Development of highly selective and sensitive probes for hydrogen
peroxide†
Lee-Chiang Lo* and Chi-Yuan Chu
Department of Chemistry, National Taiwan University, Taipei 106, Taiwan. E-mail: lclo@ntu.edu.tw; Fax: 886-2-2362-1979; Tel: 886-2-2362-1979
Received (in Cambridge, UK) 6th August 2003, Accepted 12th September 2003 First published as an Advance Article on the web 6th October 2003
Probes that react specifically with hydrogen peroxide to release chromophoric or fluorescent reporter groups were designed and synthesized.
Hydrogen peroxide is one of the major reactive oxygen species (ROS) in living systems.1 Its production is linked to the activities of certain enzymes that have been implicated in important cellular processes, including apoptosis, cytotoxicity, cell growth, and proliferation.2 Therefore, many efforts have been devoted to the determination of hydrogen peroxide in bioanalytical chemistry.3 Currently, the most widely used method is an enzymatic approach that involves the use of horseradish peroxidase (HRP), which catalyzes the oxidation of suitable substrates to generate read-out signals.4 However, it would be of great interest to develop a chemical approach that not only is sensitive to hydrogen peroxide, but also avoids possible interference resulting from the complex biological samples.
It is well known that hydrogen peroxide reacts with arylboronic acids under mild alkaline conditions to generate phenols.5 p-Dihydroxyborylbenzyloxycarbonyl (Dobz) group was later introduced as a special amine protecting group, as it could be selectively removed by hydrogen peroxide.6Based on these results, we envision that probes highly specific for hydrogen peroxide could be developed. Prototype probe 1 (1a and 1b) were designed and synthesized to test the idea, as shown in Scheme 1. In the design of probe 1, a suitable reporter group
(chromophore/fluorophore) is masked with a 2,3-butanediol ester of a Dobz derivative. Probe 1a carries a chromophoric p-nitroaniline group (lmax 383 nm), while probe 1b adopts a fluorescent coumarin reporter (lex348 nm, lem440 nm). Upon reaction with hydrogen peroxide, the reporters could be released and serve as the basis for the determination of hydrogen peroxide (Scheme 1).
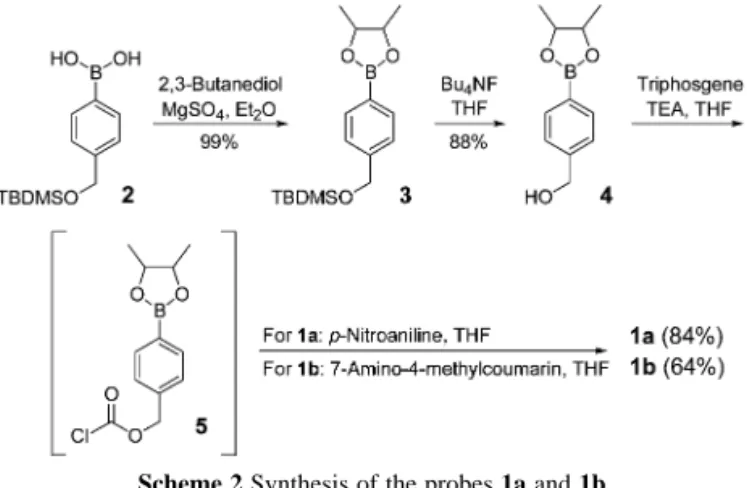
The synthesis of the probes 1a and 1b begins with arylboronic acid 27 in a straightforward manner (Scheme 2). The boronic moiety was first quantitatively converted to boronate ester 3 by reaction with 2,3-butanediol. Removal of the TBDMS group of ester 3 was achieved by treatment with Bu4NF to give borylbenzyl alcohol 4 in 88% yield. Borylbenzyl alcohol 4 is a key intermediate in this synthetic scheme. It reacted with triphosgene to generate the borylbenzylox-ycarbonyl chloride 5 in situ, which in turn could couple with the corresponding reporter group, namely p-nitroaniline and 7-amino-4-methylcoumarin, to offer probes 1a and 1b in 84% and 64% yields, respectively.
