Evaluation of the Contribution of Cyclooxygenase 2 Genotypes to Taiwan Breast
Cancer
Chen-Hsien Su1,2*, Chieh-Lun Hsiao2*, Wen-Shin Chang1,2*, Liang-Chih Liu3, Hwei-Chung Wang3, Chia-Wen Tsai2, Long-Yuan Li4, Chang-Hai Tsai2,5 and Da-Tian Bau1,2 1Graduate Institute of Clinical Medical Science, China Medical University, Taichung,
Taiwan, R.O.C.;
2Terry Fox Cancer Research Laboratory, China Medical University Hospital,
Taichung, Taiwan, R.O.C.;
3Department of Breast Surgery, China Medical University Hospital, Taichung,
Taiwan, R.O.C.;
4Center for Molecular Medicine, China Medical University Hospital, Taichung,
Taiwan, R.O.C.;
5Asia University, Taichung, Taiwan, R.O.C.
* These Authors contribute equally to this work
Correspondence to: Da-Tian Bau, Terry Fox Cancer Research Laboratory, Department of
Medical Research, China Medical University Hospital, 2 Yuh-Der Road, Taichung, 404 Taiwan, R.O.C. Tel: +886 422052121 Ext. 7534, Fax: +886 422053366, e-mail:
artbau1@yahoo.com.tw; artbau2@gmail.com
Abstract. Overexpression of cyclooxygenase 2 (COX-2) has been suggested to be
associated with breast carcinogenesis. The aim of this study is to evaluate the contribution of genotypic polymorphisms in COX-2 to breast cancer risk of Taiwan females. In total, 1232 breast cancer patients and 1232 healthy controls were genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) methodology. Six polymorphic variants of COX-2 including G-1195A (rs689466), G-765C (rs20417), T8473C (rs5275), Intron 1 (rs2745557), Intron 5 (rs16825748) and Intron 6 (rs2066826) were examined. The results showed that GC genotype of COX-2 G-765C was associated with a lower risk compared with wild-type GG genowild-type (odds ratio=0.66, 95% confidence interval=0.53-0.83, p=0.0005). The C allele of COX-2 G-765C was significantly more frequently found in controls than in cancer patients (p=0.0006). In addition, the odds ratios of the GG/AG+AA, GC/GG and GC/AG+AA at G-765C/Intron 1 combined genotypes compared with wild-type GG/GG genotype were 0.79 (95%CI=0.66-0.96; p=0.0166), 0.61 (95%CI=0.48-0.78; p=0.0001), and 0.71 (95%CI=0.36-1.37; p=0.3040), respectively. As for the combination of G-765C and Intron 6, the odds ratios of the GG/AG+AA, GC/GG and GC/AG+AA combined genotypes compared with wild-type GG/GG reference genotype were 0.79 (95%CI=0.62-1.01; p=0.0561), 0.63 (95%CI=0.50-0.81; p=0.0003), and 0.68 (95%CI=0.38-1.21; p=0.1897), respectively. Our results indicate that C allele of COX-2 G-765C was associated with decreased risk of breast
cancer in Taiwan, and could serve as an early detective and predictive marker for breast cancer risk.
Breast cancer is now ranked first among cancers affecting females throughout the world and its incidence rate is keeping on increasing in the recent decades (1). In Taiwan, breast cancer is the fourth leading cancer, important for its high incidence, high mortality, and early onset (2, 3). It is believed that breast cancer is largely multicausal and its susceptibility is conferred by environmental and hormone exposures in addition to multigenic variations of the genome. Previous studies revealed that oriental women affected by breast cancer were significantly younger than white women and had racial/ethnic difference in their survival patterns (4, 5). In recent years scientists began to explore the mechanisms underlying breast cancer formation at the molecular level. Investigations into these racial/ethnic differences from the cancer genomic angle may enhance the speed to unravel the genomic and environmental etiology of breast cancer, and for cancer early detection and prediction. Cyclooxygenase, also known as prostaglandin endoperoxide synthetase, plays a critical role in cellular metabolism through converting arachidonic acid to prostaglandins. Two isoforms of COX, COX-1 and COX-2 act quite differently. COX-1 is constitutively expressed and presents in various tissues while COX-2 is non-detected in most normal tissues. However, COX-2 can be rapidly induced by inflammatory stimuli resulting in elevated levels of prostaglandins which are closely in regulating cell proliferation, apoptosis and angiogenesis, contributing to tumor occurrence and progression (6-9). In recent years, mounting evidence showed that
elevated COX-2 in breast tissues is related to the genesis of mammary tumors (10, 11), and is associated with parameters of aggressive breast cancer, including large tumor size, positive axillary lymph node metastases (12), and HER2-positive tumor status (13). On the other way, targeted inhibition of COX-2 by selective cyclooxygenase-2 inhibitor celecoxib inhibited the proliferation of breast cancer cell lines in vitro (14).
