利用莖頂組織超低溫冷凍處理法去除甘藷病毒之研究
鄭櫻慧
1、林靜宜
2、羅淑芳
3,* 1行政院農業委員會農業試驗所植物病理組
2行政院農業委員會農業試驗所嘉義分所植保系
3行政院農業委員會農業試驗所嘉義分所農藝系
摘要
本 研 究 試 驗 材 料 為 甘 藷
[Impomoea
batatas ( L ) Lam.]栽培種「台農 57 號」、「台
農
66 號」與野生種「WT248」、「WT427」分
別感染甘藷羽狀斑駁病毒(SPFMV)、甘藷病
毒
G (SPVG)與甘藷黃矮病毒(SPCSV),取病
毒株莖頂組織為材料,以藻膠包埋玻璃質化
法進行超低溫冷凍保存,於回復生長培養基
(即 MS 基本鹽類)添加 1 μM NAA, 0.5 μM
BA, 0.5 μM Kinetin,30 g L
-1蔗糖及 9.4 g L
-1Difco agar。結果顯示,「台農 66 號」及
「
WT427 」 可 獲 得 植 株 成 活 率 最 佳
13.115.2%,而「台農 57 號」僅獲得 2.2%;
若以
1 μM IAA 替代 NAA,並且將 BA 濃度
提高
1 倍,則「台農 57 號」的植株成活率提
高為
44.6%,但野生種兩品系的植株成活率
降低為
011.1%;以感染病毒之甘藷進行超
低溫冷凍處理法去除病毒的試驗顯示,栽培
種「台農
57 號」、「台農 66 號」及野生種
「WT248」之再生株以 RT-PCR 均未測到上
述 三 種 病 毒 , 去 病 毒 率 為
100%, 野 生 種
「WT427」的 9 個再生株中,5 個測到甘藷
病毒
G,去病毒率為 44.4%。
關鍵詞︰甘藷、超低溫冷凍保存、病毒。
Effect of Cryotherapy to Shoot Tips
Culture on Elimination of Sweet
Potato Virus
Ying-Huey Cheng
1, Ching-Yi Lin
2and
Shu-Fang Lo
3,*1 Plant Pathology Division, Taiwan Agricultural
Research Institute, Wufeng District, Taichung City 41362, Taiwan ROC
2 Department of Plant Protection, Chiayi
Agricultural Experiment Station, Taiwan Agricultural Research Institute, Chiayi 60044, Taiwan ROC
3 Department of Agronomy, Chiayi Agricultural
Experiment Station, Taiwan Agricultural Research Institute, Chiayi 60044, Taiwan ROC
ABSTRACT
Sweet potato [Ipomoea batatas (L) Lam.] cultivars/lines, ‘Tainung No. 57 (TNG57)’, ‘Tainung No. 66 (TNG66)’, ‘WT248’ and ‘WT427’ infected with Sweet potato feathery mottle virus (SPFMV), Sweet potato virus G (SPVG) and Sweet
potato chlorotic stunt virus (SPCSV) were cultivated
at Wufeng District, Taichung City, for the field experiment. The treatment of cryotherapy was applied to the shoot tips of infected plants for preservation. The preserved shoot tips were put into the survival medium which consisted of MS (Murashige and Skoog) basic salted medium and supplemented with 1 μM NAA, 0.5 μM BA, 0.5 μM Kinetin, 30 g L-1 sucrose, and 9.4 g L-1 Difco
agar. The shoot regeneration rates were 13.115.2% from in vitro-grown sweet potato of ‘TNG66’ or ‘WT427’, and was 2.2% for ‘TNG57’. In the case of 1 μM IAA replaced with NAA and concentration of BA was increased twice in the survival medium, the shoot regeneration rate enhanced to 44.6% for ‘TNG57’, but decreased to 011.1% for 2 wild types of sweet potato. By multiplex RT-PCR, SPFMV, SPVG and SPCSV were undetectable in all regenerated plantlets of ‘TNG 57’, ‘TNG 66’ and ‘WT248’ (100% virus eradication); however, SPVG was detected in 5 out of 9 regenerated plantlets of ‘WT427’.
