Sexual Size and Shape Dimorphism in an Agamid Lizard, Japalura
swinhonis (Squamata: Lacertilia: Agamidae)
Chi-Yun Kuo1,3,*, Yu-Teh Lin1,2, and Yao-Sung Lin1,21Institute of Ecology and Evolutionary Biology, National Taiwan University, Taipei 106, Taiwan 2Department of Life Science, National Taiwan University, Taipei 106, Taiwan
3Biodiversity Research Center, Academia Sinica, Nankang, Taipei 115, Taiwan (Accepted August 7, 2008)
Chi-Yun Kuo, Yu-Teh Lin, and Yao-Sung Lin (2009) Sexual size and shape dimorphism in an agamid lizard,
Japalura swinhonis (Squamata: Lacertilia: Agamidae). Zoological Studies 48(3): 351-361. Sexual dimorphism
in size and shape is a widespread phenomenon in the animal kingdom. Sexual dimorphism in morphology can be explained in proximate (growth pattern/sampling effects) and ultimate (evolutionary payoffs) contexts. There are 3 mutually non-exclusive hypotheses for the evolution of sexual dimorphism: fecundity advantage, intersexual resource partitioning, and sexual selection, each of which can make specific predictions regarding a lizard’s morphology. In this study, we describe sexual dimorphism in size and shape in an agamid lizard,
Japalura swinhonis, with discussions from both proximate and ultimate perspectives. The results showed that
all body parts of males were larger than those of females. After the effect of body size was accounted for, males had proportionately longer and wider heads, and shorter limbs and body length. Sexual shape dimorphism can be proximately explained by different growth patterns between the 2 sexes. We found a correlation between morphology and perch habitat, but not between morphology and diet since the 2 sexes exhibited extensive dietary overlap. Our results rejected the resource partitioning hypothesis and provided support for the fecundity advantage hypothesis as the underlying mechanisms of sexual dimorphism in J. swinhonis.
http://zoolstud.sinica.edu.tw/Journals/48.3/351.pdf
Key words: Allometry, Intersexual resource partitioning, Life history adaptation, Morphometrics, Sexual
selection.
* To whom correspondence and reprint requests should be addressed. Tel: 886-2-27899525. E-mail:chiyunkuo@ntu.edu.tw
S
exual dimorphism is a widespreadphenomenon in the animal kingdom (Andersson 1994). Morphological differences between the 2 sexes; however, have 2 aspects: size and shape. Sexual size dimorphism (SSD) describes the situation in which the 2 sexes differ in measured values of certain morphological traits. SSD has been extensively described in reptiles (Andersson 1994). Early studies of SSD in reptiles focused on overall body size and used single traits (body weight or snout-to-vent-length in most cases) to stand for overall body size (Stamps 1993), with less emphasis on SSD of separate body parts that also have ecological relevance. Shape
dimorphism, on the other hand, can have diverse meanings depending on how “shape” is defined (Bookstein 1989). In a majority of the literature, the shape of a body part is defined as the trait value after the effect of body size is removed, expressed in the form of a proportion to or regression residual of overall body size. Shape dimorphism was not extensively studied until recently (e.g., Malhotra and Thorpe 1997, Butler and Losos 2002, Irschick et al. 2005, Schwarzkopf 2005), although there is no reason to believe that it is any less important than size dimorphism (Butler and Losos 2002).
Sexual dimorphism can be explained by both proximate (growth patterns) and ultimate
(evolutionary payoffs) causes, and both have been extensively studied, particularly the latter (see reviews in Cox et al. 2003 and Stamps 1993, respectively). Much insight can be gained by the integrated study of both the proximate and ultimate perspectives of sexual dimorphism (Watkins 1996, Duvall and Beaupre 1998, Butler and Losos 2002). The approaches used to identify the proximate causes of sexual size and shape dimorphism, however, differ. For size dimorphism, the proximate cause is the factor that produces intersexual differences in the growth rate, such as differences in growth hormone concentrations or trade-offs in allocating energy between growth and reproduction (John-Alder et al. 2007). For shape dimorphism, the proximate cause is the intersexual difference in growth trajectories, analyzed separately for each body part, that leads to shape differences (Butler and Losos 2002). In this study, we focused on the proximate cause of sexual shape dimorphism.
From the perspective of ultimate causes, at least 3 non-mutually exclusive hypotheses have been proposed for the evolution of sexual dimorphism (Katsikaros and Shine 1997). First of all, a larger abdominal volume is selected in females because it can enhance females’ fecundity through a better capacity to accommodate volumin- ous litters or eggs. According to this hypothesis, abdominal volume in females is the target of directional selection. An increase in abdominal volume can be achieved either by an increase in overall body size, as observed in some mammalian and amphibian species (Monnet and Cherry 2002, Tague 2005) or by an increase in its relative proportion to the overall body size, as observed in some reptile species (Schwarzkopf 2005, Thompson and Withers 2005). Second, sexual dimorphism may have evolved as a result of resource partitioning to avoid intersexual competition, where ecological differentiation is a consequence of disruptive selection on traits involved in resource utilization. In this hypothesis, differentiation in the morphology of body parts involved in resource utilization should occur, and intersexual resource partitioning should also be observed. Third, sexual dimorphism may have arisen as the outcome of sexual selection via competition for mates or mate choice. This hypothesis predicts that males evolve morphological features that enhance mating success, whether through improved fighting ability against other males or better chances of attracting females (Cooper and Vitt 1993, Censky 1997).