The human COX-2 gene (also known as PTGS2) is located on chromosome 1q25.2-q25.3 and consists of 10 exons spanning 8.3 kb (15). More than 15 single nucleotide polymorphisms for COX-2 have been identified, but the most extensively studied polymorphisms are the G-765C (rs20417) in the promoter and the C8473T (rs5275) in the 3’UTR of COX-2. Genetic polymorphisms at COX-2 promoter region have been shown to alter its expression and influence the susceptibility to various carcinomas, including childhood acute lymphoblastic leukemia (16), hepatocellular carcinoma (17), prostate (18), bladder cancer (19). In 2002, it has been proposed that the G-765C polymorphism on COX-2 may eliminate a Sp1-binding site but create an E2F binding site and result in altered COX-2 expression (20). Another polymorphism site C8473T (rs5275) of COX-2 on 3’UTR was suggested to be associated with the alteration of mRNA level of the gene as sequences within the 3’UTR are important for message stability and translational efficiency (21). In 2010, Yu and his colleagues have conducted a mete-analysis on the associations between several COX-2
polymorphisms and breast cancer risk and suggested no significant association was observed for the G-765C and C8473T polymorphisms (22). However, of the studies included in their meta-analysis, only two studies were conducted in Chinese population and none of the two studies investigated the rs5277 polymorphism at all (23, 24). In conclusion, the genotype of COX-2 among Chinese has not yet been well studied, which is in urgent need.
Therefore, in the present work we aimed at analyzing not only the famous polymorphic site of COX-2, G-765C (rs20417) and 3’UTR (rs5275), but also four other sites G-1195A (rs689466), Intron 1 (rs2745557), Intron 5 (rs16825748) and Intron 6 (rs2066826) in a very representative population (control/case=1232/1232), and investigated the correlation between COX-2 genotypes and risk of breast cancer among Taiwanese women.
Materials and Methods
Study population and sample collection. 1232 female cancer patients diagnosed with breast cancer were recruited at our hospital. For comparison, equal amounts of age-matched non-breast cancer healthy volunteers as controls were selected from the Health Examination Cohort of the hospital matched with age (±5 years). Our study was approved by the Institutional Review Board of the China Medical University Hospital (DMR99-IRB-108). Before recruitment, a standard questionnaire was administered through face-to-face interviews by trained interviewers to obtain
information on demographic data and related factors.
Genotyping assays. Genomic DNA was prepared using a QIAamp Blood Mini Kit (Blossom, Taipei, Taiwan) as previous studies (25, 26). The polymerase chain reaction (PCR) cycling conditions were: one cycle at 94°C for 5 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 10 min. Pairs of PCR primer sequences and restriction enzyme for each DNA product are listed in Table I.
Statistical analyses. The associations between the genotypes of the COX-2 polymorphisms and risk of breast cancer and patients’ clinical characteristics were estimated by computing odds ratios (ORs) and 95% confidence intervals (CIs) from unconditional logistic regression analysis with the adjustment for age and age at menarche. Pearson’s Chi-square test or Fisher’s exact test (when any cell was less than 5) was also used to compare the distribution of the genotypes. The data were recognized as significant when the statistical p-Value was less than 0.05.
