Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health
全文
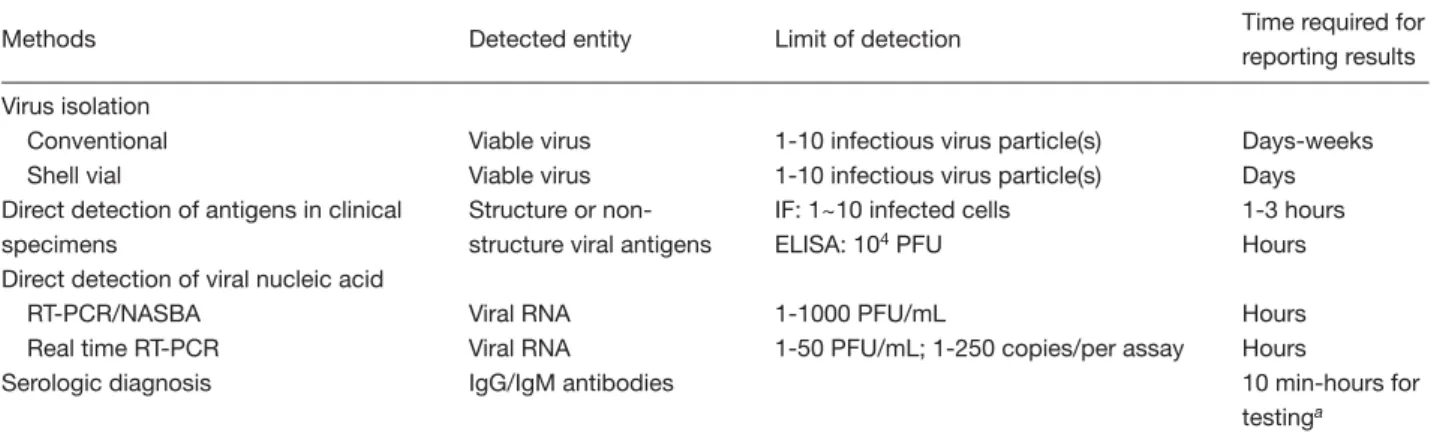
(2) Laboratory diagnosis of dengue virus infections. RNA, proteins, and antibodies will be very helpful in clarifying the epidemiology patterns of DENV infection, DF and DHF/DSS. The incubation period of DENV infection varies from 3 to 14 days (average, 4 to 7 days). Clinical laboratory findings associated with DF include a neutropenia followed by a lymphocytosis, often marked by atypical lymphocytes and possibly elevated level of aspartate aminotransferase in the serum [2]. Viremia generally lasts from 4 to 5 days. The circulating viable DENV particles remain readily detectable in the blood for up to 5 days after the onset of symptoms, and then rapidly disappear following the appearance of DENVspecific antibody. DENV-specific immunoglobulin M (IgM) antibody becomes positive on the sixth day following the onset of illness in most patients. During primary DENV infection, the IgG antibody appears a few days following IgM antibody. In secondary DENV infection, an anamnestic IgG response occurs, causing serotiter to rapidly increase initially, almost immediately following the onset of disease, and then remain high in most patients [8,9]. Therefore, laboratory diagnosis (Table 1) must consider the timing of the clinical course of dengue patients, as well as likely quantitative patterns of the tested parameters, and the advantages and limitations of each method, as discussed in detail below.. Virus Isolation and Identification Virus isolation is widely used as a gold standard for diagnosing DENV infection. Serotypes of DENV are easily determined using immunofluorescence (IF) staining in infected cells with serotype-specific. monoclonal antibody [10,11]. The isolated virus strains are important for follow-up virological study and detailed biological characterizations. The viremia appears during the onset of fever, and the rate of virus isolation is significantly improved in those samples collected within 6 days after the onset of disease [8,9]. Although the most sensitive virus isolation method is in vivo amplification through mosquito inoculation, the mosquito-derived cell cultures, such as C6/36 (Aedes albopictus), AP61 (Aedes pseudoscutellaris) and TRA284 (Toxorhynchites amboinensis), are widely used in clinical laboratories [8]. The diluted serum sample is inoculated on to the cell culture monolayer on screw cap tubes, dishes or flasks. Following incubation at 28°C for 1 week, the inoculated cells are screened for DENV by use of IF stain with 4 different serotype-specific monoclonal antibodies [10,11]. The disadvantages of this method are the time-consuming procedures required to obtain the culture results, and the need for cell culture facilities and experienced laboratory professionals. Reducing the time required for final culture results is a high priority in laboratory diagnosis. Roche et al used rapid centrifugation assay to isolate DENV-2 [12]. The specimen was inoculated on to monolayers of cells grown in 24 plastic wells. The inoculated cells were centrifuged for 1 h at 1000 × g at 33°C. After adding the maintenance medium, the inoculated cells were observed daily for cytopathic effect (CPE). Once CPE was detected, or after incubation for 11 days (in those cases without CPE) to increase the detection sensitivity, the DENV was identified by IF stain. This rapid centrifugation allowed the isolation of virus as early as 2 days post-inoculation, and achieved a 16.6%. Table 1. Methods used for the laboratory diagnosis of dengue virus infections Methods Virus isolation Conventional Shell vial Direct detection of antigens in clinical specimens Direct detection of viral nucleic acid RT-PCR/NASBA Real time RT-PCR Serologic diagnosis. Detected entity. Limit of detection. Time required for reporting results. Viable virus Viable virus Structure or nonstructure viral antigens. 1-10 infectious virus particle(s) 1-10 infectious virus particle(s) IF: 1~10 infected cells ELISA: 104 PFU. Days-weeks Days 1-3 hours Hours. Viral RNA Viral RNA IgG/IgM antibodies. 1-1000 PFU/mL 1-50 PFU/mL; 1-250 copies/per assay. Hours Hours 10 min-hours for testinga. Abbreviations: RT-PCR = reverse transcriptase-polymerase chain reaction; NASBA = nucleic acid sequence-based amplification; IgG = immunoglobulin G; IgM = immunoglobulin M; IF = immunofluorescence; ELISA = enzyme-linked immunosorbent assay; PFU = plaque-forming units aIf paired sera are required for comparison, 2-3 weeks are needed to obtain the final results.. 6.