However, it can be difficult to differentiate between the latter 2 hypotheses since some body parts are involved in both foraging and mating. As a consequence, resource partitioning may occur as a secondary phenomenon after morphological differentiation caused by sexual selection. To distinguish between these 2 hypotheses, resource utilization of the 2 sexes has to be included in the analysis if a sexually dimorphic body part is involved in both mating and utilizing a resource. When intersexual partitioning of that resource is not observed, we can then conclude that sexual selection is the primary mechanism for sexual dimorphism.
Japalura swinhonis (Günther 1864) is an
endemic species as well as the largest Japalura species in Taiwan. They can reach 31 cm in total length (Shan 2001). The species is found in locations with sufficient sunlight below 1500 m in elevation throughout the entire island, ranging from wooded areas to habitats with intense human activities, such as school campuses. Japalura
swinhonis is arboreal. Individuals often perch
on tree trunks and sometimes on the ground or branches. Individuals are sexually dimorphic. Males have a bright yellow stripe running along both sides of the body. Females have a brownish body coloration. Some females have a wide, deep-brown stripe running anteriorly-posteriorly along the dorsum. The aims of this study were 2 fold: we first describe sexual dimorphism in size and shape for body parts involved in resource use and/or mating behaviors of J. swinhonis. We then provide integrated analyses of the proximate and ultimate causes of sexual dimorphism in this lizard.
MATERIALS AND METHODS Study site
The study site was a deserted hiking trail in a wooded area inside the Taipei City Zoo (24°24'N, 121°21'E). The canopy was fairly open, and much light reached the understory, producing a combination of light and shade at the study site. The understory was structurally complex, with various grass and shrub species.
Data collection
We walked at a speed of 0.3 m/s along the trail once in the morning at 08:00-12:00 and again in the afternoon, at 14:00-17:00 during Mar.-June
2005. Lizards were caught either by hand or a noose. The following morphological variables were measured using an electronic caliper: snout-to-vent-length (SVL), left forelimb length (FLIMB, measured as the length from where the forelimb connects to the body to the wrist joint), left hindlimb length (HLIMB, measured as the length from where the hindlimb connects to the body to the ankle joint), length of the longest finger and toe of the left limbs (FIN and TOE), head width (HW, measured as the widest distance between the 2 sides), head length (HL, measured as the length from the tip of the snout to posterior of the HW measurement site), and tail length (TAIL, measured as the length from posterior of the cloaca to the tip of the tail). The value for body length (BL) was obtained as the difference between SVL and HL. All morphological variables were measured to the nearest 0.01 mm, except TAIL, which was measured to the nearest 1 cm. Two habitat variables, initial perch height and perch diameter, were measured as well. Lizards were given a unique number on the back using water-insoluble paint as a temporary mark and toe-clipped in a unique combination as a permanent mark. A captured lizard was then returned to the point of capture and released.
Morphometric analyses
All morphological variables were natural log (ln)-transformed prior to the statistical analyses. Sexual dimorphism was described using principal component analyses (PCA) and unpaired t-tests. To separate shape from size, we used Mosimann’s (1970) method as described in Butler and Losos (2002) to produce shape variables for each individual: the size variable (SIZE) is defined as the geometric mean of 8 measured morphological variables (Table 1). Shape variables were then produced as the ratio between a morphological variable and SIZE.
To investigate the allometric patterns of the 2 sexes, we applied the following allometric equation (Lindeman 2000):
Y = β0SIZEβ1
where Y is a morphological variable.
The equation can be converted to a linear form by applying the natural logarithm to both
sides. β0 and β1 respectively became the
y-intercept and slope of the regression function. A slope of > 1, < 1, and = 1 respectively denote positive allometry, negative allometry, and isometry. The regression slope of each morphological
variable against SIZE was compared between the 2 sexes to examine intersexual differences in allometric patterns.
Diet analysis
Stomach contents were obtained in July-Oct. 2005 by stomach-flushing. Immediately upon capture, 5-10 ml of saline was injected into the mouth using a 5 ml modified syringe. Each captured lizard was flushed no more than once in 3 d to minimize the influence of stomach-flushing on behavior. The stomach contents were preserved in 70% alcohol. Insect items were identified to order, and other food items to class with the aid of reference collections of arthropods from the study area (Znari and Mouden 1997). The proportions of stomachs containing a given prey taxon were calculated (occurrence frequency) as the number of stomachs containing that particular prey taxon divided by the total number of stomachs surveyed. The wet weight of every prey taxon was measured to the nearest 0.001 g. Prey taxa that weighed < 0.001 g were considered unimportant in that stomach. To prevent bias caused by the 0.001 g limit imposed by our scale, stomachs containing prey taxa that were considered unimportant were excluded from further analysis if the total weight of the stomach contents in that stomach was < 0.02 g. This procedure guaranteed that even if in 1 stomach a prey taxon in reality weighed as much as 0.001 g and was considered unimportant, it would account for no more than 5% of the total weight. The percent weight of every prey taxon was then obtained on a stomach-by-stomach basis. The index of relative importance (IRI) was calculated for those prey taxa with occurrence frequencies exceeding 1/20, using the method described by Bjorndal et al. (1997) with percent volume replaced by percent weight:
IRI = 100(FiWi)
Σn
1(FiWi)
where F denotes the occurrence frequency, W the percent weight, and n the number of prey taxa.
