Contents lists available atScienceDirect
Lung Cancer
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / l u n g c a n
The efficacy of pemetrexed as a third- or fourth-line therapy and the significance
of thymidylate synthase expression in patients with advanced non-small cell
lung cancer
Myung Hee Chang
a, Jin Seok Ahn
a,∗, Jeeyun Lee
a, Kyoung Ha Kim
a, Yeon Hee Park
a, Joungho Han
b,
Myung-Ju Ahn
a, Keunchil Park
aaDivision of Hematology–Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Irwon-dong, Seoul 135-710,
Republic of Korea
bDivision of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
a r t i c l e i n f o
Article history:
Received 16 September 2009 Received in revised form 15 November 2009 Accepted 5 December 2009
Keywords:
Non-small cell lung cancer Pemetrexed
Thymidylate synthase Predictors
Prognostic factors
a b s t r a c t
Background: Pemetrexed is one of the standard second-line therapies in advanced non-small cell lung cancer (NSCLC). Currently, there are no standard cytotoxic treatments beyond second-line therapy. We evaluated the efficacy and safety of pemetrexed as a salvage regimen in heavily pretreated NSCLC patients. We also analyzed thymidylate synthase (TS) expression in tumor tissues to determine whether TS expres-sion is correlated with the clinical efficacy of pemetrexed.
Methods: One hundred and ten NSCLC patients who received pemetrexed as third- or fourth-line therapy at the Samsung Medical Center between June 2006 and June 2008 were retrospectively reviewed. TS expression was analyzed by immunohistochemical staining in 55 NSCLC tissue specimens. The relation-ships between TS expression and clinicopathological factors were evaluated. Univariate and multivariate analyses were performed to define the predictive factors and prognostic significances.
Results: The median age of patients in this study was 59 years (range: 24–84), 50.9% were men, and 27 (24.6%) were smokers or previous smokers. Sixty-five patients (59.1%) received pemetrexed as third-line treatment, and 95 (86.4%) had non-squamous cell carcinoma. Platinum-based chemotherapy (84.6%) was the most common first-line therapy, and EGFR TKIs [erlotinib (17.3%) or gefitinib (43.6%)] were a common second-line therapy. The median time from date of diagnosis to the date of the first pemetrexed treatment was 12.8 months (range: 1.8–62.2 months) and the median number of pemetrexed treatments was 4 (range 1–22). Eighteen patients achieved PR (16.3%), 41 patients SD (37.3%), and 43 patients PD (39.1%), with a disease control rate of 53.6%. The median follow-up duration was 16.1 months, the median progression-free survival (PFS) was 3.2 months (95% CI: 1.9–4.5 months), and the median overall survival (OS) was 11.6 months (95% CI: 9.0–14.1 months). Male gender was the only independent variable for poor PFS (HR = 1.673, 95% CI: 1.103–2.535), with poor performance status (HR = 2.454, 95% CI: 1.405–4.287) and history of smoking (HR = 1.856, 95% CI: 1.087–3.168) being independent adverse factors for OS. Thirteen of 55 tumor tissues (23.6%) showed TS expression; however, there were no significant correlations between TS expression and the clinicopathological factors.
Conclusion: Pemetrexed was suggested as a third- or fourth-line therapy due to its favorable efficacy and tolerable toxicity. Further studies are warranted to define the adequate sequence of salvage treatments, especially in patients with adenocarcinoma lung cancer.
© 2009 Elsevier Ireland Ltd. All rights reserved.
1. Introduction
The standard first-line therapy for patients with advanced non-small cell lung cancer (NSCLC) is platinum-based doublet com-bination chemotherapy, which offers a modest survival advantage
∗ Corresponding author. Tel.: +82 2 3410 3453; fax: +82 2 3410 1754. E-mail address:ajis@skku.edu(J.S. Ahn).