(3) Kao et al. higher success rate in isolating the virus compared with conventional tube methods [12]. By using the protein A-gold-silver staining method to detect DENV antigens in cultured C6/36 mosquito cells, the virus antigen appeared at 2 to 3 days post-inoculation, significantly earlier than for the traditional direct IF stain method [13]. To improve the cell culture method used for public health surveillance of DENV activities in Taiwan, Kao et al initially used flow cytometry assay and monoclonal antibodies (MAb 16-4) against non-structure protein 1 (NS1) after the amplification of the tested virus preparation in the C6/36 mosquito-infected cell culture, and detected the antigen of DENV-1 as much as 10 h earlier than conventional IF stain [14]. It was also found that MAb 16-4 to NS1 was much more sensitive than MAb 8-8 to envelope protein in detection of the virus by using similar technique. When this method was applied to clinical samples, MAb 16-4 against the NS1 could detect 7 of 12 conventional virus isolation positive samples even at 3 days postinoculation. Therefore, the selection of the most effective monoclonal antibodies against DENV integrated with flow cytometry can be used for rapid laboratory diagnosis and pathogenesis studies in patients with different levels of clinical severity. Additionally, using reverse transcriptase-polymerase chain reaction (RT-PCR) following conventional inoculation of C6/36 cells with tested clinical samples can detect DENV-1 at 3 days earlier than the IF stain method [15].. Detection of Virus Antigen The presence of DENV antigen in acute sera and peripheral blood mononuclear cells (PBMC) from DENV-infected patients has been determined using biotin-streptavidine enzyme-linked immunosorbent assay (ELISA) [16]. The PBMC of dengue patients generally displayed a higher detection rate of DENV than that in serum samples (53.8 vs 18.9%, p<0.001). The DENV antigens were detectable in both serum and PBMC collected between days 2 and 7 after disease onset, with the detection rate being highest in PBMC collected on day 4 and in serum samples collected on day 5. Besides using PBMC, an amplified fluorogenic ELISA (F-ELISA) was developed for increasing the sensitivity to detect DENV-3 antigen in serum samples [17]. This assay utilized biotinylated hyperimmune mouse IgG antibody directed against dengue antigens. as a detector and also employed DENV-3 monoclonal antibody as the captured antibody. After the reaction of antigen and antibody, methylumbelliferyl-β-Dgalactopyranoside was used as the fluorogenic substrate for generating fluorescence. The fluorescence counts were quantitated using a fluorometer. The high affinity of the biotin for the multivalent binding sites of streptavidine-labelled β-galactosidase and the combined amplification effect of enzyme-fluorogenic substrate interactions increase the sensitivity of fluorogenic detection methods. By comparing with virus isolation, the sensitivity and specificity of F-ELISA were 90% and 99%, respectively, and there was 98% agreement between this method and virus isolation. The disadvantages of this test include the availability of anti-dengue monoclonal antibodies with high affinity and high specificity. For pathological studies, the immunohistochemical detection of DENV in the biopsies or autopsy tissues provides very useful evidence confirming dengue viral etiology. Various techniques, such as immunoperoxidase staining with dengue-specific monoclonal antibody or serotype-specific polyclonal antibody, have been devised for detecting DENV antigens in frozen and fixed tissues from autopsy and necropsy specimens [18-21]. Recently, the high circulating levels of DENV-1 and DENV-2 NS1 in the acute phase of disease were demonstrated by use of the ELISA method [22,23]. Levels of free NS1 in plasma correlated more closely with higher viremia in DHF patients than in DF patients [23]. Thus, early detection of NS1 might be very useful in case management of DHF patients and future studies on the correlation of NS1 in viral pathogenesis of DHF.. Detection of Virus Nucleic Acid Molecular diagnosis typically provides more sensitive and rapid detection than traditional virus isolation methods, because it amplifies nucleic acid even for inactivated virus. However, the specimens and RNA must be carefully handled to avoid RNA degradation. Nested RT-PCR, using the primers at different regions of the dengue viral genome, has been developed for detecting and typing DENV in clinical samples [24]. Two sets of primers corresponding to the C/prM region of the virus, designed by Lanciotti et al, are frequently used to identify DENV and its serotypes via 2-step nested RT-PCR, including the D1 (nt 134-161) and D2 (nt 616-644) primers used in the first run of RT-PCR for rapidly identifying dengue cases, and 7.
(4) Laboratory diagnosis of dengue virus infections. 4 serotype-specific primers [TS1 (nt 568-586), TS2 (nt 232-252), TS3 (nt 400-421) and TS4 (nt 506-527)] used in the second run of RT-PCR for serotyping [25]. The potential diagnostic usefulness of RT-PCR assay is to detect DENV in human serum. This assay demonstrates sensitivities of 94% with DENV-1, 93% with DENV-2 and 100% with DENV-3 and DENV-4, compared with the traditional method of virus isolation [25]. The disadvantages of the assay by Lanciotti et al include: (1) 100 copies of viral genome equivalents and 2 steps of RT-PCR are required to increase the detection sensitivity; (2) false-negative results occurred in DENV1 and DENV-2; and (3) ease of cross-contamination can lead to false-positive results, which is a general problem associated with RT-PCR [25]. In order to reduce the rates of false negatives in molecular diagnosis and remedy the inefficient subsequent public health alarming impact on dengue control activity due to frequent virus mutation in RNA viruses, possible solutions include selecting primers of the “conserved regions” of the viral genome from the gene bank, and use of degenerate primers or modified primers according the emerged mutant virus strain sequence [26]. To minimize contamination and maximize costeffectiveness, Harris et al established a modified singletube multiplex RT-PCR [27]. The detection limits of Harris’ protocol were around 1 plaque-forming unit (PFU) for DENV-1, 50 PFU for DENV-2, 1 PFU for DENV-3, and 30 PFU for DENV-4. De Paula et al also showed that 1-tube RT-PCR protocols obtained a higher rate of DENV detection than the 2-step methods, and these data correlated well with serologic diagnosis [28]. In fact, 2-step RT-PCR is extremely useful for detecting and amplifying multiple messages simultaneously from a single RNA sample via a single RT reaction. Such a method is extremely useful in conjunction with random hexamer in the first RT reaction and amplifying different regions of viral RNA in PCR to avoid misdiagnosis due to mutation of viral RNA. However, 1-tube RT-PCR is easier to use when processing large numbers of samples, and helps to minimize carry-over contamination resulting from unopened tubes between cDNA synthesis and amplification. The drawback of 1-tube reaction is amplification of only a single target per reaction. The selected primer sets and viral regions, the amount of viral RNA available, the numbers of messages to be amplified, the sample size, the enzymes for reverse transcription and cost are all critical determinants of whether the 1-tube or 2-step methods should be used. 8. To avoid thermal cycling, the possibility of crosscontamination and reduce the time to results, a singlestep isothermal RNA-specific amplification method, known as “nucleic acid sequence-based amplification (NASBA)”, was developed for DENV detection [29]. This method uses an isothermal enzyme that requires only a water bath to control the reaction temperatures, without requiring the need for an expensive thermal cycler. The NASBA product is RNA, which is far less stable than DNA (PCR product) but is less likely to serve as a contaminant, and is thus an advantage of NASBA. However, its current signal detection still requires an electrochemiluminescence (ECL) reader. The NASBA assay could detect dengue viral RNA in clinical samples at plaque titers below 25 PFU/mL. Using normal human plasma spiked with DENV, the NASBA assay had a detection threshold of 1 to 10 PFU/mL. The sensitivity of RT-PCR methods with different designs for detecting DENV varies [30]. It is difficult to generalize which molecular detection test is better, since primers, enzymes, buffers, reaction conditions, targets of viral genomic regions, and machines used for PCR reaction can all influence the results of PCR. In numerous circumstances, these comparisons depend on primarily empiric data. Future development should consider using multiplex PCR and/or genotyping/ serotyping methods to simultaneously obtain all important virological information. Quantification of DENV RNA in human plasma samples can provide more clues for performing pathogenesis studies and monitoring the progress of clinical manifestations. Quantitative competitive RT-PCR at C-prM or 3'-non-coding region has been developed for determining dengue viral load, and has sensitivity of 10 to 250 RNA copies [31,32]. Fluorogenic RT-PCR based on 3'-noncoding sequence provided an alternative quantitative method for measuring DENV RNA, and had a detection limit of 20-50 PFU/mL [33, 34]. Real-time RT-PCR assay represents another choice for quantifying DENV RNA. PCR products were detected in real time on a Light Cycler instrument with 5'-nuclease technology with detection limits ranging from 8.6 to 16 RNA copies per assay [35]. Compared with TaqMan probe assay, SYBR green I-based realtime RT-PCR is inexpensive, easy to use, and was developed for detecting 4 serotypes of dengue viruses with low detection limits ranging from 4.1-10 PFU/mL [36]. However, the methods have almost similar detection limits. The disadvantages of SYBR green I.