For the diet analysis, we first compared the mean number of prey taxa found per stomach to see if individuals of 1 sex consumed more prey taxa than did the other sex. We then compared the occurrence frequency of every prey taxon to examine dietary similarities in taxon composition. Last, the mean percent weight of every prey taxon
was compared between males and females to quantify the importance of that given prey taxon relative to the others. Only those prey taxa with occurrence frequencies exceeding 1/20 with an IRI of > 10 were included in the latter 2 parts of the analysis. Also, stomachs with unidentified prey items weighing more than 5% of the total content weight were excluded from the 3rd part of the analysis.
Feeding niche overlap
We applied 2 indices to measure the extent of feeding niche overlap. The 1st index measures the overlap in resource utilization functions, adapted from Goodall (1973):
Cxy = 1 - 12
(
Σn1
|
pxi - pyi|
)
, where pxi = xi/X, pyi = yi/Y;where n is the number of prey taxa utilized by both
sexes, xi and yi are the respective numbers of male and female individuals utilizing prey taxa i, and X and Y are the respective summations of individuals utilizing prey taxa 1 to prey taxa n by males and females. The Cxy value ranges from 0 (mutually exclusive resource utilization) to 1 (completely overlapping resource utilization).
The 2nd index is the product-moment correlation coefficient (Hurlbert 1978):
Cp = Σ n 1
(
xi - X n)(
yi - Yn)
Σn 1(
xi - X n)
2 Σn 1(
yi - Y n)
2 1/2where symbols are the same as those defined for the previous index. This index measures the correlation of resource use between the 2 sexes, under the assumption that resources are uniformly available to both sexes.
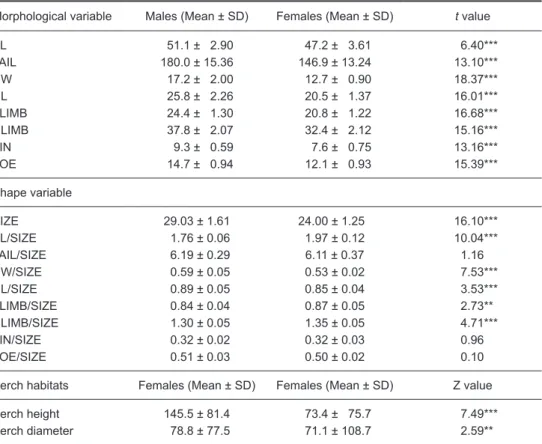
Table 1. Mean and standard deviation (SD) of size and shape variables. All
morphological variables, and perch diameter are in millimeters (mm). Perch heights are in centimeters (cm). Statistical tests applied on all morphological and ln-transformed shape variables were unpaired t-tests; those on perch height and diameter were Wilcoxon Z tests (2-tailed, α = 0.05)
Morphological variable Males (Mean ± SD) Females (Mean ± SD) t value
BL 051.1 ± 02.90 047.2 ± 03.61 6.40*** TAIL 180.0 ± 15.36 146.9 ± 13.24 13.10*** HW 017.2 ± 02.00 012.7 ± 00.90 18.37*** HL 025.8± 02.26 020.5 ± 01.37 16.01*** FLIMB 024.4± 01.30 020.8 ± 01.22 16.68*** HLIMB 037.8 ± 02.07 032.4 ± 02.12 15.16*** FIN 009.3 ± 00.59 007.6 ± 00.75 13.16*** TOE 014.7± 00.94 012.1 ± 00.93 15.39*** Shape variable SIZE 29.03 ± 1.61 24.00 ± 1.250 16.10*** BL/SIZE 01.76 ± 0.06 01.97 ± 0.120 10.04*** TAIL/SIZE 06.19 ± 0.29 06.11 ± 0.370 1.16 HW/SIZE 00.59 ± 0.05 00.53 ± 0.020 7.53*** HL/SIZE 00.89 ± 0.05 00.85 ± 0.040 3.53*** FLIMB/SIZE 00.84 ± 0.04 00.87 ± 0.050 2.73** HLIMB/SIZE 01.30 ± 0.05 01.35 ± 0.050 4.71*** FIN/SIZE 00.32 ± 0.02 00.32 ± 0.030 0.96 TOE/SIZE 00.51 ± 0.03 00.50 ± 0.020 0.10 Perch habitats Females (Mean ± SD) Females (Mean ± SD) Z value Perch height 145.5 ± 81.4 073.4 ± 075.7 7.49*** Perch diameter 078.8 ± 77.5 71.1 ± 108.7 2.59**
RESULTS Sexual dimorphism in size
Morphological measurement of 47 males and 40 females were included in the analyses. Males had significantly greater values of all size variables and therefore were also greater in SIZE (unpaired
t-tests, Table 1). The 1st principal component
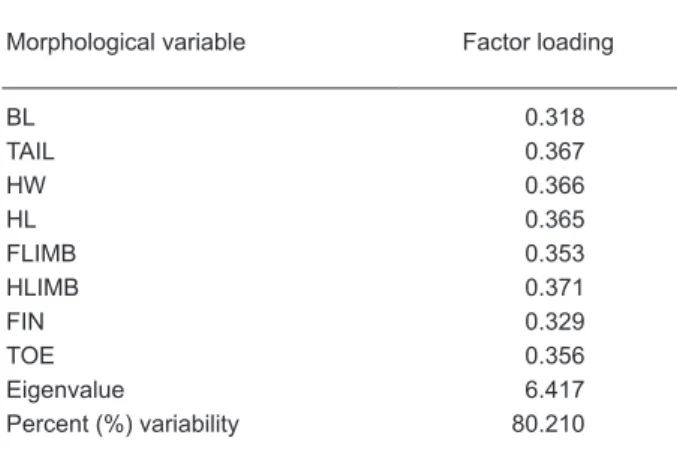
based on ln-transformed size variables positively and equally loaded for all morphological variables and accounted for 80.21% of the variation (Table 2). Consistent with the univariate approach, the PC1 scores of males and females significantly differed (unpaired t-test, t = 18.84, p < 0.0001).