Key words: Sweet potato, Cryopreservation, Virus.
* 通信作者, losf@dns.caes.gov.tw 投 稿 日 期:2015 年 7 月 7 日 接 受 日 期:2015 年 9 月 15 日
作 物 、 環境 與生 物 資 訊 12:132-141 (2015)
Crop, Environment & Bioinformatics 12:132-141 (2015) 189 Chung-Cheng Rd., Wufeng District, Taichung City 41362, Taiwan ROC
前言
感染甘藷的病毒種類相當多,超過
9 科
30 種(Clark et al. 2012),臺灣有紀錄危害甘
藷的病毒有甘藷羽狀斑駁病毒
(Sweet potato
feathery mottle virus; SPFMV) (Yang 1972)、
甘 藷 潛 隱 病 毒
(Sweet potato latent virus;
SPLV) (Liao et al. 1979a, b)、甘藷病毒 G
(Sweet potato virus G; SPVG) (Wang et al.
2013)、甘藷病毒 Y [Sweet potato virus Y;
SPVY, 又稱甘藷病毒 2 (Sweet potato virus 2,
SPV2)]、甘藷黃萎病毒(Sweet potato yellow
dwarf virus; SPYDV)、甘藷捲葉病毒(Sweet
potato leaf curl virus; SPLCV) (Chung et al.
1985, 1986, Green et al. 1989)和甘藷黃矮病
毒(Sweet potato chlorotic stunt virus; SPCSV)
(Cheng et al. 2012)等 7 種。國外報導感染甘
藷的病毒中,以
SPFMV 和 SPCSV 最為常見
(Loebenstein et al. 2003, 2009, Tairo et al.
2005),二病毒單獨感染對產量影響不大,但
若複合感染協力作用造成嚴重的甘藷病毒病
(Sweet potato virus disease; SPVD)
(Schaefers and Terry 1976),大幅降低塊根產
量
(Karyeija et al. 2000, Mukasa et al.
2006)。臺灣感染甘藷的 7 種病毒中以 SPFMV
最為普遍,若與
SPLV 複合感染,使「台農
57 號」減產 24.5%,
「台農
63 號」減產 35.6%,
明顯減少甘藷產量(Chung et al. 1981)。
Brison et al. (1997)以超低溫冷凍法進行
梅樹種原保存,可獲得
50%的健康株,而傳
統切取生長點去除病毒的技術僅可獲得
20%
的健康株;根據
Helliot et al. (2002)指出香蕉
利用傳統的切取生長點去病毒方法,無法去
除
CMV (Cucumber mosaic virus)病毒,若以
超低溫冷凍法可獲得無
CMV 的植株達 2%;
Wang and Valkonen (2008)利用藻膠包埋甘
藷莖頂組織,成功去除
SPFMV 和 SPCSV2
種 病 毒 , 二 位 學 者 亦 利 用 組 織 化 學 免 疫 定
位,發現不管單獨或複合感染,SPFMV 位於
第
4、5 葉原體,SPCSV 只存在於第 5 葉原
體,內層第
3、2、1 葉原體與頂端分生組織
無病毒存在。以莖頂組織為培植體進行藻膠
包埋之液態氮處理時,外圍細胞會凍死,存
活 的 為 內 層 之 未 分 化 葉 原 體 與 頂 端 分 生 組
織,誘導再生後可以得到去病毒的小苗。
本研究旨在以甘藷栽培種「台農
57 號」、
「台農
66 號」與野生種品系「WT248」、
「WT427」等 4 品種(系)病毒株之莖頂組織進
行藻膠包埋超低溫冷凍法處理,據以探討此
一方法去除病毒病的效率。
一、供試材料及方法
供試材料為截取甘藷「台農
57 號」、「台