(5) Kao et al. include: it binds to any double-stranded DNA in the reaction, including primer-dimers and other nonspecific products, causing overestimation of the target concentration. Therefore, the method of SYBR green I for RNA detection requires extensive optimization of the primers employed. On the other hand, the hybridization fluoro/quench probe Taqman method requires separate probes for each mRNA target and these probes are expensive to synthesize. Recently, real-time PCR assay, measuring positive as well as negative sense RNA, was also used for detecting replicating DENV in PBMC from dengue patients, a good indicator showing that the virus in the infected cells had not been cleared by the cell-mediated immunity [37]. Remaining problems in molecular diagnosis of viral RNA include the lack of commercially available reagent kits and the lack of standardization of the existing tests.. Detection of Anti-dengue Virus Antibody Historically, hemagglutination inhibition (HI), complement fixation test (CF) and neutralization tests were used extensively for measuring anti-DENV antibodies [38,39]. The HI test, which is sensitive and reproducible, possesses the advantages of using reagents that can be prepared locally and being applicable to differentiate primary and secondary infection based on HI serotiters. Three main disadvantages of the HI test have limited its wide application. First, tested serum samples must be pretreated with acetone or kaolin, to remove nonspecific inhibitors of hemagglutination, and then must be absorbed using gander or type O human red blood cells, to remove nonspecific agglutinins. Second, the accurate HI test requires paired (acute and convalescent) serum samples. Four-fold or greater changes in HI titers between paired serum samples are considered as confirmed “recent” infections. Third, difficulty in discrimination between the DENV infections from other closely related flaviviruses [e.g., Japanese encephalitis virus (JEV) or West Nile virus (WNV)] limits the applicability of the HI test to those geographical areas in which other flaviviruses besides DENV are also prevalent. Such a problem of cross-reactivity also extends to serotyping DENV for understanding past infections of the tested individual. To minimize the disadvantages of the HI test, the plaque reduction neutralization test (PRNT) is mainly used to determine the serotype of DENV with which the patients are infected [8,40]. In this test, dilutions of. 56°C heat-inactivated tested human serum are incubated with defined amounts of virus, which is required to show sufficient plaques in the tested system. The endpoint of the titration is the highest dilution of serum required to reduce the number of plaques by 50-90%, depending on the preference of the authors. The initial emphasis on original antigenic sin and the experiments to support the hypothesis on the “primary infection” of DENV frequently used 90% plaque reduction for PRNT [41]. The PRNT data supporting the hypothesis on secondary infection and experiments of antibodydependent enhancement were likely obtained using 50-70% reduction cut-offs for reading the positive reaction [42]. A 4-fold or greater increase in titers between the acute and convalescent samples indicates a current infection. PRNT serotyping is especially useful in primary DENV infection, because the laboratory results generally exhibit a monotypic reaction to the infecting virus. In secondary DENV infections, high-titer neutralizing antibody is produced against at least 2 types of DENV and is generally cross-reacted with all 4 serotypes of DENV, and other flaviviruses. The disadvantages of PRNT include the time-consuming titration of DENV and the careful handling of cell culture techniques required in each step [43]. An ELISA-format microneutralization test for dengue viruses possesses the advantages of ease of performance and reading results, low cost, and increased test efficiency [44]. This new method provided results that were essentially the same as those from PRNT for samples from primary DENV infection, but the measurement results correlated poorly with the samples from patients with secondary DENV infection. Unlike traditionally complicated HI and PRNT tests, a simple and rapid ELISA method has been adapted to detect antibodies against DENV [45-51]. Using this method, a single medical technologist or public health laboratory individual can screen numerous serum samples per day. Moreover, ELISA requires no troublesome serum pretreatment, and a few microliters of sample are sufficient for a test. Several formats of ELISA are designed for detecting DENV antibodies. Classical indirect ELISA and immunoglobulin antibody capture ELISA are the 2 most common formats. Indirect ELISA uses the viral antigens to coat the microtiter plates. Following serial incubation with patient serum and enzyme conjugated anti-human immunoglobulin, the chromogen substrate is added during the final step for color development. The color is then read using a spectrophotometer. The first target layer of 9.