Results
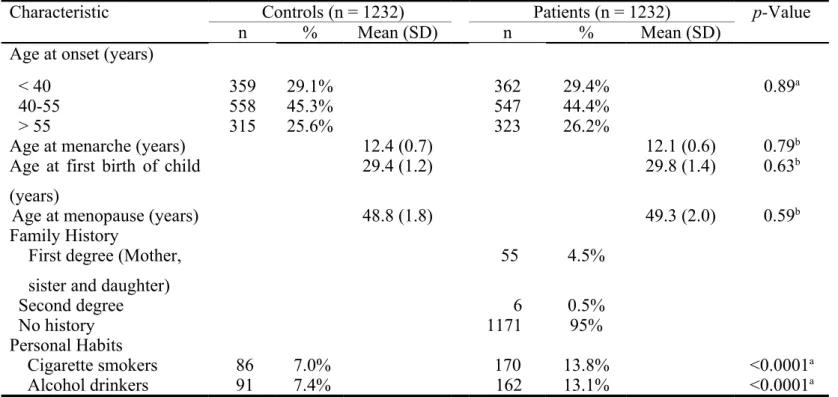
The frequency distributions of selected demographic and life-style characteristics of 1232 breast cancer patients and 1232 non-cancer controls are shown in Table II. The age-related characteristics of patients and controls are all well matched (p>0.05). About five percent of patients are with family history. As for the individual life-style, the cigarette smoking and alcoholism were both risk factors for breast cancer in this population (p<0.05) (Table II).
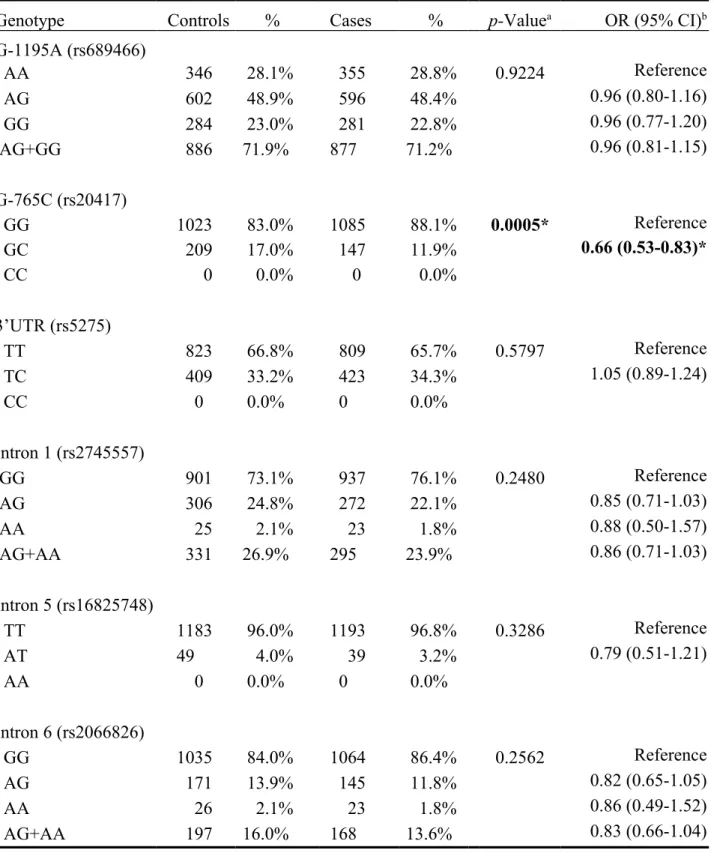
The frequencies of the genotypes for the COX-2 G-1195A, G-765C, 3’UTR, Intron 1, Intron 5 and Intron 6 in controls and breast cancer patients are shown in Table III. The genotype distribution of the COX-2 G-765C was significantly different between breast cancer and control groups (p=0.0005), while those for COX-2 G-1195A, 3’UTR, Intron 1, Intron 5 or Intron 6 polymorphisms were not (p>0.05) (Table III). Those who carried GC genotype at COX-2 G-765C have 0.66-fold odds ratio of breast cancer risk compared with those with GG genotype (95% CI=0.53-0.83). There was no COX-2 G-765C CC carrier (Table III).
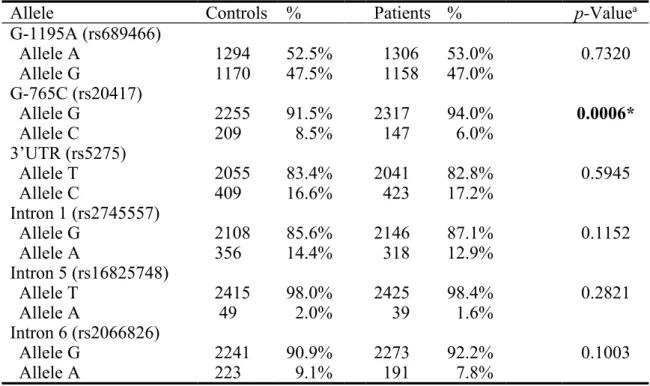
The frequencies of the alleles for COX-2 polymorphisms in controls and breast cancer patients are shown in Table IV. Neither of the allele of the COX-2 of the polymorphisms were found to be associated with breast cancer (p>0.05) except that of COX-2 G-765C (p=0.0006) (Table IV).