In the preliminary study, 200 mM of probe 1a was incubated
with various amounts of hydrogen peroxide (10 ~ 50 mM) in the
presence of 140 mM NaHCO3(pH 8.3) at 25 °C. The UV/vis spectra of probe 1a and p-nitroaniline in DMF are shown in Fig. 1. Although there is some overlapping in their UV/vis spectra, the incubation mixtures were monitored and recorded with a microplate reader at 405 nm to reduce the interference from the unreacted probe 1a. The amount of released p-nitroaniline (A405) quickly increased with time and reached plateau ( > 90% yield) after 80 ~ 90 min. A good linear correlation was observed between the released p-nitroaniline and the hydrogen peroxide in the mixture (Fig. 2), indicating that the design of the probe could function as depicted in Scheme 1 and that probe 1a could
† Electronic supplementary information (ESI) available: general methods. See http://www.rsc.org/suppdata/cc/b3/b309393j/
Scheme 1 Structure of the probe 1 (1a and 1b) and its reaction with
hydrogen peroxide to release the reporter signals.
Scheme 2 Synthesis of the probes 1a and 1b.
Fig. 1 UV/vis spectra of probe 1a (Ã) and p-nitroaniline (—) in DMF.
T h i s j o u r n a l i s © T h e R o y a l S o c i e t y o f C h e m i s t r y 2 0 0 3 2 7 2 8 C H E M . C O M M U N . , 2 0 0 3 2 7 2 8 – 2 7 2 9
be successfully used for the determination of hydrogen peroxide. Although lower probe concentrations could be used when working with low concentrations of hydrogen peroxide, we suggest keeping the probe concentration at 200 mM in order
to obtain a good response range within a reasonable time. We have also investigated the potential interfering effect of hydroperoxide and thiol compounds on the reaction of probe 1a with hydrogen peroxide. The results indicated that tert-butyl hydroperoxide reacted with probe 1a at a much slower rate ( ~ 2%). As for cysteine, it only produced a negligible increase in A405when incubated alone with probe 1a. However, when added in the incubation mixture of probe 1a and hydrogen peroxide, it lowered the amount of the released p-nitroaniline, indicating that the thiol group might consume hydrogen peroxide8and should be avoided in the assay mixture.
Since hydrogen peroxide is also the co-product of many oxidases for some important metabolites, measurements of the concentration of these substrates or the activity of the responsible enzymes could be indirectly achieved by monitor-ing the hydrogen peroxide resultmonitor-ing from the coupled reactions. Therefore, the probes developed in this study could be linked to these studies and find wide applications. As a demonstration, experiments for the determination of glucose concentrations were carried out. In this model study, various amounts of glucose (10 ~ 50 mM) were first treated with glucose oxidase to
generate hydrogen peroxide, of which the concentrations were determined by using probe 1a as described above. A good linear correlation again was successfully established between glucose and hydrogen peroxide as shown in Fig. 3.
One of the major advantages in the design of the probes in this study is the flexibility in the choice of the reporter groups. Different reporter groups, which carry special properties yet do not affect the detecting mechanism, could be used to meet the demands. In the meantime, the preparation of different probes basically follows the same synthetic scheme. For example, probe 1b, which carries a fluorescent coumarin derivative, was also conveniently constructed. Probe 1b behaves similarly to that of probe 1a when employed in excess (200 mM) for the
determination of hydrogen peroxide (Fig. 4), except a fluores-cence microplate reader (lex355 nm, lem460 nm) was utilized. It offers a more sensitive detection range (0.1 ~ 5 mM) than that
of probe 1a.