Japalura swinhonis exhibited size dimorphism in
all measured body parts.
Sexual dimorphism in shape
Mean values of the 5 shape variables of males and females markedly differed, with males having greater values for HW/SIZE and HL/SIZE, and females having greater values for BL/SIZE, FLIMB/SIZE, and HLIMB/SIZE (unpaired t-tests, Table 1). Values of TAIL/SIZE, FIN/SIZE, and TOE/SIZE did not significantly differ between males and females. Results of the multivariate analyses were again consistent with those of the univariate analyses. The 1st 3 principal components accounted for 69.27% of the total variation (Table 3). The 1st principal component accounted for 36.27% of the variation and strongly
and positively loaded for ln(FLIMB/SIZE) and ln(HLIMB/SIZE), and negatively for ln(HW/SIZE) and ln(HL/SIZE). The 2nd principal component accounted for another 20.23% of the variation and strongly and positively loaded for ln(BL/SIZE) and negatively for ln(FIN/SIZE) and ln(TOE/SIZE). The 3rd principal component accounted for another 12.78% of the total variation and strongly and positively loaded for ln(TAIL/SIZE). PC1 and PC2 scores of males and females significantly differed, but PC3 scores did not (unpaired t-test. PC1,
t = 6.61, p < 0.0001; PC2, t = 4.69, p < 0.0001;
PC3, t = 0.13, p = 0.89). Sexual dimorphism in shape was evident: males had more-prominent heads and females had longer bodies and limbs.
Allometric patterns
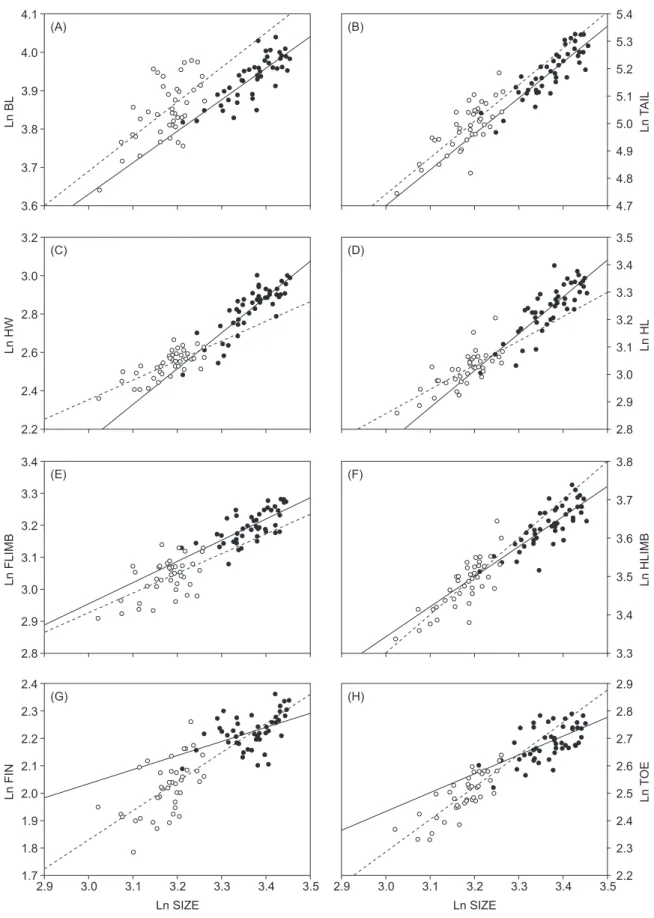
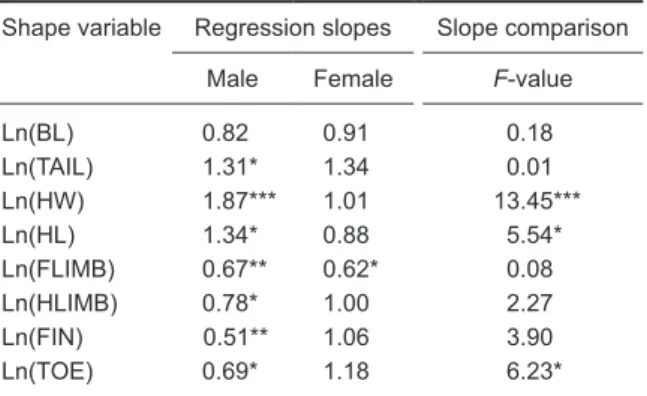
By examining the regression slopes, we found that males exhibited allometry in all morphological variables except BL, which exhibited isometry (Fig. 1, Table 4). Among the allometric body parts, TAIL, HW, and HL were positively allometric and FLIMB, HLIMB, FIN, and TOE were negatively allometric. In females, only FLIMB exhibited negative allometry. All other morphological variables were isometric. Comparisons of regression slopes between the 2 sexes revealed that regression slopes of HW, HL, and TOE significantly differed between males and females, with males being more positively allometric in HW and HL but more negatively allometric in TOE than females. The regression slopes for FLIMB, HLIMB, FIN, TAIL,
Table 2. Factor loadings of the 1st principal
component on size variables
Morphological variable Factor loading
BL 0.318 TAIL 0.367 HW 0.366 HL 0.365 FLIMB 0.353 HLIMB 0.371 FIN 0.329 TOE 0.356 Eigenvalue 6.417 Percent (%) variability 80.210 BL: body length; TAIL: tail length; HW: head width; HL: head length; FLIMB: forelimb length; HLIMB: hindlimb length; FIN: length of the longest finger; TOE: length of the longest toe. See text for more detailed definitions.