To further investigate the contribution of COX-2 genotype to breast cancer susceptibility, the two-polymorphic interactions among COX-2 genotypes were
investigated by genotype analysis. In Tables V and VI, the two combinations with statistically significant p-Values were shown. As for the combination of G-765C and Intron 1, the ORs of the GG/AG+AA, GC/GG and GC/AG+AA combined genotypes compared with wild-type GG/GG reference genotype were 0.79 (95%CI=0.66-0.96; p=0.0166), 0.61 (95%CI=0.48-0.78; p=0.0001), and 0.71 (95%CI=0.36-1.37; p=0.3040), respectively (Table V). As for the combination of G-765C and Intron 6, the ORs of the GG/AG+AA, GC/GG and GC/AG+AA combined genotypes compared with wild-type GG/GG reference genotype were 0.79 (95%CI=0.62-1.01; p=0.0561), 0.63 (95%CI=0.50-0.81; p=0.0003), and 0.68 (95%CI=0.38-1.21; p=0.1897), respectively (Table VI). As for other combinations, there was no significant difference in frequencies of any combined genotypes between the two groups for each combined genotype (data not shown). We have also analyzed the joint effects of COX-2 genotypes and environmental factors, including smoking and alcohol drinking while no significant interaction was found (data not shown).
Discussion
The abnormal expression of COX-2 has been reported to play an important role in breast carcinogenesis (10, 11). In order to reveal the role of COX-2 from the genomic viewpoint and to find potential detective markers for breast cancer, up to six polymorphic site of the COX-2 gene have been chosen and investigated their
association with the breast cancer susceptibility in a representative Taiwanese population. We found that for the promoter site G-765C of COX-2, the variant GC genotype and C allele were associated with a decreased risk for breast cancer, compared with the wild-type GG genotype and G allele, respectively (Tables III and IV), while those for other polymorphic sites were not (Tables III and IV). It is reasonable that the polymorphic site at promoter region may interact with transcription factors, determining the individual difference at transcriptional and translational levels of COX-2 at the initiation or progression periods of breast carcinogenesis (20). Also, the COX-2 G-765C may interact with other polymorphic sites, such as Intron 1 (rs2745557) and Intron 6 (2066826), to conduct their influences on regulating both COX-2 expression levels and cancer risk (Tables V and VI)
COX-2 played a role in the etiology of breast cancer, which is an outcome of complex genetic and environmental interactions. In the analysis of the synergistic effects of genotypes together with smoking and alcoholism life-style on breast cancer, no significant finding was found (data not shown). Although we could not find positive interaction of COX-2 genotype with these environmental risk factors, the environmental factors could not be excluded for their influence on the transcriptional, translational and post-translational levels of COX-2. For instance, the expression level of COX-2 is reported to be regulated by reactive oxygen species (ROS) in hepatocytes (27), and intracellular ROS may be elevated by various environmental stimulus
including smoking and alcohol drinking. Since it is known that transcriptional modulation of COX-2 is cell-specific (28), the correlation between ROS and COX-2 expression in breast carcinogenesis may be investigated in breast cancer cell models. In 2014, Gao and his colleagues have found that GC/CC genotypes at COX-2 G-765C were associated with higher cancer risk and larger tumor size, suggesting that variant genotypes of COX-2 promoter polymorphism may not only participate in cancer susceptibility determination but the progression of breast carcinogenesis (29).
In conclusion, this is the study which focuses on the single or combined COX-2 genotypes and their effects on breast cancer risk in Taiwan. Further investigations of multiple genotypes of other cancer related genes, gene-gene and gene-environment interactions, and phenotypic assays of the cancer-associated polymorphic sites are urgently needed in the future. The presence of C allele at G-765C of COX-2 was not only associated with a lower cancer risk, but involved in the early breast carcinogenesis.