(6) Laboratory diagnosis of dengue virus infections. this indirect ELISA can employ different antigen preparations, including infected mosquito cells grown on the microtiter plate [46], coated monoclonal antibody to capture virus antigen [47], infected cell culture supernatant [48,49], and polyethylene glycolconcentrated DENV antigen [45]. Future evaluation of different methods to prepare viral antigen in order to achieve better sensitivity and specificity is needed. Owing to the difficulty in preparing uniform infected cells, complexity in processing the infected cells and cost considerations, use of amplified DENV in mosquito cells in microwells for antigen preparation is not a practical ELISA method in epidemiological investigation and clinical use. Therefore, monoclonal antibody capture antigens or concentrated antigens are frequently used in dengue ELISA. However, the monoclonal antibody capture antigen method needs the selection of high quality monoclonal antibodies and optimization of the reaction protocol. Methods for concentrating DENV also require extensive evaluation. Recently, anti-human IgM capture ELISA (MACELISA) and anti-human IgG ELISA (GAC-ELISA) were developed and widely used for detecting human anti-dengue IgM and IgG antibodies. In this technique, anti-human IgM and anti-human IgG are coated onto different wells of microtiter plates. The viral antibody, virus antigen and secondary antibody conjugated with enzyme are then added and incubated sequentially. The developed color solution is measured using a spectrophotometer. Mouse brain-derived virus hemagglutinin antigens [50] or cell culture-derived virus antigen [51] served as the virus antigen in the IgM and IgG capture ELISA tests, and the sensitivity and specificity did not differ significantly between these 2 antigen preparations [51]. Other simple and quick modifications of ELISA have been developed, marketed, and assessed, including the immunochromatographic (IC) method (PanBio, Australia), dipstick enzyme immunoassay (INDX Integrated Diagnostics, Baltimore, MD, USA), and dot blot assay (Genelabs Diagnostics, Singapore) for the detection of anti-dengue antibodies. Evaluation study based on a panel of 132 serum samples from suspected dengue patients and control patients showed that the sensitivity of these dengue antibody assays varied between 96 and 100% for IgM and between 52 and 97% for IgG, whereas the specificity varied from 86 to 92% and 95-100% for IgM and IgG, respectively [52]. The IC method, which takes less than 10 min and is highly convenient for bedside diagnosis and field 10. epidemiology, showed the lowest sensitivity for dengue IgG detection (52%) among immunoassays. However, the sensitivity and specificity of this method of dengue IgM detection need further evaluation with regard to primary versus secondary infections. Besides rapid test kits, commercialized ELISA kits for measuring dengue antibody are also available, and have variable sensitivities and specificities [52-59]. Strengths of the ELISA test include: ease of performance and user friendliness, provision of evidence of recent dengue infection by IgM-ELISA, and ability to distinguish primary versus secondary DENV infection by comparing the ratio of optical density readings of dengue-IgM to dengue-IgG. However, the ELISA test also had 2 major problems. First, the presence of rheumatoid factor in patient serum may influence the specificity of the dengue IgM test [60]. Second, ELISA using whole virus antigens for the detection of dengue-specific antibody still has certain cross-reactivity with all 4 serotypes of dengue viruses, particularly in experiencing secondary, tertiary, and quaternary dengue viral infections. Purified serotype-specific recombinant proteins expressed in Escherichia coli (11~14 kDa), corresponding to the B domains of DENV glycoprotein E, were successfully used for detecting DENV serotypespecific antibody with immunoblot strip assay [61]. Serotype-specific epitopes of DENV were also identified recently, using phage displayed peptide library. The serotype-specific DENV antibody can be clearly distinguished using phage-based synthetic peptides [62,63]. An alternative design using NS1 serotypespecific IgG ELISA has been developed and used to determine serotype-specific antibody [64]. Recently developed DENV envelope/membrane and NS1 serotype-specific capture IgM ELISA were successful (detection rate, 86.1 and 83.3%, respectively) for DENV serotyping in primary infections [65]. Dengue antibodies can be detected not only in serum using the traditional method, but also in other body fluids such as saliva and cerebrospinal fluid (CSF) [66,67]. The sensitivity and specificity of dengue-IgM detection in saliva correlated closely with IgM detection in serum samples. Saliva-based dengue-IgM assay should be especially useful for large-scale epidemiologic studies and for diagnosing acute disease in situations when blood collection is difficult due to cultural factors or difficult venous access, particularly in very young children. The dengue IgM titer in CSF was invariably lower than that in serum, causing low detection of dengue.
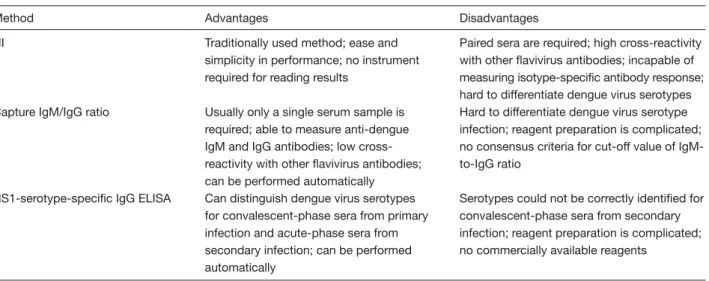
(7) Kao et al. IgM in CSF. To date, the detection of dengue IgM in CSF has not been a reliable method for diagnosing dengue with central nervous system involvement. However, the finding of dengue in CSF implies its involvement in disease pathogenesis in the central nervous system [68]. Numerous techniques have been developed for serodiagnosis of dengue, and variations in parameters and conditions are main concerns in quality assurance of laboratory diagnosis. The parameters used to compare different serologic tests (sensitivity, specificity, predictive values), and how those specimens are selected, including using tested samples and carefully selected positive and negative controls are the most important factors in method validation [54,69].. Differentiation of Primary Versus Secondary Infection The pathogenesis of DHF and DSS remains controversial. Two main theories are generally proposed. The secondary infection or immune enhancement hypothesis has been the most commonly accepted hypothesis to date [70]. The other hypothesis regarding primary infection emphasizes virologic factors involved in the pathogenesis of dengue infection [71]. Dengue viruses might change due to selection pressures and dynamic evolution as they undergo cross-species replication in alternating human and mosquitoes, thus increasing the number of DHF cases as an epidemic progresses in the same epidemic center [72]. Certain immune-selected virus strains in viral quasispecies populations are very likely to increase the potential for severe illness or larger epidemics of DHF/DSS (Chao, Chang and King, personal communication). Definitive differentiation of primary infection versus secondary infection is a key issue to comprehensive understanding of the pathogenesis of DHF. The detection of DENV antibody provides the only method of differentiating these 2 infections. The World Health Organization has previously recommended that the comparison of HI titers between paired acute and convalescent serum samples can be used to differentiate primary and secondary infections [39]. Since a primary dengue infection leads to the slow elevation of the HI antibody, the identification of a primary