[1]. Unfortunately, all NSCLC patients eventually experience dis-ease progression and require salvage therapy. Recently, ASCO and NCCN guidelines recommend docetaxel, pemetrexed, or erlotinib as second-line therapies. Shepherd et al. showed that docetaxel was superior to the best supportive care (median survival: 7.0 months vs. 4.6 months, respectively)[2]. Hanna et al. compared pemetrexed with docetaxel, which showed equivalent outcomes (median sur-vival: 8.3 months vs. 7.9 months, respectively)[3]. In a BR.21 trial, erlotinib showed survival benefits when given as a second- or
third-0169-5002/$ – see front matter © 2009 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.lungcan.2009.12.002
line therapy, compared to a placebo (overall survival: 6.7 months vs. 4.7 months, respectively)[4]. A recent retrospective study demon-strated that docetaxel and EGFR (epidermal growth factor receptor) tyrosine kinase inhibitor (TKI) were reasonable therapeutic options as third-line therapy[5]. Currently, there is no standard chemother-apeutic regimen for third-line or beyond therapy in NSCLC patients. However, EGFR TKI is frequently used in East Asia as a second-line therapy owing to its efficacy. Thus, pemetrexed is more often administered as a third-line or beyond therapy in Korea.
Given its mild toxicity profile and promising anti-tumor activ-ity in previous trials, pemetrexed may be a good drug candidate for third-line or beyond therapy in NSCLC patients[6,7]. Peme-trexed, a multitargeted antifolate agent, inhibits at least three of the enzymes involved in DNA synthesis and folate metabolism: thymidylate synthase (TS), dihydrofolate reductase (DHFR) and glycinamide ribonucleotide formyl transferase (GARFT). Among them, TS is a key enzyme that catalyzes the methylation of fluoro-dUMP, the precursor of DNA synthesis, into dTMP [8]. Previous studies showed that higher TS levels were associated with poor prognosis in NSCLC patients [9,10], and could be a predictor of TS-inhibiting agents[11].
In view of these data, we retrospectively analyzed the efficacy and safety of pemetrexed as a salvage regimen in heavily pre-treated NSCLC patients. In addition, we investigated the correlation between TS expression in tumor tissues and the clinical efficacy of pemetrexed.
2. Materials and methods
2.1. Patients and data collection
One hundred and ten patients with NSCLC who received peme-trexed as third- or fourth-line therapy at Samsung Medical Center between June 2006 and June 2008 constituted the study cohort. The relationships between TS expression and the clinicopatho-logical factors were evaluated in available tissue specimens for additional immunohistochemical assays. Clinical data was retro-spectively reviewed from medical records. Pathologic diagnoses of NSCLC and immunostained sections were reviewed by one of the authors (J Han). The patients were followed up for a minimum of 7 months to a maximum of 30.8 months (median follow-up: 16.1 months). This study was approved by the Institutional Review Board of Samsung Medical Center.
2.2. Treatment
All patients received pemetrexed 500 mg/m2every 21 days. Oral
daily doses of folic acid (1000g) were given 1–2 weeks before the first dose of pemetrexed and for 3 weeks after therapy. Injections of vitamin B12 (1000g IM) were given 1–2 weeks before the first dose of pemetrexed and at 9-week intervals during treatment. Dex-amethasone (4 mg, oral, twice daily) was given the day before, the day of, and the day after each dose of pemetrexed therapy. 2.3. Treatment response
Appropriate imaging studies, including chest CT scans, were per-formed every two cycles (or sooner if needed) to evaluate treatment responses for documentation of disease progression. Responses were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria[12].
