1990; Häfneret a!, 1991) which may contribute to delaying the onset in women. In addition, others have suggested that a greater vulnerability of the male foetus to perinatal central nervous system aetiological factors creates a neurodevelopmentally more severe disorder in males, which has an early onset (Castle & Murray, 1991). Our results cannot determine the causes of gender differences but they do suggest that further work in this area will be worthwhile.
Acknowledgements
The authors thank Jerry Fleming for providing us with the life table
for Iowa in 1940 that he had calculated. Preparation of this article was supported in part by the Veterans Administration's Medical
Research and Health Services Research and Development Programs
and the National Institute of Mental Health Grants MH 42604.
This manuscript was prepared in part while Dr Tsuang was a Fritz
Redlich Fellow, 1991—1992,at the Center for Advanced Study in the Behavioral Sciences, Stanford, and while Dr Goldstein was a
Fellow in the NIMH Clinical Research Program, MH 16259. We are grateful for the financial support provided to the Center for Dr Tsuang's fellowship year by the John D. & Catherine T. MacArthur Foundation and the Foundations Fund for Research in Psychiatry Endowment.
References
AMERICAN PSYCHIATRIC AssoaAlloN (1980) Diagnostic and Statistical
Manual of Mental Disorders (3rd edn) (DSM-lII). Washington, DC: APA.
(1987) Diagnostic and Statistical Manual of Mental Disorders (3rd cdii revised) (DSM-III-R). Washington, DC: APA.
ANGERMEYER, M. C. & KUHN (1988) Gender differences in age at
onset of schizophrenia: an overview. European Archives of
Psychiatry and Neurological Sciences,237, 351—364.
BARON, M., Riscis, N. & MENDLEWICZ, J. (1983) Age at onset in
bipolar-related major affective illness: clinical and genetic
implications. Journal of Psychiatric Research, 17, 5—18. CASTLE, D. J. & MURRAY, R. M. (1991) The neurodevelopmental
basis of sex differences in schizophrenia. Psychological Medicine, 21, 565—575.
CAVALLI-SFORZA, L. L. & BODMER, W. F. (1971) The Genetics of Human Populations, pp. 289—301. San Francisco: Freeman. CHEN, W. J., FARAONE, S. V. & TSUANO, M. T. (1992) Linkage
studies of schizophrenia: a simulation study of statistical power. GeneticEpidemiology,9, 123—139.
& TSUANG,M. T. (1992b) Estimating age at onset
distributions: a review of methods and issues. Psychiatric
Genetics, 2, 219—238.
ORAV, E. J., et al (1993) Estimating age at onset distributions: the bias from prevalent cases and its impact on
risk estimation. Genetic Epidemiology, 10, 43—60.
CROWE, R. R. & SMOUSE, P. E. (1977) The genetic implications of
age-dependent penetrance in manic—depressive illness. Journal
of PsychiatricResearch,13,273—285.
DELIsI, L. E., CROW,T. J., DAVIES,K. E., et a! (1991) No genetic linkage detected for schizophrenia to Xq27-q28. British Journal of Psychiatry,158,630—634.
DAUPHINAIS, I. D. & HAUSER, P. (1989) Gender differences
in the brain: are they relevant to the pathogenesis of
schizophrenia? Comprehensive Psychiatry, 30, 197—208.
EATON, W. W. (1985) Epidemiology of schizophrenia. Epidemiology
Review, 7, 105—126.
GOLDSTEIN, J. M., TSUANG, M. T. & FARAONE, S. V. (1989)
Gender and schizophrenia: implications for understanding the heterogeneity of the illness. Psychiatry Research, 28, 243—253.
HAFNER, H. (1987) Epidemiology of schizophrenia. In Search for
theCausesof Schizophrenia(edsH. Häfner,W. F. Gattaz&
W. Janzarik), pp. 47—74.Berlin: Springer-Verlag.
BEHRENS, S., Dit VRY, J., et a! (1991) An animal model
for the effects of estradiol on dopamine-mediated behavior:
Implications for sex differences in schizophrenia. Psychiatry Research, 38, 125—134.
HEIMBUCH, R. C., MATFHYSSE, S. & KIDD, K. K. (1980) Estimating
age-of-onset distributions for disorders with variable onset. American Journalof Human Genetics,32, 564—574.
KENDLER, K. S., GRUENBERG, A. M. & TSUANG, M. T. (1985)
Psychiatric illness in first-degree relatives of schizophrenic and
surgical control patients. Archives of General Psychiatry, 42,
770—779.
TSUANG, M. T. & HAYS, P. (1987) Age at onset in
schizophrenia: a familial perspective. Archives of General
Psychiatry, 44, 881-890.
LEWINE, R. R. J. (1981) Sex differences in schizophrenia: timing
or subtypes?PsychologicalBulletin,90, 432—444. (1988) Gender and schizophrenia. In Handbook of
Schizophrenia. Vol. 3. Nosology, Epidemiology and Genetics of Schizophrenia (eds M. T. Tsuang & J. C. Simpson), pp. 379-397. Amsterdam: Elsevier.
MORRISON, J., CLANCY, J., CROWE, R., et a! (1972) The Iowa 500.
I. Diagnostic validity in mania, depression, and schizophrenia.
Archives of General Psychiatry, 27, 457—461.
PREsS, W. H., FLANNERY, B. P., TEUKOLSKY, S. A., et a! (1986)
Numerical Recipes: The Art of Scientific Computing. Cambridge: Cambridge University Press.
S@sJ4AN,M. V. (1983) Interaction of sex, age and neuroleptic dose. Comprehensive Psychiatry, 24, 125—128.
& LANG,M. (1990) The role of estrogens in schizophrenia gender differences. Schizophrenia Bulletin, 16, 185—194.
TSUANG, M. T. & WINOKUR, G. (1975) The Iowa 500: field work
in a 35-year follow-up of depression, mania, and schizophrenia. Canadian Psychiatric Association Journal, 20, 359-365.
—¿ & WOOLSON, R. F. (1977) Mortality in patients with
schizophrenia, mania, depression and surgical conditions. British Journal of Psychiatry, 130, 162—166.
—¿, —¿ & SIMPSON, J. C. (1980) The Iowa Structured
Psychiatric Interview: Rationale, Reliability and Validity. Ada Psychiatrica Scandinavia, (supplement 283).
WYATr, R. J., ALEXANDER,R. C., EGAN, M. F., ci ci (1988)
Schizophrenia,just the facts: what do we know, how welldo
we know it? Schizophrenia Research, 1, 3—18.
*Stephen V. Faraone, PhD,Associate Professor of Psychology, Department of Psychiatry, Harvard Medical
School, Brockton- West Roxbury Veterans Affairs Medical Center, Wei J. Chen, MD, ScD, Lecturer, Institute of Public Health, National Taiwan University College of Medicine; Jill M. Goldstein, PhD,Assistant Professor of Psychiatry, Harvard Medical School; Ming T. Tsuang, MD, PhD, Stanley Cobb Professor of Psychiatry and Epidemiology, and Chief, Division of Psychiatric Epidemiology and Genetics, Department of Psychiatry, Harvard Medical School, Brockton- West Roxbury Veterans Affairs Medical Center, and Massachusetts Mental Health Center, USA
*Correspondence: Psychiatry Service (116A), Brocklon Veterans Affairs Medical Center, 940 Belmont Street, Brockton, MA 02401, USA