antibody response must be inferred from the low level or absence of detectable antibody in the acute-phase serum drawn before day 5, and then converted to the higher levels of antibody titers elicited in the convalescent. stage. The secondary antibody response to dengue is characterized by the rapid increase of “much higher titers” of HI antibody, with 4-fold or even greater increases in titers between acute and convalescent serum samples. The peak titers always exceed 1:1280 in secondary responses, but generally fall below this ratio in primary dengue infection. Recently, MAC-ELISA has been devised to replace the HI test, which requires complicated procedures and suffers problems of cross-reactivity with other flaviviruses. Innis et al used IgM and IgG capture ELISA tests to detect both the dengue IgM and IgG antibodies [50]. A dengue IgM-to-IgG ratio ≥1.8:1 was defined as a primary dengue infection, whereas such a ratio <1.8:1 reflected a secondary dengue infection [9]. With serial specimens, a 4-fold increase in IgG to dengue indicated a secondary infection in the absence of IgM to dengue infection [49]. Kuno et al used the IgM capture method and the classical indirect ELISA method, a different approach from Innis, for measuring dengue IgM and IgG, respectively, to define primary versus secondary infections. The case with dengue IgM-to-IgG ratio >1.4 was interpreted as a primary infection and such a ratio <1.4 was inferred to be secondary infection [45]. A commercial capture ELISA for detecting dengue-specific IgM and IgG antibodies (PanBio Dengue Duo, Australia) showed excellent sensitivity and specificity for distinguishing primary and secondary infections. In comparison to the provided IgM and IgG reference sera, the dengue case was defined as secondary infection when the serum sample:calibrator absorbance ratio for IgG was ≥3.0 and there was no detectable IgM [73]. Schilling et al combined anti-dengue IgM and IgG antibodies with detection of virus or virus RNA for diagnosis of primary and secondary DENV infection [74]. Recently, NS1 serotype-specific IgG ELISA was also found to be a reliable method for differentiating primary and secondary virus infections [75]. The advantages and disadvantages of HI, capture IgM-to-IgG ratio, and NS1-serotype-specific IgG ELISA in the differentiation of primary and secondary DENV infection are summarized in Table 2.. Unsolved Problems and Future Perspectives of Laboratory Diagnosis Recently, Shu and Huang [76], and Guzman and Kouri [77] also reviewed advances in the laboratory diagnosis of dengue. Regardless of numerous recently developed 11.
(8) Laboratory diagnosis of dengue virus infections. Table 2. The advantages and disadvantages of hemagglutination inhibition (HI), capture immunoglobulin M to immunoglobulin G (IgM-to-IgG) ratio, and non-structure protein 1 (NS1)-serotype-specific IgG enzyme-linked immunosorbent assay (ELISA) in the differentiation of primary and secondary dengue virus infections Method. Advantages. Disadvantages. HI. Traditionally used method; ease and simplicity in performance; no instrument required for reading results. Capture IgM/IgG ratio. Usually only a single serum sample is required; able to measure anti-dengue IgM and IgG antibodies; low crossreactivity with other flavivirus antibodies; can be performed automatically Can distinguish dengue virus serotypes for convalescent-phase sera from primary infection and acute-phase sera from secondary infection; can be performed automatically. Paired sera are required; high cross-reactivity with other flavivirus antibodies; incapable of measuring isotype-specific antibody response; hard to differentiate dengue virus serotypes Hard to differentiate dengue virus serotype infection; reagent preparation is complicated; no consensus criteria for cut-off value of IgMto-IgG ratio. NS1-serotype-specific IgG ELISA. laboratory tests for diagnosing DENV infection, several unsolved problems warrant further research efforts in the future. Obtaining virus isolation results is tedious. Serologic diagnosis of the dengue viruses is complicated by the existence of cross-reactive antigenic determinants shared by all 4 DENV serotypes and also by other members of the flavivirus family, especially in secondary dengue infection. Using recombinant technology integrated with current understanding of structure biology to minimize the expression of cross-reactive amino acids for serologic diagnosis offers the most feasible method of solving this cross-reactivity problem in the future. Quality control and quality assurance of laboratory tests is a key concern, especially as the incidence of severe DHF cases has increased worldwide. Facing different sensitivities and specificities in various viral antigen or nucleic acid detection methods, how to select the most appropriate methods during the critical clinical stages of DENV infection is a key issue in laboratory diagnosis of dengue. Consensus guidelines established by international committees or academic societies are necessary for monitoring virus activity in surveillance, patient care and treatment. The difficulty in detecting primary and secondary infection is also an obstacle for the laboratory diagnosis and pathogenesis exploration of dengue. Kuno also pointed out the difficulty in forecasting serologic outcomes based on mixed flaviviral infections or distinguishing mixed infections from sequential secondary, tertiary, quaternary infections without corroborating information on the medical history of the tested patient [69]. How to standardize 12. Serotypes could not be correctly identified for convalescent-phase sera from secondary infection; reagent preparation is complicated; no commercially available reagents. and assure the quality of the various commercially available serologic test reagent kits is another persistent problem. Because of the above problems and the varying availability of reagents, not all clinical or public health laboratories can perform the full scale of different tests. Larger laboratories or central government-supported agencies with more resources may offer the largest number of tests and serve as reference laboratories for small laboratories. Quality control programs and proficiency testing must be performed at each level of the laboratories. Donoso Mantke et al initiated an external quality assurance program for serologic diagnosis in Germany. The participants reported concurrent and correct results for 71% of the IgGpositive samples, 89% of the IgG-negative samples, 58% of the IgM-positive samples, and 97% of the IgMnegative samples [78]. Disagreement was also found between the results of participants and the reference center in the quality control surveillance of serologic diagnosis performed in the Americas [79]. Higher discrepancy rate was also observed in the nucleic acid amplification techniques in European countries [80]. However, most Asian countries/areas with high hyperendemicity of DENV activities lack reports on quality assurance results. Future studies must consider several interesting issues related to the pathogenesis of severe cases of dengue. The first question is whether any immune complex of DENV with antibody exists in the blood circulation between the late stage of viremia and the early stage of antibody appearance. During secondary infection, the virus may be bound with the pre-existing.