Table 3. Factor loadings of the 1st 3 principal
component (PC) axes on shape variables
Shape variable PC1 PC2 PC3 Ln(BL/SIZE) 0.314 0.394 -0.048 Ln(TAIL/SIZE) -0.251 0.313 0.663 Ln(HW/SIZE) -0.499 -0.144 -0.238 Ln(HL/SIZE) -0.494 0.108 -0.143 Ln(FLIMB/SIZE) 0.303 0.273 -0.419 Ln(HLIMB/SIZE) 0.359 0.309 0.089 Ln(FIN/SIZE) 0.244 -0.589 -0.184 Ln(TOE/SIZE) 0.256 -0.444 0.514 Eigenvalue 2.90 1.62 1.02 Percent (%) variability 36.27 20.23 12.78 Cumulative percent (%) 36.27 56.49 69.27 Symbols are the same as defined as in table 2.
Fig. 1. Allometric scaling patterns of 8 ln-transformed size variables against Ln SIZE. Open circles and dashed lines are females; filled
circles and solid lines are males.
Ln FLIMB 3.2 3.0 2.9 2.8 3.1 3.4 3.3 (F) Ln HLIMB 3.8 3.6 3.5 3.4 3.3 3.7 Ln FIN 2.4 2.2 2.0 1.9 1.8 1.7 2.9 3.0 3.1 3.2 3.3 3.4 3.5 (G) 2.3 2.1 Ln SIZE Ln TOE 2.9 2.7 2.5 2.4 2.3 2.2 2.9 3.0 3.1 3.2 3.3 3.4 3.5 (H) 2.8 2.6 Ln SIZE Ln HW 3.2 2.8 2.4 2.2 (C) 3.0 2.6 Ln HL 3.5 3.3 3.1 3.0 2.9 2.8 (D) 3.4 3.2 (E) Ln BL 4.1 3.9 3.7 3.6 (A) 4.0 3.8 Ln TAIL 5.4 5.2 5.0 4.9 4.8 4.7 (B) 5.3 5.1
and BL did not differ between the sexes. We also detected a significant intersexual difference in the y-intercept of the BL regression lines (two-tailed test: t0.05, 84 = 8.04, p < 0.001).
Perch habitats
Males perched significantly higher and on wider surfaces than did females (Table 1). Although both sexes perched primarily on tree trunks, females were more frequently found on the ground than males (7 of 72 sightings vs. none
of 184 sightings, Fisher’s exact test: X2 = 16.75,
d.f. = 1, p = 0.0002). We concluded that males
and females markedly differed in perch habitats.
Diet analysis
Female lizards ingested more types of prey than did males (Table 5). The mean number of prey taxa per stomach was significantly greater in females (mean, 2.77 for males; mean, 3.77 for females; unpaired t-value = 2.06, p = 0.044). This observation might reflect differences in prey composition in different habitats. Females, which perched near the ground, might encounter more-diverse prey than males. Despite the presence of more prey taxa per female stomach, most prey taxa were shared between the 2 sexes (Table 5). The main exception was termites, which were only found in male stomachs. Ants and Lepidopteran larvae were the only 2 prey taxa with considerable
Table 4. Results of the regression analysis of
ln-transformed shape variables, with Ln SIZE as the independent variable. Regression slopes were tested for significance under the null hypothesis of β0 = 1. Regression slopes were compared between the 2 sexes
Shape variable Regression slopes Slope comparison Male Female F-value
Ln(BL) 0.82 0.91 0.18 Ln(TAIL) 1.31* 1.34 0.01 Ln(HW) 1.87*** 1.01 13.45*** Ln(HL) 1.34* 0.88 5.54* Ln(FLIMB) 0.67** 0.62* 0.08 Ln(HLIMB) 0.78* 1.00 2.27 Ln(FIN) 0.51** 1.06 3.90 Ln(TOE) 0.69* 1.18 6.23*
*p < 0.05, ** p < 0.01, *** p < 0.001. Symbols are the same as
defined in table 2.
Table 5. Occurrence frequency, mean percent weight, and index of relative
importance (IRI) of food taxa found in stomachs of male (M) and female (F)
Japalura swinhonis. Sample sizes are in parentheses. The sample sizes of
percent weight were smaller than the sample sizes of occurrence frequency (see main text for details)
Prey taxa Occurrence frequency Mean percent weight IRI M (n = 29) F (n = 27) M (n = 22) F (n = 22) M F Class Arachnida 10 15 3.32 3.13 2.26 3.49 Class Diplopoda 3 2 0 4.02 0 0.60 Class Chilopoda 2 3 1.60 1.51 0.22 0.34 Class Crustacea Isopoda 2 5 0.80 1.35 0.11 0.50 Class Gastropoda 3 3 1.35 8.06 0.28 1.80 Class Insecta Coleoptera 6 10 5.91 7.15 2.42 5.32 Hemiptera 5 7 6.14 5.75 2.09 3.00 Homoptera 5 4 11.20 5.62 3.81 1.67 Hymenoptera Formicidae 24 25 29.22 22.45 47.76 41.77 Isoptera 2 0 1.89 - 0.26 Lepidoptera larvae 15 15 32.33 38.55 33.03 43.03 Orthoptera 4 6 3.18 0.54 0.87 0.24 Psocoptera 3 2 0 0 0 0
importance in both male and female stomachs (IRI > 10, Table 5). The importance values of other prey taxa were similar between the 2 sexes. High values of both Cxy (0.87) and Cp (0.95) indicated extensive overlap in resource utilization between the 2 sexes. The possibility that the mean prey size between the 2 sexes differs was refuted in a previous study (Kuo et al. 2007). Therefore, we concluded that male and female J. swinhonis do not exhibit trophic resource partitioning.
