E L S E V I E R
l August 1997
Chemical Physics Letters 274 (1997) 37-40
CHEMICAL
PHYSICS
LETTERS
Reaction dynamics of Mg(3 ip , 4 1S 0) with
H2:
insertion versus
harpoon mechanism
Dean-Kuo Liu, King-Chuen Lin
*Department ~f Chemistry, National Taiwan University, and Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei 10764, Taiwan, Republic of China
Received 1 March 1997; in final form 12 May 1997
Abstract
We have obtained nascent rotational distributions of MgH (v = 0 and 1) in the reaction of Mg(4 tS 0) with H 2. The resultant bimodal features of the MgH distributions are similar to those obtained from Mg(3 ~ P~ ). The spectral analysis and potential energy surfaces calculation suggest that the Mg(4 ~S 0) atom proceeds via a harpoon-type reaction pathway, in contrast to a direct insertion followed by Mg(3 1P1). The Mg(41S 0) system is closely associated with the Mg(3 I P i ) - H 2
reaction coordinate, through evolution of a series of surface crossings along the ion-pair coordinate. © 1997 Elsevier Science B.V.
1. Introduction
The reaction dynamics of Mg(3s3p I P 1) with H 2 has been well studied more than a decade [1-7]. The produced nascent M g H exhibits a bimodal rotational distribution. The insertion mechanism is found to be the only channel that causes the bimodality, in the light of the measurements of isotope and temperature effects on the MgH distribution [2,3]. Potential en- ergy surfaces (PES) calculation also indicates that the reactive coordinate along the 1B 2 surface in C2v geometry is attractive, while the collinear approach suffers from a substantial energy barrier [6]. Our recent study of a two-dimensional PES calculation reveals that the reactive surface of the excited state
* Corresponding author. Fax: 886-2-3636359, e-mail: kclin @ hp9k720.iams.sinica.edu.tw.
crosses non-adiabatically to the ground state, on which the M g H 2 intermediate complex begins to break apart through two possible trajectories leading to the M g H ( 2 ~ + ) and H products [8,9]. A corre- sponding quasiclassical trajectory calculation lends support to the above prediction [9].
By analogy with the M g ( I P ~ ) - H 2 reaction, Kleiber and coworkers in a study of Na(4p) reaction with H 2 found a similar rotational bimodality of the Nail product [10,11]. The bimodal nature was antici- pated primarily to stem from a side-on attack along an attractive surface, which determined the micro- scopic branching late in the exit channel. In contrast, the reactions of excited K or Cs with H 2 are domi- nated by a harpoon mechanism [12-14]. As the alkali atom approaches collinearly to some distance, K or Cs eject an electron to the H 2 molecule, and then the H atom flies apart from the ion-pair inter- mediate. Due to the instability of H~, the resulting
0009-2614/97/$17.00 © 1997 Elsevier Science B.V. All rights reserved. PH S 0 0 0 9 - 2 6 1 4 ( 9 7 ) 0 0 6 3 5 - 0
38 D.-K. Liu, K.-C. Lin / Chemical Physics Letters 274 (1997) 37-40
product energy disposal is predominantly partitioned into translation [13]. Bersohn and coworkers sug- gested that the size of alkali elements may be an important factor to determine the mechanism prefer- ence [14]. Such a competition of insertion versus harpoon-type mechanism has also been found in the alkaline earth atom with OH-containing molecules [15,16]. The former pathway proceeds via a long- lived intermediate complex, which then evolves to different product channels, whereas the latter one forms only the metal hydroxide product.
From the energetic point of view, the ionization potential of the metal element is lowered by the electronic excitation, and thereby the rate of electron transfer is enhanced. In this note, we look into such an effect of excitation energy on the reaction mecha- nisms of Mg(3p) and Mg(4s)plus H 2 collisions. The ionization potential of Mg(4s) is lowered relative to Mg(3p) by 1.1 eV.
2. Experiment
A similar experimental apparatus with a p u m p - probe technique has been illustrated in detail else- where [1-5,7], so only a brief description is given here. Two dye lasers, each pumped by an individual Nd:YAG laser, were employed as the radiation sources. The pump laser was operated to emit at 459.7 nm for excitation of Mg(4s) by two-photon absorption. After a brief time delay of less than 10 ns, the probe laser was fired to monitor the laser-in- duced fluorescence (LIF) spectra of the resulting MgH product in the A 2 I I - X 2~+ band. The laser energies of both lasers were adjusted to avoid optical saturation or unwanted multiphoton excitation.
The Mg metal, deposited in a five-armed cross heat-pipe oven, was heated to 750-760 K, corre- sponding to a vapor pressure of 4 0 - 5 0 mTorr. The pressure of H 2 in the oven was maintained at less than 4 Torr to allow for the MgH produced to be detected in a nascent state.
