Received October 10, 2003 Address for correspondence: YUH-SHAN HO
Citation review of Lagergren kinetic rate equation
on adsorption reactions
YUH-SHAN HO
a School of Public Health, Taipei Medical University, Taipei (Taiwan) b Bibliometric Centre, Taipei Medical University - Wan-Fang Hospital, Taipei (Taiwan)
This study presents a literature review concerning the preciseness of over 170 publications citing the original Lagergren’s paper in kinetics equation for solute adsorption on various adsorbents. This equation applies to a range of solid-liquid systems such as metal ions, dyestuffs and several organic substances in aqueous systems onto various adsorbents. The main objectives are to manifest different forms of citations presented and offers a correct reference style for citing the original Lagergren’s paper published in 1898.
Introduction
Adsorption kinetics depends on the adsorbate-adsorbent interaction and system condition and has been investigated for their suitability for application in water pollution control. Two vital evaluation elements for an adsorption process operation unit are the mechanism and the reaction rate. Solute uptake rate determines the residence time required for completing the adsorption reaction and can be enumerated from kinetic analysis. Numerous attempts were made in formulating a general expression to describe the kinetics of adsorption on solid surfaces for the liquid-solid adsorption system. In 1898, Lagergren presented the first order rate equation for the adsorption of ocalic acid and malonic acid onto charcoal (LAGERGREN, 1898).
Lagergren’s kinetics equation may have been the first one in describing the adsorption of liquid-solid systems based on solid capacity.
In order to distinguish kinetics equation based on sorption capacity of solid from concentration of solution, Lagergren’s first order rate equation has been called pseudo-first order (HO & MCKAY, 1998a, 1998b, 1998c, 1998d). Earlier known application of Lagergren’s kinetics equation to the adsorption was undertaken by TRIVEDI et al. (1973)
During the last three decades, the kinetics equation has also been reported for the adsorption of pollutants, such as metals, dyes and organims from aqueous solution.
This study presented a literature review of approximately 170 published papers citing Lagergren’s pseudo-first order kinetics equation in various styles.
Discussion
Lagergren’s kinetics equation has been most widely used for the adsorption of an adsorbate from an aqueous solution. Vast majority of the adsolutes in the adsorption systems from the articles studied were aqueous phase pollutants such as metal inos, dysstuffs, and contaminating organic compounds. At large, the adsorbents were activated carbon (ONGANER & TEMUR, 1998; KADIRVELU & NAMASIVAYAM, 2000;
DAI, 1994), materials of biological organic compounds (YAMUNA & NAMASIVAYAM, 1993; KANDAH, 2001), agricultural by-products such as banana pith (NAMASIVAYAM &
KANCHANA, 1992), palm-fruit bunch (NASSAR, 1997), coir pith (NAMASIVAYAM et al., 2001), cow dung (DAS et al., 2000), sago (QUEK et al., 1998), coconut husk (MANJU et
al., 1998), and orange peel (NAMASIVAYAM et al., 1996) and inorganic adsorbents such as fly ash (VIRARAGHAVAN & RAMAKRISHNA, 1999; PANDAY et al., 1985),
polyacrylamide grafted hydrous tin(IV) oxide gel (SHUBHA et al., 2001), Fe(III)/Cr(III) hydroxide (NAMASIVAYAM et al., 1994), chrome sludge (LEE et al., 1996), magnetite
(ORTIZ et al., 2001), kaolinite (ATUN & SISMANOGLU, 1996), and bituminous shale (TÜTEM et al., 1998).
Lagergren’s original paper expressed the pseudo-first order rate equation for the liquid-solid adsorption system in 1898 and was summarised as follows:
) (X x k dt
dx = (1)
X and x (mg g-1) are the adsorption capacities at equilibrium and at time t, respectively. k (min-1) is the rate constant of pseudo-first order adsorption.
Equation (1) was intergrated with boundary conditions t = 0 to t = t and x = 0 to x = x: kt x X X )= ln( (2) and
Equation (2) may be rearranged to the linear form:
(
X x)
( )
X k t 303 . 2 log log = . (4)The most popular form used is:
(
qm qt)
( )
qm k t 303 . 2 log log = , (5)qm and qt (mg g-1) are the adsorption capacities at equilibrium and at time t respectively.
k (min-1) is the rate constant of pseudo-first order adsorption.