DISCUSSION
Our study showed that J. swinhonis exhibits SSD in general body size and several body parts. We are aware of the possibility that SSD can be observed in a sample even if the phenomenon itself does not exist in natural populations (Stamps 1993). It could have simply been a consequence of different age distributions between the sexes in our sample or have arisen because of a sampling bias introduced by behavioral factors of individuals. However, male and female J. swinhonis, were previously shown to differ in asymptotic body size (Lin and Lu 1982). SSD that we observed in this study is therefore unlikely an artefact.
It has also been proposed that SSD is more likely to arise in species occupying open habitats, such as Japalura lizards, than species occupying closed habitats for 2 reasons. The 1st reason is equivalent to the sexual selection hypothesis. In open habitats, individuals are more likely to see each other, and, consequently, social displays or territorial disputes will be more frequent. Therefore, a larger size of males will be advantageous in mate acquisition (see Butler et al. 2000 and references therein). The 2nd reason is equivalent to the resource partitioning hypothesis, i.e., open habitats provide better chances to spot prey from a distance, and sit-and-wait foraging is more likely to be effective in such environments. Since optimal feeding models (Schoener 1969) predict bimodal optimal body sizes in sit-and-wait predators, the 2 size modes could respectively occur in males and females. The 1st, but not the 2nd, selective pressure is plausible for J.
swinhonis, which occupies open habitats and
displays on locations 1-2 m above the ground (Lin and Lu 1982, Kuo pers. observ.), but the diets of the 2 sexes did not differ.
From a proximate perspective, shape dimorphism of a given body part can arise through different allometric growth patterns between the 2
sexes under 2 conditions (Butler and Losos 2002). First, sexual shape dimorphism arises because the 2 sexes differ in the degree of allometry, i.e., their regression slopes differ. Alternatively, sexual shape dimorphism can result from the 2 sexes growing to different asymptotic sizes, when allometric growth is present in both sexes. For example, in the case where a body part exhibits positive allometry in both sexes, the relative length of that body part increases with body size, and relative length of that body part in the sex with a smaller asymptotic size will also be smaller. On the contrary, when a body part exhibits negative allometry in both sexes, the relative length of that body part will decrease as body size increases, and the relative length of that body part in the sex with a smaller asymptotic size will be longer. In J. swinhonis, shape dimorphism in HW, HL, and TOE fit the scenario of the 1st mechanism. Shape dimorphism in FIN seemed to be accounted for by the 1st mechanism as well, although the comparison of regression slopes yielded insignificant results. Shape dimorphism in FLIMB and HLIMB, on the other hand, conformed to the 2nd mechanism. Sexual dimorphism in BL, however, did not fit either mechanism. Both sexes exhibited isometry in BL, and smaller-sized females had greater relative body lengths. We propose a 3rd mechanism to explain such a pattern. Recall we observed that the y-intercept of the female regression line was significantly greater than that for males. Thus, it could be that the 2 sexes differed in the initial relative BL, and the initial difference was maintained by isometric growth in both sexes. Consequently, the relative length of BL remained constant as body size increased.
The 2 most universal patterns of shape dimorphism found in many lizard species were also found in J. swinhonis: males had relatively larger heads, and females relatively longer interlimb lengths. Males possessing relatively larger heads has been reported in major lizard clades. (Agamidae: Thompson and Withers 2005; Gekkonidae: Johnson et al. 2005; Iguanidae: Butler and Losos 2002; Lacertidae: Hofmann and Henle 2006; Scincidae: Schwarzkopf 2005). This phenomenon is generally considered the consequence of sexual selection to enhance success in copulation or male-male rivalry (Andersson 1994, Bull and Pamula 1996; Herrel et al. 1996, Olsson et al. 2002), although several studies also demonstrated the association between head size and diet (Preest 1994, Verwaijen et al. 2002), suggesting that intraspecific resource
partitioning may be an alternative driving force. In J. swinhonis, jaws are involved both in male-male agonistic behaviors and in the process of copulation (Wei and Lin 1981). Individuals with larger jaws also have a greater biting force (Kuo, pers. observ.). Moreover, intersexual diet differentiation was not observed in our study. We are aware that our diet data were collected only during the breeding season. However, as the population size rapidly declined by about 90% after Oct. (Kuo et al., pers. observ.), intersexual dietary differentiation, even if it existed, would contribute little to mitigating dietary competition. Therefore, our results suggest that the intersexual resource partitioning hypothesis is unlikely to be the underlying mechanism for the evolution or maintenance of sexual dimorphism in head morphology.
Female lizards in numerous species have longer interlimb lengths in spite of smaller overall body sizes (Agamidae: Thompson and Withers 2005; Gekkonidae: Shine 1992; Iguanidae: Cooper and Vitt 1989, Shine 1992; Scincidae: Schwarzkopf, 2005; Teiidae: Vitt 1983, Shine 1992). This phenomenon has consistently been interpreted as an adaptation to provide room for eggs in species with variable clutch sizes (Vitt and Congdon 1978, Cox et al. 2003), although the trait was directly proven to be a target of selection in only a small number of species (e.g., Olsson et al. 2002). Females of J. swinhonis have variable clutch sizes (Lin 1979), and our results provide support for the fecundity advantage hypothesis.