3. Results and discussion
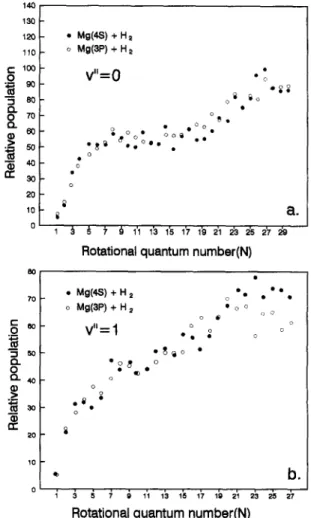
The obtained LIF spectra of MgH (v = 0 and 1) between Mg(4s) and Mg(3p) are very similar. Taking
14I:1 130 120 110 c- too 0 "5 BO 0 70 Q. 3O 20 --I Q.. 0 Q- 4o 0,) r,r' 20 • Mg(4S)+H= o Mg(3P) + H:z
V"=O
t o ° B e o o • o @ • o ~ @ • e ° o • • o +. o i . + 0 a . ~ ~ ~ 1'I lb 1~ 1Y lb 2'1 2'3 2's 2Y Rotational quantum number(N)• M0(+ + . , ° o M g ( 3 P ) + H 2 • V " - 1 ° o • o+~ ,. o o o o o o o o o - b .
Rotational quantum number(N)
Fig. 1. Comparison of the rotational distributions of M g H ( v = 0 and 1) resulting from Mg(3 tPI) and Mg(41S0).
into account the HiSnl-London factor and intensity correction for the low-N component ( N < 5) [7], we found that the normalized MgH (v = 0 and 1) rota- tional distributions obtained from Mg(4s) are essen- tially consistent with those from Mg(3p), except for a few high-N lines in the (0,1) band. The compari- son of MgH distributions, represented by the Pl branch, is shown in Fig. 1. Note that a computer simulation was applied to resolve the spectra around the band head. (The N = 11-20 lines for the (0,0) band and N = 8 - 1 4 lines for the (0,1) band [7].)
One should be cautious in studying a reaction initiated by a highly excited atom that electronic relaxation is negligible, i.e. that lower states popu- lated by relaxation do not form the same product to interfere with the study. We examine the probability
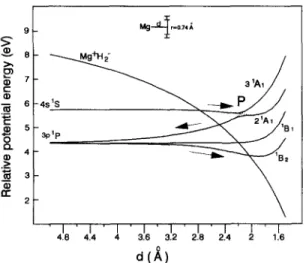
D.-K. Liu, K.-C. Lin / Chemical Physics Letters 274 (1997) 37-40 39 9 v 5 >= i g ~ r=O.74/~
/
31A1 / -3pip ~ 21A1 ~B1 I ' l i i ' l 2!8 2!, 1!6 4.8 4.4 4 3.6 3.2d(~)
Fig. 2. Calculation of potential energy surfaces as a function of internuclear distance.
of product contribution by these states through colli- sional and radiative relaxation. Since the obtained MgH is in a nascent state, the product contribution associated with the process of collisional relaxation is negligible under our experimental conditions. The radiative relaxation for the 4s ~ 3p transition gives rise to a spontaneous emission coefficient of 2.6 ×
107 s - l, corresponding to a lifetime of 39 ns. During the delay time adopted, a < 25% population contri- bution of MgH may result from the relaxed 3p state. In fact, we have shortened the delay time, but the obtained bimodal feature of the MgH distributions does not show any significant change.
To provide insight into the reaction pathway asso- ciated with the Mg(4s) state, we present in Fig. 2 the potential energies of Mg(3p, 4 s ) - H 2 reactions as a function of the internuclear distance in a C2v sym- metry. The calculations were done using basis sets in 6-31G * * level for the Mg and H atoms. Configura- tion interaction calculations with single excitation (CIS) contained in the GAUSSIAN 92 package were used for the computation. The ]Pl state is splitted into three surfaces (2 IA 1, IB 1 and IB 2) as Mg and H 2 approach. The corresponding PESs are consistent with those computed by Chaquin and coworkers [6]. At an internuclear distance about 2.2 .&, the repulsive 2 ~A~ curve has an avoided crossing with the upper 3 1A ~ curve, which correlates with the 4 ] S O state.
The potential diagram for the Mg(3p, 4 s ) - H 2 reaction is found to be similar to that in the Na(3p,
4 s ) - H 2 reaction [17]. Therefore, we propose a reac- tion pathway for the Mg(4s) system analogous to the N a - H 2 case [10,11,17]. The M g ( 4 s ) - H 2 collision begins to go along the attractive 3 IA 1 surface, and then crosses to the 2 1A 1 surface. The 4s state, which lies close to the ionization continuum, gains more ionic character than the 3p state. The mixing of valence and partial ionic characters allows for a certain probability for the non-adiabatic transition. For jumping to the reactive 1B 2 surface, Mg and H 2 have to fly apart to the vicinity of the asymptotic 3p state. Finally, the M g - H 2 tracks along the I B 2 surface, leading to a similar rotational distribution as in the 3p state reaction. An analogous interpretation has been applied to the Na(4s, 4 p ) - H 2 reactions [10,11,17]. However, here the probability for curve crossing from 2 IA l to tB z is questionable. Since a large repulsive force is exerted on the M g - H 2 inter- mediate as it tracks along 2 ~Aj, the moiety should break apart rapidly to Mg(3p) plus H 2. A secondary collision of Mg(3p) seems to be required to produce MgH.