Acknowledgements
We thank Tzu-Chia Wang, Yun-Ru Syu, Lin-Lin Hou and Chia-En Miao for their technical assistance. This study was supported by research grants from Terry Fox Cancer Research Foundation, China Medical University and Hospital to Dr. Liu (DMR-103-019) and Taiwan Ministry of Health and Welfare Clinical Trial and
References
1 Siegel R, Naishadham D and Jemal A: Cancer statistics, 2013. CA Cancer J Clin 63: 11-30, 2013.
2 Cheng SH, Tsou MH, Liu MC, Jian JJ, Cheng JC, Leu SY, Hsieh CY and Huang AT: Unique features of breast cancer in Taiwan. Breast Cancer Res Treat 63: 213-223, 2000.
3 Kuo WH, Yen AM, Lee PH, Chen KM, Wang J, Chang KJ, Chen TH and Tsau HS: Cumulative survival in early-onset unilateral and bilateral breast cancer: an analysis of 1907 Taiwanese women. Br J Cancer 100: 563-570, 2009.
4 Hsu JL, Glaser SL and West DW: Racial/ethnic differences in breast cancer survival among San Francisco Bay Area women. J Natl Cancer Inst 89: 1311-1312, 1997.
5 Natarajan N, Nemoto D, Nemoto T and Mettlin C: Breast cancer survival among Orientals and whites living in the United States. J Surg Oncol 39: 206-209, 1988.
6 Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C and Kaidi A: The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30: 377-386, 2009.
7 Sobolewski C, Cerella C, Dicato M, Ghibelli L and Diederich M: The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010: 215158, 2010.
8 Kalinski P: Regulation of immune responses by prostaglandin E2. J Immunol 188: 21-28, 2012.
9 Ke HL, Tu HP, Lin HH, Chai CY, Chang LL, Li WM, Li CC, Lee YC, Yeh HC, Wu WJ and Bau DT: Cyclooxygenase-2 (COX-2) up-regulation is a prognostic
marker for poor clinical outcome of upper tract urothelial cancer. Anticancer Res 32: 4111-4116, 2012.
10 Half E, Tang XM, Gwyn K, Sahin A, Wathen K and Sinicrope FA: Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res 62: 1676-1681, 2002.
11 Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK and Hung MC: Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell 6: 251-261, 2004.
12 Spizzo G, Gastl G, Wolf D, Gunsilius E, Steurer M, Fong D, Amberger A, Margreiter R and Obrist P: Correlation of COX-2 and Ep-CAM overexpression in human invasive breast cancer and its impact on survival. Br J Cancer 88: 574-578, 2003.
13 Subbaramaiah K, Norton L, Gerald W and Dannenberg AJ: Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem 277: 18649-18657, 2002.
14 Dai ZJ, Ma XB, Kang HF, Gao J, Min WL, Guan HT, Diao Y, Lu WF and Wang XJ: Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in Vitro and in Vivo. Cancer Cell Int 12: 53, 2012. 15 Piranda DN, Festa-Vasconcellos JS, Amaral LM, Bergmann A and
Vianna-Jorge R: Polymorphisms in regulatory regions of cyclooxygenase-2 gene and breast cancer risk in Brazilians: a case-control study. BMC Cancer 10: 613, 2010.
16 Wang CH, Wu KH, Yang YL, Peng CT, Wang RF, Tsai CW, Tsai RY, Lin DT, Tsai FJ and Bau DT: Association study of cyclooxygenase 2 single nucleotide polymorphisms and childhood acute lymphoblastic leukemia in Taiwan.
Anticancer Res 30: 3649-3653, 2010.
17 Chang WS, Yang MD, Tsai CW, Cheng LH, Jeng LB, Lo WC, Lin CH, Huang CY and Bau DT: Association of cyclooxygenase 2 single-nucleotide polymorphisms and hepatocellular carcinoma in Taiwan. Chin J Physiol 55: 1-7, 2012.
18 Wu HC, Chang CH, Ke HL, Chang WS, Cheng HN, Lin HH, Wu CY, Tsai CW, Tsai RY, Lo WC and Bau DT: Association of cyclooxygenase 2 polymorphic genotypes with prostate cancer in taiwan. Anticancer Res 31: 221-225, 2011. 19 Chang WS, Tsai CW, Ji HX, Wu HC, Chang YT, Lien CS, Liao WL, Shen WC,
Tsai CH and Bau DT: Associations of cyclooxygenase 2 polymorphic genotypes with bladder cancer risk in Taiwan. Anticancer Res 33: 5401-5405, 2013.
20 Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE and Laurent GJ: Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol 22: 1631-1636, 2002.
21 Cok SJ and Morrison AR: The 3'-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational efficiency. J Biol Chem 276: 23179-23185, 2001.