(9) Kao et al. antibody, making it undetectable using most current virus isolation techniques. Acid treatment can detect immune-complex-dissociated NS1 antigen in patients with acute dengue viruses infections [81]. Wang et al used a modified immunoprecipitation assay to demonstrate that the plasma dengue viruses persisting during defervescence were contained in the immune complexes for most DHF patients [82]. The role played by immune complexes in the pathogenesis of DHF/ DSS and linking to primary and secondary infection remains unclear. Therefore, methods of detecting the dengue immune complex in serum or tissue should be investigated. Second, the question of whether any new biomarker could be used to predict and diagnose DF and DHF/DSS should be examined. Antibodies against structural and non-structure proteins of DENV have been studied. Valdes et al showed that anti-NS1 and anti-NS3 antibodies were detectable mainly in secondary cases, whereas anti-E, and anti-NS5 antibodies were frequently detected in both primary and secondary DENV infection by Western blotting [83]. Se-Thoe et al found that similar Western blot profiles of anti-E were observed in both primary and secondary DENV infections, but only in approximately one-third of patients with primary or secondary DENV infections was antibody to NS1 detected, with a rate of less than 10% for antibody to NS3 protein [84]. Churdboonchart et al reported that primary dengue cases, whose serum samples were obtained during the convalescent phase, exhibited low titers of IgG class antibodies to E proteins and 2 nonstructure proteins NS1 and NS5. Meanwhile, secondary dengue-infected patients whose sera samples were gathered during the acute phase always displayed IgG antibodies to E proteins, while those whose sera samples were collected during the convalescent phase showed high titers of IgG antibodies to NS1, NS3, NS5 and C proteins [85]. By using an immunodominant NS1 synthetic peptide and ELISA, Huang et al indicated that anti-NS1 antibodies were detected in both primary and secondary infection [86]. The discrepancy among the above findings resulted from variations in viral protein preparation, case definitions of primary and secondary DENV infection, dates of sample collection, and study sample size. Recently, Shu et al developed NS1 serotype-specific IgG ELISA for differentiation of primary and secondary DENV infections by using flavivirus group-specific monoclonal antibody as capture antibody and NS1-containing culture supernatants of DENV as detecting antigens to overcome the pitfalls in antigen selection [75]. Besides antibodies. against these viral proteins, expressions of certain cytokines, chemokines, innate immunity, and cellularmediated immune responses may be alternative approaches for diagnosing dengue and managing patients. To improve and extend currently used techniques, more reliable and practical methods are required for research and development. The application of modern techniques, nucleic acid chips, protein chips and new biomarkers in studies on dengue diagnosis and pathogenesis represents a significant challenge for the future.. Acknowledgments The authors would like to thank Dr. Duane Gubler for his professional guidance over many years since the very beginning of this research in Taiwan. We also sincerely appreciate several laboratory methods for detecting dengue virus developed by Dr. Wei-June Chen, Dr. WeiKung Wang, Dr. Han-Chung Wu, Dr. Shuen-Ju Wu, Dr. Yi-Lin Lin, Dr. Li-Kuang Chen, strong technical support from Mei-Yin Liao, Yen-Huei Chiu, Hui-Ting Wang, and frequent scientific exchanges and discussions with Dr. Betty Wu-Hsieh, Dr. Wen Chang, Dr. ChinLuen Liao, Dr. Han-Chung Wu, Dr. Jyh-Hsiung Huang, Dr. Pei-Yun Shu, and Dr. Huan-Yao Lee in our Taiwan Dengue Research Team. This work has been supported by the National Health Research Institute since 1994 (NHRI Grant DD01-86IX-CR-501P; NHRICN-CL8903P; NHRI-CN-CL9302P, NSC 92-2312B002-357). Finally, the authors would like express our sincere gratitude to Dr Shuenn-Ju Wu at the Naval Medical Research Center, USA, and Dr. Goro Kuno at the Centers for Disease Control and Prevention, USA for their careful review of this manuscript.. References 1. Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health 1987;18:392-7. 2. Kalaynarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis 1997; 176:313-21. 3. Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998;11:480-96. 4. Sinniah M, Igarashi A. Dengue hemorrhagic fever. Rev Med Virol 1995;5:193-203. 5. Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA 1994;91:2395-400. 6. Wu YC. Epidemic dengue 2 on Liouchyou Shiang, Pingtung 13.
(10) Laboratory diagnosis of dengue virus infections. County in 1981. Chinese J Microbiol Immunol 1986;19:203-11. 7. King CC, Wu YC, Chao DY, Lin TH, Chow L, Wang HT, et al. Major epidemics of dengue in Taiwan in 1981-2000: related to intensive virus activities in Asia. Dengue Bulletin 2000;24:1-10. 8. Vorndam V, Kuno G. Laboratory diagnosis of dengue virus infections. In: Gubler DJ, Kuno G, eds. Dengue and dengue hemorrhagic fever. CAB International; 1997:313-33. 9. Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis 1997;176:322-30. 10. Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg 1982;31:830-6. 11. Gubler DJ, Kuno G, Sather GE, Velez M, Oliver A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am J Trop Med Hyg 1984;33:158-65. 12. Roche RR, Alvarez M, Guzman MG, Morier L, Kouri G. Comparison of rapid centrifugation assay with conventional tissue culture method for isolation of dengue 2 virus in C6/36HT cells. J Clin Microbiol 2000;38:3508-10. 13. Chen WJ, Chen SL, Fang AH, Wang MT. Detection of dengue virus antigen in cultured cells by using protein A-gold-silver staining (pAgs) method. Microbiol Immunol 1993;37:359-63. 14. Kao CL, Wu MC, Chiu YH, Lin JL, Wu YC, Yueh YY, et al. Flow cytometry compared with indirect immunofluorescence for rapid detection of dengue virus type 1 after amplification in tissue culture. J Clin Microbiol 2001;39:3672-7. 15. Oliveira De Paula S, Malta Lima D, Clotteau M, Pires Neto Rd Rda J, Lopes da Fonseca BA. Improved detection of dengue-1 virus from IgM-positive serum samples using C6/36 cell cultures in association with RT-PCR. Intervirology 2003;46: 227-31. 16. Kittigul L, Meethien N, Sujirarat D, Kittigul C, Vasanavat S. Comparison of dengue virus antigens in sera and peripheral blood mononuclear cells from dengue infected patients. Asian Pac J Allergy Immuol 1997;15:187-91. 17. Malergue F, Chungue E. Rapid and sensitive streptavidinebiotin amplified fluoregenic enzyme-linked immunosorbentassay for direct detection and identification of dengue viral antigens in serum. J Med Virol 1995;47:43-7. 18. Bhoopat L, Bhamarapravati N, Attasiri C, Yokasarn S, Chaiwun B, Khunamornpong S, et al. Immunohistochemical characterization of a new monoclonal antibody reactive with dengue virus-infected cells in frozen tissue using immunoperoxidase technique. Asian Pac J Allergy Immunol 1996;14:107-13. 19. Ramos C, Sanchez G, Pando RH, Baquera J, Hernandez D, Mota J, et al. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol 1998;4:465-8. 14. 20. Miagostovich MP, Ramos RG, Nicol AF, Nogueira RM, CuzziMaya T, Oliveira AV, et al. Retrospective study on dengue fatal cases. Clin Neuropathol 1997;16:204-8. 21. Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 2004;189:1141-8. 22. Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamana M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol 2002;40:376-81. 23. Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002;186:1165-8. 24. Lanciotti RS. Molecular amplification assays for the detection of flaviviruses. Adv Virus Res 2003;61:67-99. 25. Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992;30:545-51. 26. Reynes JM, Ong S, Mey C, Ngan C, Hoyer S, Sall AA. Improved molecular detection of dengue virus serotype 1 variants. J Clin Microbiol 2003;41:3864-7. 27. Harris E, Roberts TG, Smith L, Selle J, Kramer LD, Valle S, et al. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol 1998;36:2634-9. 28. De Paula SOD, Lima CDM, Torres MP, Pereira MR, Lopes Da Fonseca BA. One-step RT-PCR protocols improve the rate of dengue diagnosis compared to two-step RT-PCR approaches. J Clin Virol 2004;30:297-301. 29. Wu SJ, Lee EM, Putvatana R, Shurtliff RN, Porter KR, Suuharyono W, et al. Detection of dengue viral RNA using a nucleic acid sequence-based amplification assays. J Clin Microbiol 2001;39:2794-8. 30. Raengsakulrach B, Nisalak A, Maneekarn N, Yenchitsomanus PT, Limsomwong C, Jairungsri A, et al. Comparison of four reverse transcription-polymerase chain reaction procedures for the detection of dengue virus in clinical specimens. J Virol Methods 2002;105:219-32. 31. Wang WK, Lee CN, Kao CL, Lin YL, King CC. Quantitative competitive reverse transcription-PCR for quantification of dengue virus RNA. J Clin Microbiol 2000;38:3306-10. 32. Sudiro TM, Zivny J, Ishiko H, Green S, Vaughn DW, Kalayanarooj S, et al. Analysis of plasma viral RNA levels during acute dengue virus infection using quantitative.