In the following we propose an alternative mecha- nism to proceed via a harpoon-type pathway. An ion-pair curve, dominated by the Coulombic force, is included in the potential diagram of Fig. 2. The electron affinity of H 2 is adopted as - 2 . 1 eV. As Mg(4s) approaches towards H 2, a series of non- adiabatic curve crossings are found along the Mg+H2 coordinate, and including finally one to the reactive ]B 2 surface. Note that the crossing region lies in the attractive entrance channel of ~ B 2 about 2 ,~ prior to crossing to the ground state surface, where the anisotropy causes the bimodal nature of the rotational distribution. The similarity of the corre- sponding MgH distributions obtained by 3~Pl and 4]S0 is indicative of two things. First, the Mg(4s) collision by H 2 is closely associated with the M g ( 3 p ) - H 2 reaction coordinate through evolution of a series of surface crossings. Second, the product energy disposal in the Mg(4s) reaction, which has 1.1 eV more excitation energy than the 3p state, is dominated by translation. In the process of electron transfer, the strong instability of H~ tends to cause most of the energy to be partitioned into translation [13]. Unlike the case of the 3p state, the excitation energy facilitates the Mg(4s) plus H 2 reaction via the pathway of electron transfer, rather than by
40 D.-K. Liu, K.-C. Lin / Chemical Physics Letters 274 (1997) 37-40
formation of a stable insertive intermediate complex. By analogy with our work, in the reactions of Ca( ~ S 0, 3pj) with H202, the Ca(3pi ) state produces CaOH, while the ground state atom leads to only CaO [16]. The corresponding pathways proceed along an inser- tion either into an O - O or an O - H bond. A competi- tion occurs between dissociation of the intermediate complex and migration of the H atom. The reaction with Ca(3pj) is ascribed to an electron transfer, such that the ion-pair intermediate formed has no time to allow for H atom migration prior to dissociation.
In summary, the analysis of MgH rovibrational spectra suggests that the Mg(41S 0) atom proceeds via a harpoon-type reaction pathway, in contrast to a direct insertion mechanism for the Mg(3 i p~) plus H 2 reaction. The competition of electron transfer versus insertion depends on excitation energy. As the exci- tation energy increases, the harpoon process is fa- vored, through which the reaction coordinate is joined to the reactive ~B 2 surface.
Acknowledgements
This work is supported by the National Science Council, the Republic of China, under the contract No. NSC86-2113-M001-044-L2.
References
[1] W.H. Breckenridge, H. Umemoto, J. Chem. Phys. 80 (1984) 4168.
[2] W.H. Breckenridge, J.H. Wang, Chem. Phys. Lett. 82 (1985) 4945.
[3] K.C. Lin, C.T. Huang, J. Chem. Phys. 91 (1989) 5387. [4] P.D. Kleiber, A.M. Lyyra, K.M. Sando, S.V. Zafiropulos,
W.C. Stwalley, J. Chem. Phys. 85 (1986) 5493.
[5] P.D. Kleiber, A.M. Lyyra, K.M. Sando, S.P. Heneghan, W.C. Stwalley, Phys. Rev. Lett. 54 (1985) 2003.
[6] P. Chaquin, A. Sevin, H. Yu, J. Phys. Chem. 89 (1985) 2813. [7] D.K. Liu, T.L. Chin, K.C. Lin, Phys. Rev. A 50 (1994) 4891. [8] D.K. Liu, T.L. Chin, Y.R. Ou, C.T. Huang, K.C. Lin, J.
Chin. Chem. Soc. 42 (1995) 293. [9] Y.R. Ou and K.C. Lin (to be published).
[10] S. Bililign, P.D. Kleiber, W.R. Kearney, K.M. Sando, J. Chem. Phys. 96 (1992) 218.
[11] S. Bililign, P.D. Kleiber, J. Chem. Phys. 96 (1992) 213. [12] A.G. Urena, R. Vetter, Int. Rev. Phys. Chem. 15 (1996) 375. [13] D.K. Liu, K.C. Lin, J. Chem. Phys. 105 (1996) 9121. [14] X. Huang, J. Zhao, G. Xing, X. Wang, R. Bersohn, J. Chem.
Phys. 104 (1996) 1338.
[15] P. de Pujo, O. Sublementier, J.P. Visticot, J. Berlande, J. Cuvellier, C. Alcaraz, T. Gustavsson, J.M. Mestdagh, P. Meynadier, J. Chem. Phys. 99 (1993) 2533.
[16] M.D. Oberlander, R.P. Kampf, J.M. Parson, Chem. Phys. Lett. 176 (1991) 385.