2.4. Immunohistochemistry
Tissue sections were deparaffinized in xylene and then rehy-drated in serially graded alcohol. TS antigen retrieval consisted of
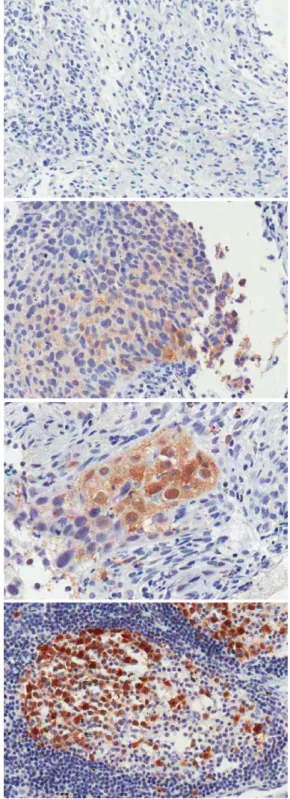
Fig. 1. Immunohistochemical staining for TS in tumor tissues.
microwave heating (three cycles of 5 min at 700 W) in 10 mM cit-rate buffer at pH 6.0 and cooling to room temperature for 20 min. After washing in distilled water, the slides were preincubated in 4% BSA and dextran solution for 30 min to reduce nonspecific binding. The slides were incubated 1 h at room temperature with mouse monoclonal anti-TS (Zymed Laboratories, CA) at a 1:100 dilution in a humidified chamber. After washing, the tissue sec-tion was observed with peroxidase-labeled polymer conjugated to goat anti-mouse immunoglobulins in Tris–HCl buffer (Envision plus System-HRP labeled polymer, Dako, Glostrup, Denmark) and
Table 1
Characteristics of patients.
Third line Fourth line p-Value Total
Total 65 45 110
Median age (years) 61 (24–84) 56 (39–75) 0.219 59 (24–84)
Gender 0.007 Male 26 (40%) 30 (67%) 56 (51%) Female 39 (60%) 15 (33%) 54 (49%) Disease status 0.868 Recurred 15 (23%) 11 (24%) 26 (24%) Advanced 50 (77%) 34 (76%) 84 (76%)
ECOG performance status 0.802
0–1 53 (82%) 36 (80%) 89 (81%)
2–3 12 (19%) 9 (20%) 21 (19%)
Smoking history 0.012
Never 53 (82%) 27 (60%) 80 (73%)
Former and current 10 (15%) 17 (38%) 27 (25%)
Unknown 2 (3%) 1 (2%) 3 (3%)
Histology 1.000
Non-squamous cell carcinoma 57 (88%) 39 (86.7) 96 (87%)
Squamous cell carcinoma 8 (12%) 6 (13.3) 14 (13%)
First-line therapy Gemcitabine-platinum 48 (74%) 27 (60%) 75 (68%) Other-platinum 8 (12%) 11 (24%) 19 (17%) TKIs 6 (9%) 5 (11%) 11 (10%) Others 3 (5%) 2 (4%) 5 (5%) Second-line therapy TKIs 52 (80%) 15 (33%) 67 (61%) Docetaxel 4 (6%) 15 (33%) 19 (17%) Others 9 (14%) 15 (33%) 24 (22%) Third-line therapy TKIs 25 (56%) Docetaxel 6 (13%) Others 14 (31%)
Best response to first-line therapy 1.000
PR-SD 44 (68%) 29 (65%) 73 (66%)
PD 20 (31%) 14 (31%) 34 (31%)
Unknown 1 (2%) 2 (4%) 3 (3%)
ECOG: Eastern Cooperative Oncology Group.
incubated for 30 min at room temperature. The slides were then washed and the chromogen was developed for 5 min with liq-uid 3,30-diaminbenzidine (Dako, Glostrup, Denmark). Finally, the sections were counterstained with Mayer hematoxylin. Negative controls were processed as above, without the primary antibody. To date, there are several commercially available anti-TS antibod-ies, but there are no validated scoring methods for interpreting the immunohistochemical staining. Colon cancer tissues were stained to serve as positive controls. Adjacent normal-appearing bronchial epithelium within each tissue section served as internal references. The intensity of the TS expression was scored on a scale of 0, 1+, 2+, 3+, where scores of 2+ and above were classified as high[10](Fig. 1).