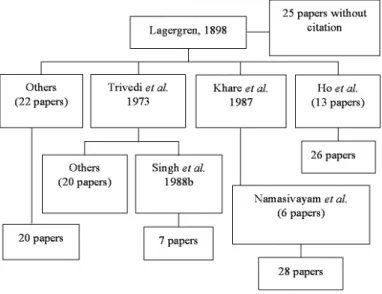
Figure 1 indicates how the equation was cited. However, all direct citations except from HO et al. have made numerous mistakes in representing the author, journal title,
year, volume or page number. It is recommended to cite directly from the original article but vast majority of the authors used so called second-handed references due to the difficulty of maintaining the original information. Therefore, they cited the desired references without realizing the citation mistakes.
The correct reference style citing the original Lagergren’s paper was first presented by HO and MCKAY in 1998 (HO & MCKAY, 1998a, 1998b, 1998c, 1998d). That was ‘Lagergren, S. (1898), Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar, Band 24, No. 4, 1-39.’ Its English translation was ‘Lagergren, S. (1898), About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens. Handlingar, Band 24, No. 4, 1-39.’ and the abbreviated version ‘Lagergren, S. (1898), Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakad. Handl., Band 24, No. 4, 1-39.’ This correct citing style, presented in Ho’s 13 papers since 1998, has been widely used by more than 20 other authors.
The paper published by TRIVEDI et al. (1973) has been the most applied style. In
their paper, the authors cited Lagergren’s original paper as follows, ‘S. Lagergren, Bil.K. Svenska Vetenskapsakad Handl. 24, (2), No. 4 (1898)’. Mistakes occurred in authors, journal name and the page number of the article was also missing. More than 20 papers cited Lagergren’s paper via TRIVEDI et al. reference style (PANDAY et al.,
1985; GUPTA et al., 1990; ONGANER & TEMUR, 1998; VIRARAGHAVAN & RAMAKRISHNA, 1999; KADIRVELU & NAMASIVAYAM, 2000).
KHARE et al. (1987) has also been widely referred. KHARE et al. (1987) reported that the adsorption of Victoria Blue by fly ash was diffusion controlled and the process followed the pseudo-first order adsorption rate expression of Lagergren. From their paper, the KHARE et al. cited Lagergren’s original paper as follows, ‘Lagergren. (1898)
Bil. K. Svenska Ventenskapakad. Handl. 24’. Mistakes again occurred in authors, journal name and the page number of the article was also missing. Seven papers cited Lagergren’s paper were from KHARE et al. (1987), such as NAMASIVAYAM & KANCHANA (1992); YAMUNA & NAMASIVAYAM (1993); NAMASIVAYAM et al. (1994);
and LEE et al. (1996).
SINGH et al. (1988b) reported that the sorption of As(III) by haematite followed the
pseudo-first order sorption rate expression of Lagergren. From the paper, SINGH et al. cited Lagergren’s paper from TRIVEDI et al. (1973). However, there were seven papers
cited Lagergren’s paper from SINGH et al. (1988b), for instance, PERIASAMY & NAMASIVAYAM (1994); NASSAR, (1997);ZHANG et al. (1998); and KANDAH (2001).
The pseudo-first order rate equation of Lagergren has also been widely discussed in many sorption systems by India researcher Namasivayam. Namasivayam’s style of reference has been utilized by more than 20 papers citing Lagergren’s paper, for
More than 20 papers (DAI, 1994; ATUN & SISMANOGLU, 1996; QUEK et al., 1998;
TÜTEM et al., 1998; MANJU et al., 1998; DAS et al., 2000) showed other citing styles for referring the pseudo-first order rate equation of Lagergren, but the citation of these papers is not as frequent as compared to the papers mentioned above. In addition, more than 20 papers have discussed the pseudo-first order rate equation of Lagergren in their publications without citing Lagergren’s paper.
Besides the discussion made above, there was another false phenomenum that can be found in some papers. Researchers who cited the reference without actual discussion on pseudo-first order equation of Lagergren such as: SINGH et al. (1988a); MARTIN &
HOLDICH (1986); and SANKAR et al. (1999).