(11) Kao et al. competitor reverse transcription-polymerase chain reaction. J Med Virol 2001;63:29-34. 33. Houng HH, Hritz D, Kanesa-thasan N. Quantitative detection of dengue 2 virus using fluorogenic RT-PCR based on 3'-noncoding sequence. J Virol Methods 2000;86:1-11. 34. Houng HH, Chung-Ming Chen R, Vaughn DW, Kanesa-thasan N. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1-4 using conserved and serotype-specific 3' noncoding sequences. J Virol Methods 2001;95:19-32. 35. Drosten C, Gottig S, Schilling S, Asper M, Panning M, Schmitz H, et al. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Vallery fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol 2002;40:2323-30. 36. Shu PY, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, et al. Development of group-and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus. J Clin Microbiol 2003;41:2408-16. 37. Wang WK, Sung TL, Tsai YC, Kao CL, Chang SM, King CC. Detection of dengue virus replication in peripheral blood mononuclear cells from dengue virus type 2-infected patients by a reverse transcription-real-time PCR assay. J Clin Microbiol 2002;40:4472-8. 38. Anonymous. Dengue diagnostic laboratory procedures for the Americas: a manual. Centers for Disease Control, 1981. 39. WHO. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: World Health Organization; 1997. 40. Russell PK, Nisalak A, Sukhaavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967;99:285-90. 41. Barnes WJS, Rosen L. Fatal hemorrhagic disease and shock associated with primary dengue infection on a Pacific island. Am J Trop Med Hyg 1974;23:495-506. 42. Morens D M, Halstead SB, Larsen LK. Comparison of dengue virus plaque reduction neutralization by macro and “semimicro” methods in LLC-MK2 cells. Microbiol Immunol 1985; 29:1197-205. 43. Morens DM, Halstead S, Repik PM, Putvatana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J Clin Microbiol 1985;22:250-4. 44. Kuno G. A manual of plaque assay and plaque reduction neutralization test with an emphasis on dengue viruses. Centers for Disease Control, USA; 1987. 45. Kuno G, Gomez I, Gubler DJ. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods 1991;33:101-13.. 46. Figueiredo LTM, Simoes MC, Cavalcante SMB. Enzyme immunoassay for the detection of dengue IgG and IgM antibodies using infected mosquito cells as antigen. Trans R Soc Trop Med Hyg 1989;83:702-7. 47. Chungue E, Marche R, Plichart R, Boutin JP, Roux J. Comparison of immunoglobulin G enzyme-linked immunosorbent assay (IgG-ELISA) and haemagglutination inhibition (HI) test for the detection of dengue antibodies. Prevalence of dengue IgG-ELISA antibodies in Tahiti. Trans R Soc Trop Med Hyg 1989;83:708-11. 48. Hooi P, Malasit P. Anti-dengue IgG detection by an indirect ELISA. Southeast Asian J Trop Med Public Health 1995; 26:673-6. 49. Rossi CA, Drabick JJ, Gambel JM, Sun W, Lewis TE, Henchal EA. Laboratory diagnosis of acute dengue fever during the united nations mission in Haiti, 1995-1996. Am J Trop Med Hyg 1998;59:275-85. 50. Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 1989;40:418-27. 51. Cardosa MJ, Tio PH, Nimmannitya S, Nisalak A, Innis BL. IgM capture ELISA for detection of IgM antibodies to dengue virus: comparison of 2 formats using hemagglutinins and cell culture derived antigens. Southeast Asean J Trop Med Public Health 1992;23:726-9. 52. Groen J, Koraka P, Velzing J, Copra C, Osterhaus AD. Evaluation of six immunoassays for detection of dengue virusspecific immunoglobulin M and G antibodies. Clin Diagn Lab Immunol 2000;7:867-71. 53. Tan R, Kurniawan H, Hartati S, Widjaja S, Jennings GB. Comparative sensitivity of laboratory methods to diagnose dengue virus infections at Husada Hospital Jakarta. Southeast Asian J Trop Med Public Health 1994;25:262-5. 54. Kuno G, Cropp BC, Wong-Lee J, Gubler DJ. Evaluation of an IgM immunoblot kit for dengue diagnosis. Am J Trop Med Hyg 1998;59:757-62. 55. Branch SL, Levett PN. Evaluation of four methods for detection of immunoglobulin M antibodies to dengue virus. Clin Diagn Lab Immunol 1999;6:555-7. 56. Wu SJ, Paxton H, Hanson B, Kung CG, Chen TB, Rossi C, et al. Comparison of two rapid diagnostic assays for detection of immunoglobulin M antibodies to dengue virus. Clin Diagn Lab Immunol 2000;7:106-10. 57. Chakravarti A, Gur R, Berry N, Mathur MD. Evaluation of three commercially available kits for serological diagnosis of dengue haemorrhagic fever. Diagn Microbiol Infect Dis 2000; 36:273-4. 58. Parida MM, Upadhyay C, Saxena P, Dash PK, Jana AM, et al. 15.