2.5. Statistical analyses
Progression-free survival (PFS) was defined as the time from the first date of pemetrexed therapy to the date of documented progression or death from any cause. The overall survival (OS) after pemetrexed use was measured from the first date of pemetrexed therapy to the date of death or the last follow-up. The Kaplan–Meier product-limit method was used to estimate PFS and OS. Survival rates were compared using the log-rank test. Multivariate analysis of the independent prognostic factors for survival was performed using the Cox proportional hazard regression model with a 95% confidence interval (CI).
3. Results
3.1. Patient characteristics
The main clinical characteristics of the patients are shown in Table 1. The median age was 59 years (range: 24–84), of which 50.9% were men, and most patients (81.8%) had a good East-ern Cooperative Oncology Group (ECOG) performance status of 0–1. Twenty-seven patients (24.6%) were either current or pre-vious smokers. Sixty-five patients (59.1%) received pemetrexed as third-line therapy and 45 patients (40.9%) as fourth-line ther-apy. Ninety-five (86.4%) patients had non-squamous cell carcinoma (adenocarcinoma: N = 90, mucoepidermoid carcinoma: N = 1, undif-ferentiated carcinoma: N = 3, sarcomatoid carcinoma: N = 1) and 15 had squamous cell carcinoma. The most frequently administered
Table 2
Best responses to pemetrexed.
Best response Number of patients (%)
Third line Fourth line Total Partial response 9 (13.8) 9 (20.0) 18 (16.3) Stable disease 27 (41.5) 14 (31.1) 41 (37.3) Progressive disease 25 (38.5) 18 (40) 43 (39.1)
Disease control 36 (55.4) 23 (51.1) 59 (53.6) p = 0.770 Not evaluable 4 (6.2) 4 (8.9) 8 (7.3)
Table 3
Univariate and multivariate analyses for PFS and OS.
Feature PFS (Mo) Univariate (p) Multivariate (p, HR) OS (Mo) Univariate (p) Multivariate (p, HR)
All 3.22 11.57 Age (years) 0.162 0.815 ≤65 4.67 11.57 >65 2.56 12.95 Gender 0.014 0.015 0.215 Male 2.07 HR = 1.673 10.98 Female 3.65 (1.103–2.535) 11.90 ECOG 0.420 0.001 0.002 0–1 3.39 13.02 HR = 2.454 ≥2 1.35 6.67 (1.405–4.287) Smoking history 0.116 0.019 0.023 Has smoked 2.07 9.50 HR = 1.856 Never smoked 3.22 12.95 (1.087–3.168) Histologic type 0.050 0.064 Non-squamous 3.65 12.20 Squamous 1.78 6.51 Treatment line 0.979 0.213 Third 3.22 11.05 Fourth 3.19 13.02 Response to first-line CT 0.129 0.158 PR + SD 2.79 12.85 PD 3.19 9.14 Response to pemetrexed <0.001 <0.001 PR + SD (59) 6.08 15.45 PD (43) 1.25 6.67
first-line and second-line treatment regimens were platinum-based chemotherapy (85.5%) and EGFR-TKIs [erlotinib (17.3%) or gefitinib (43.6%)], respectively. The best responses to the first-line treatments were as follows: PR in 48 patients (44.8% of evaluable patients), SD in 25 patients (23.4%), and PD in 34 patients (31.8%). The median time from the date of diagnosis to the date of peme-trexed treatment was 12.8 months (range: 1.8–62.2 months). At the time of the pemetrexed treatment, 33 patients (30%) had treated, stable brain metastases.
3.2. Efficacy of pemetrexed
The median cycle of pemetrexed administration was 4 (range: 1–22). Nineteen patients (17.3%) received six cycles of pemetrexed. Of note, 26 patients (23.6%) received more than six cycles and 10 patients (9.1%) received 12–22 cycles. In this study cohort, the dis-ease control rate was 53.6% with 18 (16.3%) PRs, 41 (37.3%) SDs and 43 (39.1%) PDs (Table 2). The median follow-up duration was 16.1 months, the median PFS was 3.2 months (95% CI: 1.9–4.5 months) and the median OS was 11.6 months (95% CI: 9.0–14.1 months) (Fig. 2). At the time of final analysis, 68 patients (61.8%) were dead and eight patients were lost to follow-up.