22 Yu KD, Chen AX, Yang C, Qiu LX, Fan L, Xu WH and Shao ZM: Current evidence on the relationship between polymorphisms in the COX-2 gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 122: 251-257, 2010. 23 Gao J, Ke Q, Ma HX, Wang Y, Zhou Y, Hu ZB, Zhai XJ, Wang XC, Qing JW, Chen WS, Jin GF, Liu JY, Tan YF, Wang XR and Shen HB: Functional polymorphisms in the cyclooxygenase 2 (COX-2) gene and risk of breast cancer in a Chinese population. J Toxicol Environ Health A 70: 908-915, 2007.
polymorphism of the cyclooxygenase-2 gene associated with breast cancer. Clin Oncol (R Coll Radiol) 21: 302-305, 2009.
25 Wang HC, Liu CS, Chiu CF, Chiang SY, Wang CH, Wang RF, Lin CC, Tsai RY and Bau DT: Significant association of DNA repair gene Ku80 genotypes with breast cancer susceptibility in Taiwan. Anticancer Res 29: 5251-5254, 2009.
26 Chang CH, Chiu CF, Liang SY, Wu HC, Chang CL, Tsai CW, Wang HC, Lee HZ and Bau DT: Significant association of Ku80 single nucleotide polymorphisms with bladder cancer susceptibility in Taiwan. Anticancer Res 29: 1275-1279, 2009.
27 Lim W, Kwon SH, Cho H, Kim S, Lee S, Ryu WS and Cho H: HBx targeting to mitochondria and ROS generation are necessary but insufficient for HBV-induced cyclooxygenase-2 expression. J Mol Med (Berl) 88: 359-369, 2010. 28 Casado M, Callejas NA, Rodrigo J, Zhao X, Dey SK, Bosca L and Martin-Sanz
P: Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. Faseb J 15: 2016-2018, 2001.
29 Gao J, Kang HF, Ma XB, Tang W, Liu D, Zhao Y, Zhang SQ, Guan HT, Lin S, Ren HT, Wang XJ and Dai ZJ: Functional promoter -765 G > C variant in COX-2 gene is associated with the susceptibility of breast cancer in Chinese Han women. Cancer Cell Int 14: 38, 2014.
Table I. The primer sequences, polymerase chain reaction and restriction fragment length
polymorphism conditions for cyclooxygenase 2 (COX-2) gene polymorphisms.
Polymorphism (location)
Primers sequences (5’ to 3’) Restriction enzyme SNP sequence DNA fragment size (bp) G-1195A (rs689466) F: CCCTGAGCACTACCCATGAT R: GCCCTTCATAGGAGATACTGG Hha I A G 273 220 + 53 G-765C (rs20417) F: TATTATGAGGAGAATTTACCTTTCGC R: GCTAAGTTGCTTTCAACAGAAGAAT PvuⅡ C G 100 74 + 26 3’UTR (rs5275) F: GTTTGAAATTTTAAAGTACTTTTGAT R: TTTCAAATTATTGTTTCATTGC Bcl I T C 147 124 + 23 Intron 1 (rs2745557) F: GAGGTGAGAGTGTCTCAGAT R: CTCTCGGTTAGCGACCAATT Taq I G A 439 353 + 76 Intron 5 (rs16825748) F: GCGGCATAATCATGGTACAA R: CAGCACTTCACGCATCAGTT BsrG I T A 417 314 + 103 Intron 6 (rs2066826) F: ACTCTGGCTAGACAGCGTAA R: GCCAGATTGTGGCATACATC Aci I A G 327 233 + 94 F and R indicate forward and reverse primers, respectively.
Table II. Distributions of demographic and life-style of breast cancer patients and the age-matched controls.
Characteristic Controls (n = 1232) Patients (n = 1232) p-Value
n % Mean (SD) n % Mean (SD)
Age at onset (years)
< 40 359 29.1% 362 29.4% 0.89a
40-55 558 45.3% 547 44.4%
> 55 315 25.6% 323 26.2%
Age at menarche (years) 12.4 (0.7) 12.1 (0.6) 0.79b
Age at first birth of child (years)
29.4 (1.2) 29.8 (1.4) 0.63b
Age at menopause (years) 48.8 (1.8) 49.3 (2.0) 0.59b
Family History
First degree (Mother, sister and daughter)
55 4.5% Second degree 6 0.5% No history 1171 95% Personal Habits Cigarette smokers 86 7.0% 170 13.8% <0.0001a Alcohol drinkers 91 7.4% 162 13.1% <0.0001a
Table III. Distribution of cyclooxygenase 2 (COX-2) genotypes among breast cancer patient and control groups.
