Short Reports 1863
Acetate: mp 80”; Found: C, 54.31; H, 5.1% Cs2Hs601s reqtures C, REFERENCES 54.2; H,
S.is;
‘H NMR (CDCl,): 66.26 (lH, d, J = 10 Hz, H-3),7.65 (lH, d, J = 10 Hz, H-4X 7.48 (lH, d, J = 9 Hz, H-S), 6.98 1. Nair, A. G. R. and Subramanian, s. s. (1975) Phytochemistry (lH, dd, .I = 9 and 2 Hz, H-6), 6.99 (lH, d, J = 2 Hz, H-8), 14, 1135.
coumarm protons 5.2 (4H, m), 4.92 (lH, s), 4.64 (lH, q, .I = 12, 2. Aqaneyulu. A. S. R., Rao, K. J., Rao, V. K., Row, L. R., 6 Hz), 4.13 (2H, d, J = 1.8 Hz); sugar protons and acetoxyl Subrahmanian, C., Pelter, A. and Ward, R. S. (1975) chemical shifts 8iven in the text. 3. Tetrahedron 31, 1277.
Aqaneyuhr, A. S. R., Rao, A. M., Rao, V. K., Row, L. R., Pelter, Acknowledgement~ur thanks are due to Prof. J. Shoji, School A. and Ward, R. S. (1977) Tetrahedron 33, 133.
of Pharmaceutical Sciences, Showa Untversrty, Halanodat, 4. Markham, K. R., Temai, B., Stanley, R., Getgler, H. and Shinagawa-Ku, Tokyo 142, Japan, for a sample of apim. One of Mabry, J. J. (1978) Tetruhedron 34, 1389.
us (PSM) gratefully acknowledges the CSIR for the award of a 5. Austm, D. J. and Meyers, M. B. (1965) Phytochemstry 4,255. senior research fellowship. 6. Sakuma, S. and Shoji, J. (1981) Gem. Pharm Bull. 29, 2431.
P~yh%emrsrry, Voi 24, No 8, pp 1863-1864, 1985 003 I -9422/85 $3.00 + 0.00
Pnntcd III Great Bntrua 0 1985 Pergamon Fress Ltd
(+)-CALOCEDRIN, A LIGNAN DIHYDROANHYDRIDE FROM
CALOCEDRUS
FORMOSANA
JIM-MIN FANG, SHYI-TAI JAN and Yu-SHIA CHENG
Department of Chemtstry, National Taiwan University, Taipei, Taiwan, Repubhc of China
(Revised received 28 November 1984)
Key Word Ldex-Calocedrus formosana; Cuprcssaccae; calocedrm; llgnan dihydroanhydride; hlbalactone.
Abstract-A novel lignan dlhydroanhydride, (+ )-calocedrin, was isolated from the wood of Colocedrusformosana. Its
structure was determined to be truns-a-(3,4-methylenedioxybenzylidene)-xy-
butanolide by spectroscopic methods. Reduction of (+)-calocedrin resulted in an optically inactive lignan lactone,
( f )-hibalactone.
INTRODUCTION m/z 368 and the base peak at m/z 135, ascribable to the 3,4- Calocedrus fownosana, a member of the Cupressaceae, is methylenedioxybenxyl fragment. The IR spectrum an economically important tree indigenous to Taiwan [ 11. showed the presence of hydroxyl (356Ot~n-~), lactone Previous investigations [2, 31 on the heartwood have (1745 cm-‘) and olefin (164Ocm-‘) groups. Analyses of shown that it contains ess&tiai oil and a large quantity of
terpenoid acids, such as shonanic, thujic and chaminic. Lignan components, such as hinokinin and hibalactone (savinin), have also been found.
RESULTS AND DISCUSSION
On continuing a study of the chemical constituents, the (’ wood of C. fonnosana was collected in our campus and o subJected to extraction with acetone. The combined extracts were concentrated and the residual contents separated on a silica gel column eluting with hexane-ethyl acetate gradients. After (-thibalactone 1 (RJ 0.30, hexane-acetone, 7: 3) [4, 51, a novel lignan, namely ( + )- caloccdrin, was eluted (R, 0.16). Calocedrin was re-
crystallized from ethanol, mp 187-188”, [a] g + 6” (c 0.9; 1 R=H acetone). The mass spectrum displayed the parent peak at 2 R=OH
0
(1
1 ;1.
,““*
T
n 1; O O-7 31864 Short Reports
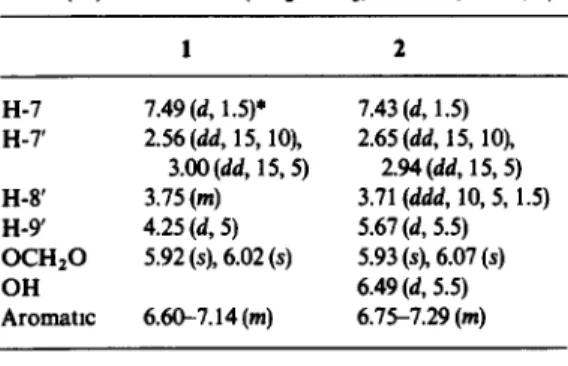
the ‘H and “C NMR spectra (Tables 1 and 2) revealed that the structure of ( +)-caloccdrin (2) was related to that of hibalactone. Calocedrin contained an unusual hemiacetal la&one (-CO,-CHOH-) moiety as character- ized in the ‘H NMR spectrum [a]. The hydroxyl proton, coupled by H-9’, exhibited as a doublet (J = 5.5 Hz) at 66.49 that was shifted by change of concentration or temperature. Similarly, the hemiacetal proton (H-9’) was coupled by the hydroxyl proton, displaying as a doublet (J = 5.5 Hz) at 6 5.67. Since irradiation at the resonance of H-8’ (6 3.71) did not cause any apparent effect on the signal pattern of H-9’, these two protons should orient nearly orthogonally (truns configuration) according to the Karplus empirical rule.