Sexual dimorphism in limb shape has been less examined in lizards. Among species that exhibit sexual dimorphism in limb shape, males always have relatively longer forelimbs and hindlimbs (Malhotra and Thorpe 1997, Butler and Losos 2002, Irschick et al. 2005, Schwarzkopf 2005). To our knowledge, this is the first report of females having relatively longer limbs than males. Limb shape is associated with habitat use, escape behavior, or both (Losos 1990, Schulte et al. 2004, Irschick et al. 2005). It has been proposed that individuals with relatively longer limbs should utilize wider perch surfaces, since longer limbs confer better sprinting ability on wide surfaces (Losos 1990, Irschick and Losos 1998, Beuttel and Losos 1999, but see Goodman et al. 2007 and references therein). In J. swinhonis, however, the pattern of sexual dimorphism in limb shape cannot be explained by such a limb shape-habitat relationship because females had relatively longer limbs but utilized narrower perch surfaces. We
suspect that differences in limb shape might be associated with differences in escape behaviors. Males, which are conspicuous and perch higher on tree trunks, more often jumped from tree trunks to the ground to escape predators. On the other hand, females may escape by running on the ground, given their lower perch height and cryptic coloration.
Gould (1975) proposed an alternative and non-adaptive explanation for sexual shape dimorphism. Differences in shape can arise as an indirect consequence of selection on body size if the body parts under examination exhibit allometric growth. Thus, it would be difficult, if not impossible, to rule out this non-adaptive possibility when shape dimorphism is observed in sexually size-dimorphic species. A recent study on water skinks,
Eulamprus quoyii, detected shape dimorphism in
the absence of size dimorphism, providing some support for the argument that shape dimorphism itself can be the direct target of selection, rather than merely being a by-product of different body sizes between the 2 sexes (Schwarzkopf 2005).
We concluded that J. swinhonis is dimorphic in both size and shape, which can be explained by different growth patterns between the 2 sexes. We found a correlation between morphology and perch habitat, but not between morphology and diet. Our results lend support to the hypothesis of life history adaptation and sexual selection. Although differentiation of habitat use was observed, we suggest that it was not the direct consequence of intersexual resource partitioning, but a secondary consequence of sexual selection.
Acknowledgments: We express our sincere
thanks to the following people for their assistance in the field: M.H. Chen, R.J. Chen, J. Kao, Y.T. Jeng, and Y.J. Kuo. We also acknowledge K.Y. Lue for providing valuable comments on experimental design. Special thanks go to L.L. Lee and 2 anonymous reviewers for their critical comments and B. Stein for her insights into manu-script writing. We also thank H.C. Lin of Taipei City Zoo for permission to conduct our study on the zoo grounds.
REFERENCES
Andersson M. 1994. Sexual selection. Princeton, NJ : Prince-ton Univ. Press.
Beuttel K, JB Losos. 1999. Ecological morphology of Caribbean anoles. Herpetol. Monogr. 13: 1-28.
Bjorndal KA, AB Bolten, CJ Lagueux, DR Jackson. 1997. Dietary overlap in three sympatric congeneric freshwater turtles (Pseudemys) in Florida. Chelon. Conserv. Biol. 2:
430-433.
Bookstein FL. 1989. Size and shape: a comment on semantics. Syst. Zool. 38: 173-180.
Bull CM, Y Pamula. 1996. Sexually dimorphic head sizes and reproductive success in the sleepy lizard Tiliqua rugosa. J. Zool. 240: 511-521.
Butler MA, JB Losos. 2002. Multivariate sexual dimorphism, sexual selection, and adaptation in Greater Antillean
Anolis lizards. Ecol. Monogr. 72: 541-559.
Butler MA, TW Schoener, JB Losos. 2000. The relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis lizards. Evolution 54: 259-272.
Censky EJ. 1997. Female mate choice in the non-territorial lizard Ameiva plei (Teiidae). Behav. Ecol. Sociobiol. 40:
221-225.
Cooper WEJ, LJ Vitt. 1989. Sexual dimorphism of head and body size in an iguanid lizard: paradoxical results. Am. Nat. 133: 729-735.
Cooper WEJ, LJ Vitt. 1993. Female mate choice of large male broad-headed skinks. Anim. Behav. 45: 683-693.
Cox RM, SL Skelly, HB John-Alder. 2003. A comparative study of adaptive hypotheses for sexual size dimorphism in lizards. Evolution 57: 1653-1669.
Duvall D, SJ Beaupre. 1998. Sexual strategy and size dimorphism in rattlesnakes: integrating proximate and ultimate causation. Am. Zool. 38: 152-165.
Goodall DW. 1973. Sample similarity and species correlation.
In RH Whittaker, ed. Ordination and classification of
communities. The Hague: Junk, pp. 106-156.
Goodman BA, AK Krockenberger, L Schwarzkopf. 2007. Master of them all: performance specialization does not result in trade-offs in tropical lizards. Evol. Ecol. Res. 9:
527-546.
Gould SJ. 1975. Allometry in primates, with an emphasis on scaling and evolution of the brain. Contrib. Primatol. 5:
244-292.
Günther A. 1864. The reptiles of British India. London: Ray Society.
Herrel A, R Van Damme, F De Vree. 1996. Testing the niche divergence hypothesis by bite force analysis. Neth. J. Zool. 46: 253-262.