Conclusion
All of the examined references from the literature review in this study were incorrect for referencing Lagergren’s equation. Research papers conventionally include an introduction, a description of the objectives and procedures of the study, an account of the results and a discussion of the results and their implications. These sections were thought as the core of a paper and always the focus of an author while writing. However, a paper’s contribution existed not only in its originality and creativity, but also in its continuity and development for the following researches. The reference section can play a key role to researchers that were interested in the paper’s statement and would like to follow the study or find useful information from the paper. Although this section was as important as the core of a paper, it was easily to be ignored by the author.
This paper offered a correct reference style for citing the original Lagergren’s paper. It also suggested an important idea that author must not only be creative but also careful while writing, in order to publish more valuable and worth of reading papers.
Nomenclature
X adsorption capacity at equilibrium (mg g–1);
x adsorption capacity at time t (mg g–1);
k rate constant of pseudo-first order adsorption (min–1);
t reaction time (min);
qm adsorption capacity at equilibrium (mg g–1);
References
ATUN, G., SISMANOGLU, T. (1996), Adsorption of 4,4'-iso propylidene diphenol and diphenylolpropane 4,4'dioxyaceticacid from aqueous solution on kaolinite, Journal of Environmental Science and Health Part A-Environmental Science and Engineering & Toxic and Hazardous Substance Control, 31 : 2055–2069.
DAI, M. G. (1994), The effect of zeta-potential of activated carbon on the adsorption of dyes from aqueous-solution. 1. The adsorption of cationic dyes: Methyl Green and Methyl Violet, Journal of Colloid and Interface Science, 164 : 223–228.
DAS,D.D., MAHAPATRA,R., PRADHAN,J., DAS,S.N.,THAKUR,R.S. (2000), Removal of Cr(VI) from aqueous solution using activated cow dung carbon, Journal of Colloid and Interface Science, 232 : 235–240.
GALIATSATOU, P.,METAXAS, M.,KASSELOURI-RIGOPOULOU, V. (2002), Adsorption of zinc by activated carbons prepared from solvent extracted olive pulp, Journal of Hazardous Materials, 91 : 187–203. GUPTA,G.S., PRASAD,G.,SINGH,V. N. (1990), Removal of chrome dye from aqueous solutions by mixed
adsorbents: Fly ash and coal, Water Research, 24 : 45–50.
HAMADI,N.K., CHEN,X.D., FARID,M.M.,LU,M.G.Q.(2001), Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust, Chemical Engineering Journal, 81 : 95–105.
HO, Y. S., MCKAY, G. (1998a), A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents, Process Safety and Environmental Protection, 76B : 332–340.
HO, Y. S., MCKAY, G. (1998b), Kinetic models for the sorption of dye from aqueous solution by wood, Process Safety and Environmental Protection, 76B : 183–191.
HO, Y. S., MCKAY, G. (1998c), Sorption of dye from aqueous solution by peat, Chemical Engineering Journal, 70 : 115–124.
HO, Y. S., MCKAY, G. (1998d), The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat, Canadian Journal of Chemical Engineering, 76 : 822–827.
KADIRVELU,K., NAMASIVAYAM, C. (2000), Agricultural by-product as metal adsorbent: Sorption of lead(II) from aqueous solution onto coirpith carbon, Environmental Technology, 21 : 1091–1097.
KANDAH, M. (2001), Zinc adsorption from aqueous solutions using disposal sheep manure waste (SMW), Chemical Engineering Journal, 84 : 543–549.
KHARE, S. K., PANDAY, K. K., SRIVASTAVA, R. M.,SINGH, V. N. (1987), Removal of Victoria Blue from aqueous solution by fly ash, Journal of Chemical Technology and Biotechnology, 38 : 99–104.
LAGERGREN, S. (1898), Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar, 24 (4) : 1–39.
LEE, C. K., LOW, K. S., CHOW, S. W. (1996), Chrome sludge as an adsorbent for colour removal, Environmental Technology, 17 : 1023–1028.
MANJU, G. N., RAJI, C.,ANIRUDHAN, T. S. (1998), Evaluation of coconut husk carbon for the removal of arsenic from water, Water Research, 32 : 3062–3070.