3.3. Univariate and multivariate analyses
With univariate analysis, male gender (p = 0.014) and squamous cell type (p = 0.050) were associated with a shorter PFS. Poor perfor-mance status (p = 0.001) and a history of smoking (p = 0.019) were significant predictive factors for poor survival (Table 3andFig. 3). There was no difference in PFS (p = 0.979) or OS (p = 0.213) between third- and fourth-line therapies.
Multivariate analysis using the Cox regression model was performed for PFS and OS. Male gender was the only indepen-dent variable in the model correlated with a poor PFS (p = 0.015, HR = 1.67, 95% CI: 1.10–2.54). Prognostic variables for a shorter OS were poor performance status (p = 0.002, HR = 2.45, 95% CI:
1.41–4.29) and a history of smoking (p = 0.023, HR = 1.86, 95% CI: 1.09–3.17).
3.4. Toxicity
Only one treatment-related death was observed, with the cause of death being pneumonia. Two patients developed herpes zoster infections. Scheduled chemotherapy was delayed in only two patients due to grade 3 neutropenia and grade 3 generalized edema, respectively (Table 4).
3.5. TS expression
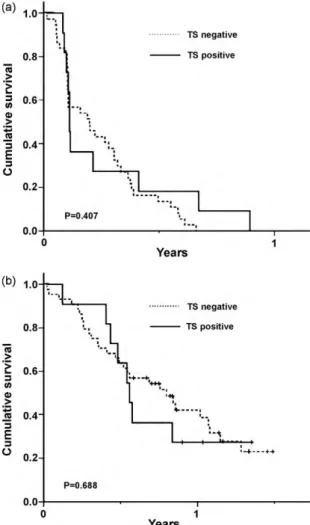
TS expression was observed in 13 of 55 available tumor tis-sues (23.6%). The staining intensity scores were as follows: none (0) in 42 (76.4%) cases, weak (+1) in two (3.6%) cases, moderate (+2) in seven (12.7%) cases and strong (+3) in four (7.3%) cases (Table 5). There were no significant differences in PFS (low TS vs.
high TS, 2.4 months, 95% CI: 0.7–4.0 months vs. 1.3 months, 95% CI: 0.8–3.0 months, respectively; p = 0.407) or OS (low TS vs. high TS, 9.5 months, 95% CI: 5.9–13.2 months vs. 6.7 months, 95% CI: 5.7–7.9 months, respectively, p = 0.688), according to TS expression levels (Fig. 4). In addition, there were no significant associations between TS expression and the clinical parameters such as age, gender, per-formance status, history of smoking, histologic type, treatment line and response rate.
4. Discussion
In this study, we evaluated the efficacy and safety of peme-trexed as a third- or fourth-line therapy in patients with advanced NSCLC. The disease control rate was 53.6% with 18 (16.3%) PRs
Fig. 3. (A) Comparison of PFS by gender, (B) comparison of OS by smoking history,
and (C) Comparison of OS by performance status.
Table 4
Toxicity profiles.
Third line (N = 65) Fourth line (N = 45) Gr 1/2 Gr 3 Gr 4/5 Gr 1/2 Gr 3 Gr 4/5 Neutropenia 0 1 0 1 0 0 Fatigue 16 0 0 7 0 0 Anorexia 10 0 0 4 0 0 Nausea 10 0 0 8 0 0 Mucositis 1 0 0 1 0 0 Constipation 1 0 0 1 0 0 Diarrhea 0 0 0 2 0 0 Pruritus 4 0 0 2 0 0 Rash 7 0 0 4 0 0 Facial edema 8 0 0 1 0 0 Edema 1 1 0 1 0 0 Neuropathy 3 0 0 2 0 0 Headache 6 0 0 1 0 0 Infectiona 0 2 1 0 0 0 General ache 1 0 0 4 0 0 Indigestion 1 0 0 3 0 0
aThe two patients (G3) had herpes zoster.