In summary, probes 1a and 1b were conveniently prepared and were demonstrated to be probes for hydrogen peroxide on a reaction mechanism basis. The skeleton of these probes consists of a butanediol ester of the Dobz derivative, masking the amino group of suitable reporter groups. These two probes are stable compounds that could be activated and release the reporter groups upon reaction with hydrogen peroxide. The probe design offers great flexibility for signal output. Probes 1a and 1b serve well in the micromolar and sub-micromolar range, respectively. In addition, in combination with a microplate reader they are well suited for high throughput screening purposes. Our ongoing study is to improve the sensitivity of the probes by exploiting new reporter groups. In the meantime, one of the major goals is to apply these probes in studying the events inside the cells. Preliminary data of the pH effect on the reaction of probe 1a and H2O2favorably indicated that the probe could still function at pH 7.0–7.5, although at a slower rate (18–32%). We are currently evaluating the feasibility of this potential application.
This work was supported by the National Science Council (NSC 92-3112-B-002-005 to LCL).
Notes and references
1 (a) J. M. McCord and I. Fridovich, J. Biol. Chem., 1969, 244, 6049–6055; (b) T. L. Vanden Hoek, L. B. Becker, Z. Shao, C. Li and P. T. Schumacker, J. Biol. Chem., 1998, 273, 18092–18098.
2 (a) R. E. Parchment and G. B. Pierce, Cancer Res., 1989, 49, 6680–6686; (b) R. D. Allen and T. K. Roberts, Am. J. Reprod. Immunol. Microbiol., 1986, 11, 59–64; (c) J. W. Tetrud and J. W. Langston, Science, 1989, 245, 519–522.
3 (a) D. M. Ziegler and J. P. Kehrer, Methods Enzymol., 1990, 186, 621–626; (b) J. H. Lee, I. N. Tang and J. B. Weinstein-Lloyd, Anal. Chem., 1990, 62, 2381–2384; (c) R. Rapoport, I. Hanukoglu and D. Sklan, Anal. Biochem., 1994, 218, 309–313.
4 (a) G. G. Guilbault, P. J. Brignac Jr. and M. Juneau, Anal. Chem., 1968,
40, 1256–1263; (b) M. Zhou, Z. Diwu, N. Panchuk-Voloshina and R. P.
Haugland, Anal. Biochem., 1997, 253, 162–168; (c) M. Zhou and N. Panchuk-Voloshina, Anal. Biochem., 1997, 253, 169–174.
5 H. G. Kuivila and A. G. Armour, J. Am. Chem. Soc., 1957, 79, 5659–5662.
6 (a) D. S. Kemp and D. C. Roberts, Tetrahedron Lett., 1975, 4629–4632; (b) M. Wakselman, Nouv. J. Chim., 1983, 7, 439–447.
7 P. R. Ashton, R. Ballardini, V. Balzani, A. Credi, K. R. Dress, E. Ishow, C. J. Kleverlaan, O. Kocian, J. A. Preece, N. Spencer, J. F. Stoddart, M. Venturi and S. Wenger, Chem. Eur. J., 2000, 6, 3558–3574.
8 B. Haliwell, M. V. Clement and L. H. Long, FEBS Lett., 2000, 10–13.
Fig. 2 The linear correlation of the released p-nitroaniline (A405) upon
reactions of probe 1a (200 mM) with 10, 20, 30, 40 and 50 mM of hydrogen
peroxide in the presence of 140 mM NaHCO3(pH 8.3) for 80–90 min.
Fig. 3 Determination of glucose using probe 1a after pretreatment with
glucose oxidase (0.9 U, 175 mM NaHCO3, pH 8.3) for 30 min. The other
conditions are the same as described in Fig. 2.
Fig. 4 The linear correlation of the fluorescence response (lex355 nm, lem
460 nm) upon reactions of probe 1b (200 mM) with 0.1, 0.2, 0.5, 1.0, 2.5 and
5.0 mM of hydrogen peroxide. The other conditions are the same as
described in Fig. 2.
2 7 2 9 C H E M . C O M M U N . , 2 0 0 3 , 2 7 2 8 – 2 7 2 9