Genotype Controls % Cases % p-Valuea OR (95% CI)b
G-1195A (rs689466) AA 346 28.1% 355 28.8% 0.9224 Reference AG 602 48.9% 596 48.4% 0.96 (0.80-1.16) GG 284 23.0% 281 22.8% 0.96 (0.77-1.20) AG+GG 886 71.9% 877 71.2% 0.96 (0.81-1.15) G-765C (rs20417) GG 1023 83.0% 1085 88.1% 0.0005* Reference GC 209 17.0% 147 11.9% 0.66 (0.53-0.83)* CC 0 0.0% 0 0.0% 3’UTR (rs5275) TT 823 66.8% 809 65.7% 0.5797 Reference TC 409 33.2% 423 34.3% 1.05 (0.89-1.24) CC 0 0.0% 0 0.0% Intron 1 (rs2745557) GG 901 73.1% 937 76.1% 0.2480 Reference AG 306 24.8% 272 22.1% 0.85 (0.71-1.03) AA 25 2.1% 23 1.8% 0.88 (0.50-1.57) AG+AA 331 26.9% 295 23.9% 0.86 (0.71-1.03) Intron 5 (rs16825748) TT 1183 96.0% 1193 96.8% 0.3286 Reference AT 49 4.0% 39 3.2% 0.79 (0.51-1.21) AA 0 0.0% 0 0.0% Intron 6 (rs2066826) GG 1035 84.0% 1064 86.4% 0.2562 Reference AG 171 13.9% 145 11.8% 0.82 (0.65-1.05) AA 26 2.1% 23 1.8% 0.86 (0.49-1.52) AG+AA 197 16.0% 168 13.6% 0.83 (0.66-1.04)
a Based on Chi-square test or Fisher’s exact test (when one or more cell is less than 5); b OR: odds ratio, CI: confidence interval;
Table IV. Distribution of cyclooxygenase 2 (COX-2) allelic frequencies among breast cancer patient and control groups.
Allele Controls % Patients % p-Valuea
G-1195A (rs689466) Allele A 1294 52.5% 1306 53.0% 0.7320 Allele G 1170 47.5% 1158 47.0% G-765C (rs20417) Allele G 2255 91.5% 2317 94.0% 0.0006* Allele C 209 8.5% 147 6.0% 3’UTR (rs5275) Allele T 2055 83.4% 2041 82.8% 0.5945 Allele C 409 16.6% 423 17.2% Intron 1 (rs2745557) Allele G 2108 85.6% 2146 87.1% 0.1152 Allele A 356 14.4% 318 12.9% Intron 5 (rs16825748) Allele T 2415 98.0% 2425 98.4% 0.2821 Allele A 49 2.0% 39 1.6% Intron 6 (rs2066826) Allele G 2241 90.9% 2273 92.2% 0.1003 Allele A 223 9.1% 191 7.8%
Table V. Frequencies of combined COX-2 G-765C and Intron 1 genotype polymorphisms among breast cancer patient and control groups.
COX-2 G-765C /Intron 1 Control Patients OR (95% CI) p-Valuea n % n % All 1232 100.0 1232 100.0 GG/GG 712 57.8 806 67.6 1.00 (reference) GG/AG+AA 311 25.2 279 20.5 0.79 (0.66-0.96)* 0.0166* GC/GG 189 15.4 131 8.4 0.61 (0.48-0.78)* 0.0001* GC/AG+AA 20 1.6 16 3.5 0.71 (0.36-1.37) 0.3040
Table VI. Frequencies of combined COX-2 G-765C and Intron 6 genotype polymorphisms among breast cancer patient and control groups.
COX-2 G-765C /Intron 6 Control Patients OR (95% CI) p-Valuea n % n % All 1232 100.0 1232 100.0 GG/GG 854 69.3 938 76.1 1.00 (reference) GG/AG+AA 169 13.7 147 12.0 0.79 (0.62-1.01) 0.0561 GC/GG 181 14.7 126 10.2 0.63 (0.50-0.81)* 0.0003* GC/AG+AA 28 2.3 21 1.7 0.68 (0.38-1.21) 0.1897