(12) Laboratory diagnosis of dengue virus infections. Evaluation of a dipstick ELISA and a rapid immunochromatographic test for diagnosis of dengue virus infection. Acta Virol 2001;45:299-304. 59. Vajpayee M, Singh UB, Seth P, Broor S. Comparative evaluation of various commercial assays for diagnosis of dengue fever. Southeast Asian J Trop Med Public Health 2001;32:472-5. 60. Jelinek T, Wastlhuber J, Proll S, Schattenkirchner M, Loscher T. Influence of rheumatoid factor on the specificity of a rapid immunochromatographic test for diagnosing dengue infection. Eur J Clin Microbiol Dis 2000;19:555-6. 61. Ludolfs D, Schilling S, Altenschmidt J, Schmitz H. Serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. J Clin Microbiol 2002;40:4317-20. 62. Yao ZJ, Kao MC, Loh KC, Chung MC. A serotype-specific epitope of dengue virus type 1 identified by phage displayed random peptide library. FEMS Microbiol Lett 1995;127:93-8. 63. Wu HC, Hung YL, Chao TT, Jan JT, Huang JL, Chiang HY, et al. Identification of B-cell epitope of dengue virus type 1 and its application in diagnosis of patients. J Clin Microbiol 2000; 39:977-82. 64. Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, et al. Potential application of nonstructural protein NS1 serotypespecific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J Clin Microbiol 2002;40:1840-4. 65. Shu PY, Chen LK, Chang SF, Su CL, Chen LJ, Lin TH, et al. Dengue virus serotyping based on envelope and membrane and nonstructural protein NS1 serotype-specific capture immunoglobulin M enzyme-linked immunosorbent assays. J Clin Microbiol 2004;42:489-94. 66. Chen WJ, Huang KP, Fang AH. Detection of IgM antibodies from cerebrospinal fluid and sera of dengue fever patients. Southeast Asian J Trop Public Health 1991;22:659-63. 67. Panchareon C, Thisyakorn U. Neurological manifestations in dengue patients. Southeast Asian J Trop Public Health 2001; 32:341-5. 68. Lum LC, Lam SK, Choy YS, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg 1996;54:256-9. 69. Kuno G. Serodiagnosis of flaviviral infections and vaccinations in humans. Adv Virus Res 2003;61:3-65. 70. Halstead SB. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med 1970;42:350-60. 71. Rosen L. The Emperor’s new clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am J Trop Med Hyg 1977;26:337-43. 72. Chao DY, Lin TH, Hwang KP, Huang JH, Liu CC, King CC. 1998 Dengue hemorrhagic fever epidemic in Taiwan. Emerg Infect Dis 2004;10:552-4. 16. 73. Vaughn DW, Nisalak A, Solomon T, Kalayanarooj S, Dung NM, Kneen R, et al. Rapid serologic diagnosis of dengue virus infection using commercial capture ELISA that distinguishes primary and secondary infections. Am J Trop Med Hyg 1999; 60:693-8. 74. Schilling S, Ludolfs D, Van An L, Schmitz H. Laboratory diagnosis of primary and secondary dengue infection. J Clin Virol 2004;31:179-84. 75. Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, et al. Comparison of capture immunolglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin Diagn Lab Immunol 2003;10:622-30. 76. Shu PY, Huang JH. Current advances in dengue diagnosis. Clin Diagn Lab Immunol 2004;11:642-50. 77. Guzman MG, Kouri G. Dengue diagnosis, advances and challenges. Int J Infect Dis 2004;8:69-80. 78. Donoso Mantke O, Lemmer K, Biel SS, Groen J, Schmitz H, Durand JP, et al. Quality control assessment for the serological diagnosis of dengue virus infections. J Clin Virol 2004;29:105-12. 79. Guzman MG, Pelegrino JL, Pumariega T, Vazquez S, Gonzalez L, Kouri G, et al. Quality control of the serological diagnosis of dengue in laboratories throughout the Americas, 1996-2001. Rev Panam Salud Publica 2003;14:371-6. 80. Lemmer K, Donoso Mantke O, Bae HG, Groen J, Drosten C, Niedrig M. External quality control assessment in PCR diagnostics of dengue virus infections. J Clin Virol 2004;30:291-6. 81. Koraka P, Burghoorn-Maas CP, Falconar A, Setiati TE, Djamiatun K, Groen J, et al. Detection of immune-complexdissociated nonstructural-1 antigen in patients with acute dengue virus infections. J Clin Microbiol 2003;41:4154-9. 82. Wang WK, Chao DY, Kao CL, Wu HC, Liu YC, Li CM, et al. High levels of plasma dengue load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virol 2003;305:330-8. 83. Valdes K, Alvarez M, Pupo M, Vazquez S, Rodriguez R, Guzman MG. Human dengue antibodies against structural and nonstructural proteins. Clin Diagn Lab Immunol 2000;7:856-7. 84. Se-Thoe SY, NgMM, Ling AE. Retrospective study of Western blot profiles in immune sera of natural dengue virus infections. J Med Virol 1999;57:322-30. 85. Churdboonchart V, Bhamarapravati N, Peampramprecha S, Sirinavin S. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am J Trop Med Hyg 1991;44:481-93. 86. Huang JH, Wey JJ, Sun YC, Chin C, Chien LJ, Wu YC. Antibody responses to an immunodominant nonstructural 1 synthetic peptide in patients with dengue hemorrhagic fever. J Med Virol 1999;57:1-8..
(13)
數據
相關文件
Iwaki et al., “Oral malignant melanoma in Japan,” Oral Surgery, Oral Medicine, Oral Pathology, vol.. Myers, “Melanoma of the head and neck: current concepts in staging, diagnosis,
IV Differential diagnosis => biopsy sample => Referral to clinician with expertise in the diagnosis and management of oral disease. Indications
The aim of this article is to review the management of oral leukoplakia. The topics of interest are clinical diagnosis, methods of management and their outcome, factors associated
In the current paper, we present an unusual case of bilateral TMJ OA in an advanced stage focusing on clinical, laboratory, and radiographic differential
(1) Provision of lifelong ART for HIV-infected women who are in need of treatment (CD4 count <350 mm -3 ) in order to protect their own health and also to prevent
Background: Gold standard for the diagnosis of oral dysplasia (OD) oral squamous cell carcinoma (OSCC) and malignant lesions is the histological examination.. Several
Hemangioma is the most common diagnosis for nodules in the masseter muscle, accounting for approximately 36% of all hemangiomas that arise in the skeletal muscle of the head and
In this study, the clinical relevance of 3D CBCT images compared to the 2D ones has been evaluated for the diagnosis and treatment planning of impacted canines.. CBCT allows