The structures of calocedrin and hibalactone are chemi-
Table 1. ‘H NMR spectral data of (-thibalactone 1 and (+)-calocedrin 2 (MezCO-& 90 MHz, TMS, a)
1 H-7 H-7’ H-8 H-9 OCHzO OH Aromatlc 7.49 (d, 1.5)’ 2.56 (dd, 15, lo), 3.00 (dd, 15,5) 3.75 (WI) 4.25 (d, 5) 5.92 (s), 6.02 (s) 6.60-7.14 (m) 7.43 (d, 1.5) 2.65 (dd, 15, 10). 2.94 (dd, 15,5) 3.71 (ddd, 10,5, 1.5) 5.67 (d, 5.5) 5.93 (s), 6.07 (s) 6.49 (d, 5.5) 6.75-7.29 (m)
*Numbers in parentheses indicate coupling cohstants (Hz).
Table 2. ‘“C NMR spectral data of (-)-hibalactone 1 and (+)- calocedrin 2 (MezCO-d~, 25.2 MHz, 6) C 1 2 c 1 2 1 2 3 4 5 6 7 8 9 OCHzO 129.2 (s) 128.8 (s) 108.8 (d) 108.7 (d) 149.7 (s) 149.7 (s) 148.6 (s) 148.4 (s) 110.0 (d) 109.8 (d) 122.9 (d) 122.7 (d) 136.7 (d) 137.4 (d) 127.8 (s) 126.9 (s) 172.0 (s) 171.3 (s) 101.7 (t), 101.6(t), 102.6 (t) 102.5 (t) 1’ 132.7 (s) 132.2 (s) 2 109.3 (d) 109.2 (d) 3 149.1 (s) 148.9 (s) 4’ 146.9 (s) 146.7 (s) 5 109.5 (d) 109.4 (d) 6 126.3 (d) 126.3 (d) 7 38.2 (t) 36.2 (t) 8 40.1 (d) 48.2 (d) 9 70.1 (t) 99.8 (d)
tally correlated. (+ )-Caloccdrin was reduced by sodium borohydride in the presence ofsodium hydroxide [7]. The product (66% yield) exhibited compatible physical and spectroscopic properties (mp, mmp, HPLC, UV, IR and ‘H NMR) with those of (-thibalactone, except optical activity. An intermediate aldehyde 3, obtained from hemiacetal opening, was presumed to undergo epimer- ization prior to reduction under the alkaline conditions.
EXPERIMENTAL
Plant material. Calocedrus formosana (Florin) Florin was collected in the campus of the National Taiwan University. The skinned and aIrdrIed wood (600 g) from branches 6-8 cm m diam. was selected for study After extraction x 3 with MezCO, the combined extracts were coned in uucuo to give 20 g of residue. Components were separated by CC on sihca gel (230g) and elution with hexane-EtOAc gradients.
(+)-Calocedrin. Crystals (105 mg) R, 0.16 (hexane-Me&O, 7:3). Recrystallization samples from EtOH exhrb&d mp 187-188”; [a]: + 6” (c 0.9; MezCO). UV n=H nm (s): 237 (10400), 294(9560), 330(11600). IR v=cm-‘: 356O(OH), 1745 (GO), 1640 (C=C), 1600 (aromatic). MS m/z (rel. int.): 368 (15) [Ml’, 350 (9), 316 (lo), 135 (100).
Reduction of (+)-calocedrin. NaOH (21 mg, 0.53 mmol) was added to a soln of (+)calocedrin (49 mg, 0.13 mmol) m MeOH. After stirring for 10 mm, NaBHI (6.5 mg, 0.17 mmol) was added and the mixture refluxed (80”) for 1 hr under N,. The mixture was cooled, acidtied (pH 2) with HCl and extracted wth CHCl,. The combined extracts were dried over NazSO,, filtered, coned and puritied by TLC (R, 0.30, hexane-Me&O, 7: 3) to afford a 66 % yield of (f)-hlbalactone (30 mg, 0.085 mmol); mp 141.5-143” (authentic (-)-iubalactone, 142-143”, ht. [5] 147”), mmp 139-142”. The synthetic and authentic samples had the same R, on HPLC (pPorasilcolumn). UV 1zH nm (E): 237 (12 lOO), 294 (10800), 332 (14140). IR v=cm-‘: 1740, 1640, 1601. MS m/z (rel. mt.): 352 (12) [Ml’, 217 (18), 135 (100).
Acknowledgement-The authors thank the National Science Councd (ROC) for financial support.
REFERENCES
1. Flora ojTaiwan (1975) Vol. 1, p. 538. Epoch, Taiwan. 2. Cheng, Y. S. and Lin, K. C. (1970) Chemrstry (Chmese) 28. 3. Cheng, Y. S. and Lin, K. C. (1971) Chemistry (Chinese) 94. 4. Schrecker, A. W and Hartwell, J. L. (1954) J. Am. Chem. Sot.
76, 4896.
5. Batterbee, J. E., Burden, R. S., Crombie, L. and Whiting, D.A. (1969) J. Chem. Sot. C 2470.
6. Moss, M. O., Robinson, F. V. and Wood, A. B. (1971)5. Gem. Sot. C 619.
7 Ferland, J M., Lefebvre, Y., Deghenghi, R. and Wiesner, K. (1966) Tetrahedron Letters 3617