and 41 (37.3%) SDs. In addition, the median PFS and OS were 3.2 months and 11.6 months, respectively. The median PFS observed in this study was comparable to other reports in third-line set-tings[5,6,13]. However, the OS was longer in this study than those reported in previous studies[4,5,14]. One plausible explanation for such a favorable outcome may be attributed to a higher propor-tion of patients who had never smoked and of non-squamous cell
Fig. 4. (A) Comparison of PFS by TS expression and (B) Comparison of OS by TS
Table 5
TS expressions in tumor tissues.
Intensity of stained tumor cells Number (%) Responder (CR + PR + SD) Non-responder (PD) Not evaluable
Grade 0 42 (76.4) 21 18 3
Grade 1 2 (3.6) 2 0 0
Grade 2 7 (12.7) 3 4 0
Grade 3 4 (7.3) 1 3 0
types in our series[15]. Another contributing factor might be eth-nic differences, which have been considered a consistently good prognostic factor, as previously reported[16,17].
Intriguingly, although the fourth-line therapy group had more unfavorable factors (i.e., male, smoker) than the third-line group, there were no differences in response rates, PFSs and OSs. The over-all survival was 13.0 months for the fourth-line therapy group and 11.0 months for the third-line therapy group. Furthermore, con-sidering the toxicity profiles, most of the patients tolerated the treatment well, even in third or fourth-line therapy. Given that many patients were still in good performance status even after failure of the second- or third-line therapies and were willing to receive further anti-cancer treatment, pemetrexed could be rec-ommended as salvage therapy. Moreover, 20% of patients with poor performance status enrolled in this study also tolerated peme-trexed well.
A recent phase III study showed a significant improvement in survival with first-line pemetrexed and cisplatin in NSCLC patients with adenocarcinoma[16]. We also recently reported the excellent outcome of Korean adenocarcinoma patients in second-line ther-apy with pemetrexed[18]. Although adenocarcinoma was not a significant prognostic factor in this study, there was a trend favor-ing non-squamous histology. Median PFSs in the non-squamous and squamous histology group were 3.7 and 1.8 months, respec-tively (p = 0.05), and the median OSs were 12.2 and 6.5 months, respectively (p = 0.06). This result might be due to the small number of squamous histology (12%) samples in this cohort.
Based on currently available data, NSCLC patients presenting an increased TS expression appear to have poor prognoses, especially in squamous cell carcinoma types[11]. Conversely, expression of TS neither influenced the prognosis nor predicted the response to pemetrexed in our series. One plausible explanation for such a negative finding could be the small sample size utilized in this study. Furthermore, the heterogeneity of TS staining within the tumors and lack of a standardized scoring system in NSCLC may have contributed to our results. Several studies have reported var-ious percentages of patients with NSCLC – ranging from 29.6% to 72.5% – presenting TS positivity[10,11,19–22]. In our study, TS pos-itivity (20%) was lower than in other studies. Third, there may be a discrepancy between TS enzymatic activity and protein expression by immunohistochemistry, as previously reported[22]. Thus, RT-PCR should be considered as an alternative method to determine the level of TS expression instead of protein expression[23,24]. Fourth, the level of reduced folate carrier (RFC), as well as that of folate binding protein (FBP), may influence the uptake and the effectiveness of this drug. As pemetrexed is a prodrug, the activity of folylpolyglutamate synthetase, the enzyme that converts peme-trexed to polyglutamate forms, may be an important determinant of the response. Therefore, in order to elucidate the correlation between TS expression and treatment outcome, further biomarker analyses should be conducted in larger numbers of patients.
In conclusion, pemetrexed was suggested as a third- or fourth-line therapy due to its favorable efficacy and tolerable toxicity, especially in women, patients with good performance status or non-smokers. Given the frequent use of EGFR-TKIs as second-line therapy in Korean NSCLC patients, further studies are warranted to define the adequate sequence of salvage treatments, especially in patients with adenocarcinoma lung cancer.