Hofmann S, K Henle. 2006. Male reproductive success and intrasexual selection in the common lizard determined by DNA-microsatellite. J. Herpetol. 40: 1-6.
Hurlbert SH. 1978. The measurement of niche overlap and some relatives. Ecology 59: 67-77.
Irschick DJ, JB Losos. 1998. A comparative analysis of the ecological significance of maximal locomotor performance in Caribbean Anolis lizards. Evolution 52: 219-226.
Irschick DJ, B Vanhooydonck, A Herrel, J Meyers. 2005. Intraspecific correlations among morphology, performance and habitat use within a green anole lizard (Anolis
carolinensis) populations. Biol. J. Linn. Soc. 85: 211-221.
John-Alder HB, RM Cox, EN Taylor. 2007. Proximate developmental mediators of sexual dimorphism in size: case studies from squamate reptiles. Integr. Comp. Biol.
47: 258-271.
Johnson JB, LD McBrayer, D Saenz. 2005. Allometry, sexual size dimorphism, and niche partitioning in the Mediterranean gecko (Hemidactylus turcicus). SW Nat.
50: 435-439.
Katsikaros K, R Shine. 1997. Sexual dimorphism in the tusked frog, Adelotus brevis (Anura: Myobatrachidae): the roles of natural and sexual selection. Biol. J. Linn. Soc. 60:
39-51.
Kuo CY, YS Lin, YK Lin. 2007. Resource use and morphology of two sympatric Japalura lizards (Iguania: Agamidae). J. Herpetol. 41: 713-723.
Lin JY. 1979. Ovarian, fat body and liver cycles in the lizards,
Japalura swinhonis formosensis (Lacertilia: Agamidae). J.
Asian Ecol. 1: 29-38.
Lin JY, KH Lu. 1982. Population ecology of the lizard Japalura
swinhonis formosensis (Sauria: Agamidae) in Taiwan.
Copeia 1982: 425-434.
Lindeman PV. 2000. Evolution of the relative width of the head and alveolar surfaces in map turtles (Testudines: Emydidae: Graptemys). Biol. J. Linn. Soc. 69: 549-576.
Losos JB. 1990. Ecomorphology, performance capability, and scaling of west Indian Anolis lizards: an evolutionary analysis. Ecol. Monogr. 60: 369-388.
Malhotra A, RS Thorpe. 1997. Size and shape variation in the Lesser Antillean anole, Anolis oculatus (Sauria: Iguanidae) in relation to habitat. Biol. J. Linn. Soc. 60: 53-72.
Monnet JM, MI Cherry. 2002. Sexual size dimorphism in anurans. Proc. R. Soc. Lond. Ser. B Biol. Sci. 269:
2301-2307.
Mosimann J. 1970. Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. J. Am. Statistical Assoc. 65:
930-945.
Olsson M, R Shine, E Wapstra, B Ujvari, T Madsen. 2002. Sexual dimorphism in lizard body shape: the role of sexual selection and fecundity selection. Evolution 56:
1538-1542.
Preest MR. 1994. Sexual size dimorphism and feeding energetics in Anolis carolinensis: Why do females take smaller prey than males? J. Herpetol. 28: 292-298.
Schoener TW. 1969. Models of optimal size for solitary predators. Am. Nat. 103: 277-313.
Schulte JA, JB Losos, FB Cruz, H Náñez. 2004. The relationship between morphology, escape behaviour and microhabitat occupation in the lizard clade Liolaemus (Iguanidae: Tropidurinae*: Liolaemini). J. Evol. Biol. 17:
408-420.
Schwarzkopf L. 2005. Sexual dimorphism in body shape without sexual dimorphism in body size in water skinks (Eulamprus quoyii). Herpetologica 61: 116-123.
Shan G. 2001. Natural portraits of lizards in Taiwan. Taipei, Taiwan: Big Tree Culture Enterprise. (in Chinese)
Shine R. 1992. Relative clutch mass and body shape in lizards and snakes: is reproductive investment constrained or optimized? Evolution 46: 828-833.
Stamps JA. 1993. Sexual size dimorphism in species with asymptotic growth after maturity. Biol. J. Linn. Soc. 50:
123-145.
Tague RG. 2005. Big-bodied males help us recognize that females have big pelvis. Am. J. Phys. Anthropol. 127:
392-405.
Thompson GG, PC Withers. 2005. Size-free shape differences between male and female Western Australian dragon lizards (Agamidae). Amphibia-Reptilia 26: 55-63.
Verwaijen D, R Van Damme, A Herrel. 2002. Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Funct. Ecol. 16:
Vitt LJ. 1983. Reproduction and sexual dimorphism in the tropical Teiid lizard Cnemiphorus ocellifer. Copeia 1983:
359-366.
Vitt LJ, JD Congdon. 1978. Body shape, reproductive effort, and relative clutch mass in lizards: resolution of a paradox. Am. Nat. 112: 595-608.
Watkins GG. 1996. Proximate causes of sexual size dimorphism in the Iguanian lizard Microlophus occipitalis.
Ecology 77: 1473-1482.
Wei SY, JY Lin. 1981. Behavioural study of Japalura swinhonis
formosensis (Sauria: Agamidae). Tunghai J. Biol. 22:
33-48. (in Chinese)
Znari M, EE Mouden. 1997. Seasonal changes in the diet of adult and juvenile Agama impalearis (Lacertilia: Agamidae) in the central Jbilet Mountains, Morocco. J. Arid Environ. 37: 403-412.