MARTIN,T.R.,HOLDICH,D.M. (1986), The acute lethal toxicity of heavy-metals to peracarid crustaceans (with particular reference to fresh-water asellids and gammarids). Water Research, 20 : 1137–1147. NAMASIVAYAM, C., KANCHANA, N. (1992), Waste banana pith as adsorbent for color removal from
wastewaters, Chemosphere, 25 : 1691–1705.
NAMASIVAYAM,C., KUMAR,M.D., SELVI,K., BEGUM,R.A., VANATHI,T.,YAMUNA,R.T. (2001), 'Waste' coir pith: A potential biomass for the treatment of dyeing wastewaters, Biomass & Bioenergy, 21 : 477–483.
NAMASIVAYAM, C., MUNIASAMY, N., GAYATRI, K., RANI, M.,RANGANATHAN, K. (1996), Removal of dyes from aqueous solutions by cellulosic waste orange peel, Bioresource Technology, 57 : 37–43.
NASSAR, M. M. (1997), The kinetics of basic dye removal using palm-fruit bunch, Adsorption Science & Technology, 15 : 609–617.
ONGANER,Y.,TEMUR,Ç. (1998), Adsorption dynamics of Fe(III) from aqueous solutions onto activated carbon, Journal of Colloid and Interface Science, 205 : 241–244.
ORTIZ,N., PIRES, M.A. F., BRESSIANI, J.C.(2001), Use of steel converter slag as nickel adsorber to wastewater treatment. Waste Management, 21 : 631–635.
PANDAY,K.K., PRASAD,G.,SINGH,V.N. (1985), Copper(II) removal from aqueous solution by fly ash, Water Research, 19 : 869–873.
PERIASAMY, K.,NAMASIVAYAM, C. (1994), Process development for removal and recovery of cadmium from wastewater by a low-cost adsorbent: Adsorption rates and equilibrium studies, Industrial & Engineering Chemistry Research, 33 : 317–320.
QUEK, S. Y., WASE, D. A. J.,FORSTER, C. F. (1998), The use of sago waste for the sorption of lead and copper, Water SA, 24 : 251–256.
SANKAR, M., SEKARAN, G., SADULLA, S.,RAMASAMI, T. (1999), Removal of diazo and triphenylmethane dyes from aqueous solutions through an adsorption process. Journal of Chemical Technology and Biotechnology, 74 : 337–344.
SHUBHA,K.P., RAJI,C.,ANIRUDHAN,T.S.(2001), Immobilization of heavy metals from aqueous solutions using polyacrylamide grafted hydrous tin(IV) oxide gel having carboxylate functional groups, Water Research, 35 : 300–310.
SINGH, A. K., SINGH, D. P., PANDAY, K. K., SINGH, V. N. (1988a) Wollastonite as adsorbent for removal of Fe(II) from water. Journal of Chemical Technology and Biotechnology, 42 : 39–49.
SINGH, D. B., PRASAD, G., RUPAINWAR, D. C.,SINGH, V. N. (1988b), As(III) removal from aqueous solution by adsorption, Water Air and Soil Pollution, 42 : 373–386.
TRIVEDI, H. C., PATEL, V. M., PATEL, R. D. (1973), Adsorption of cellulose triacetate on Calcium silicate, European Polymer Journal, 9 : 525–531.
TÜTEM,E., APAK,R.,ÜNAL, Ç.F. (1998), Adsorptive removal of chlorophenols from water by bituminous shale, Water Research, 32 : 2315–2324.
VIRARAGHAVAN,T.,RAMAKRISHNA,K.R. (1999), Fly ash for colour removal from synthetic dye solutions, Water Quality Research Journal of Canada, 34 : 505–517.
YAMUNA, R. T., NAMASIVAYAM, C. (1993), Color removal from aqueous solution by biogas residual slurry, Toxicological and Environmental Chemistry, 38 : 131–143.
ZHANG, L., ZHAO, L., YU, Y. T.,CHEN, C. Z. (1998), Removal of lead from aqueous solution by non-living Rhizopus nigricans, Water Research, 32 : 1437–1444.