Conflict of interest statement
None declared.
References
[1] Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Compar-ison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8.
[2] Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095–103.
[3] Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97.
[4] Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–32.
[5] Ng R, Loreto M, Lee R, Leighl NB. Brief report: retrospective review of efficacy of erlotinib or gefitinib compared to docetaxel as subsequent line therapy in advanced non-small cell lung cancer (NSCLC) following failure of platinum-based chemotherapy. Lung Cancer 2008;61:262–5.
[6] Melosky B, Agulnik J, Assi H. Retrospective practice review of treatment of metastatic non-small-cell lung cancer with second-line erlotinib. Curr Oncol 2008;15:279–85.
[7] Bearz A, Garassino I, Cavina R, Favaretto A, Boccalon M, Talamini R, et al. Pemetrexed single agent in previously treated non-small cell lung cancer: a multi-institutional observational study. Lung Cancer 2008;60:240–5. [8] Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, et al.
LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res 1997;57:1116–23.
[9] Huang CL, Yokomise H, Kobayashi S, Fukushima M, Hitomi S, Wada H. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehy-drogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol 2000;17:47–54.
[10] Nakagawa T, Tanaka F, Otake Y, Yanagihara K, Miyahara R, Matsuoka K, et al. Prognostic value of thymidylate synthase expression in patients with p-stage I adenocarcinoma of the lung. Lung Cancer 2002;35:165–70.
[11] Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006;107:1589–96. [12] Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al.
New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
[13] Sun JM, Lee KW, Kim JH, Kim YJ, Yoon HI, Lee JH, et al. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol 2009;39:27–32.
[14] Ailawadhi S, Derby L, Natarajan R, Fetterly G, Reid M, Ramnath N. Erlotinib for metastatic non-small-cell lung cancer: first-, second- or third-line setting – does it matter? A single-institution experience. Oncology 2009;76:85–90. [15] Itaya T, Yamaoto N, Ando M, Ebisawa M, Nakamura Y, Murakami H, et al.
Influ-ence of histological type, smoking history and chemotherapy on survival after first-line therapy in patients with advanced non-small cell lung cancer. Cancer Sci 2007;98:226–30.
[16] Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51.
[17] Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525–31.
[18] Lee HY, Ahn MJ, Park YH, Ahn JS, Kim BS, Kim HK, et al. Adenocarcinoma has an excellent outcome with pemetrexed treatment in Korean patients: a prospec-tive, multicenter trial. Lung Cancer 2009;66(3):338–43. Epub 2009 Mar 18. [19] Miyoshi T, Kondo K, Toba H, Yoshida M, Fujino H, Kenzaki K, et al. Predictive
value of thymidylate synthase and dihydropyrimidine dehydrogenase expres-sion in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur + uracil) in patients with non-small cell lung cancer. Anticancer Res 2007;27:2641–8.
[20] Huang C, Liu D, Masuya D, Nakashima T, Kameyama K, Ishikawa S, et al. Clinical application of biological markers for treatments of resectable non-small-cell lung cancers. Br J Cancer 2005;92:1231–9.
[21] Otake Y, Tanaka F, Yanagihara K, Hitomi S, Okabe H, Fukushima M, et al. Expres-sion of thymidylate synthase in human non-small cell lung cancer. Jpn J Cancer Res 1999;90:1248–53.
[22] Inoue K, Takao M, Watanabe F, Tarukawa T, Shimamoto A, Kaneda M, et al. Role of dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine against non-small cell lung cancer—in correlation with the tumoral expression of
thymidylate synthase and dihydropyrimidine dehydrogenase. Lung Cancer 2005;49:47–54.
[23] Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 2002;1:304–13.
[24] Hashimoto H, Ozeki Y, Sato M, Obara K, Matsutani N, Nakagishi Y, et al. Significance of thymidylate synthase gene expression level in patients with adenocarcinoma of the lung. Cancer 2006;106:1595–601.