農
66 號」、「WT248」及「WT427」等 4 品
種
( 系 ) 同 時 感 染 甘 藷 羽 狀 斑 駁 病 毒
(SPFMV)、甘藷病毒 G (SPVG)與甘藷黃矮病
毒(SPCSV)的病毒株莖段,經 1%次氯酸鈉溶
液振盪
10 min,無菌水清洗 34 次後,將培
植體切成單節置於含
MS 基本鹽類並添加 30
g L
-1蔗糖及 9.4 g L
-1Difco agar 及 pH 5.7 ±
0.1 的培養基,其培養環境為光照 16 h,溫度
25℃。經 30 d 生長後,切取莖頂 1.0 mm 大
小 , 置 於 藻 膠 培 養 液
[含 2% (w/v) Na –
alginate 及 30 g L
-1蔗糖,但不含
Ca
2+的
MS
基本鹽類培養液]。以滴管將莖頂與藻膠培養
液一同滴入包埋培養液(MS 基本培養液、0.4
M 蔗糖及 0.1 M CaCl
2),並於 25℃中靜置 30
min,待其形成直徑約 0.5 cm 之球形,再將
藻膠球培養於含有
30 g L
-1蔗糖之
MS 基本鹽
類液體培養基,每錐形瓶含有
10 粒藻膠球及
50 mL 的培養基。以震盪速率為 90 rpm 培養
1 d,再移至含有 0.3 M 蔗糖之 MS 基本鹽類
液體培養基培養
16 h。將上述處理的藻膠球
置於
LS 溶液(2 M 甘油 + 1.6 M 蔗糖,pH 5.8,
Hirai and Sakai 2003),處理 300 min,將 LS
溶液吸出,置入
PVS2 溶液(標準配方為 30%
w/v 甘油 + 15% w/v 乙二醇 + 15% w/v 二
甲基亞碸(DMSO) + 0.4 M 蔗糖, Sakai et al.
1990)的錐形瓶,每瓶含有 10 粒藻膠球,以
震盪速率為
60 rpm 培養 60 min。將處理後
的藻膠球移入含有
1 mL 的 PVS2 溶液的冷凍
管中,並置於液態氮中保存
1 h 後,取出冷
凍管直接置入
40℃溫水中回溫 2 min,將冷
凍管中的
PVS2 溶液吸出,再加入含 1.2 M 蔗
糖
MS 基本鹽類培養液,靜置 15 min。最後,
將藻膠球移至含
MS 基本鹽類培養基添加 1
μM NAA, 0.5 μM BA, 0.1 μM Kinetin, 30 g
L
-1蔗糖及
9.4 g L
-1Difco agar (Yu et al.
2012)或含 1 μM IAA, 1 μM BA, 30 g L
-1蔗糖
及
9.4 g L
-1Difco agar 的培養基,暗培養 7 d
後繼代培養一次,再置於光照
16 h,溫度 25
℃條件下,培養
30 d 後,調查癒合組織形成
率及植株成活率持續
90 d。
二、引子之設計
偵測
SPFMV、SPVG 及 SPCSV 等病毒
的簡併式引子對係根據
GenBank 網站登錄
的核苷酸序列資料比對設計,試驗中使用之
引子對如
Table 1。
(一)RNA 之抽取及反轉錄-聚合酶連鎖反應
取
100 mg 的病葉,利用總量 RNA 純化
試劑組(Plant Total RNA mini kit, Viogene,
New Taipei, Taiwan)進行 RNA 之萃取,得
到總量
RNA。以純化所得之總量 RNA 進行
反 轉 錄
- 聚 合 酶 連 鎖 反 應 (reverse
transcription-ploymerase chain reaction;
RT-PCR),RT-PCR 以單一步驟 RT-PCR 試
劑組(Genemark Co., Taichung, Taiwan)進
行,25 uL 的反應液中加入 1 uL 的 RNA,各
2.5 μL 之 20 μM 簡併式或專一性引子(Table
1) 。 RT-PCR 之 進 行 於 於 熱 循 環 反 應 儀
(GeneAmp model 2400, Perkin-Elmer Co.,
Norwalk, CT, USA)中,設定反應程序為 50
℃下進行反轉錄
30 min,94℃變性 1.5 min
後,進行
30 個 PCR 循環反應:94℃下變性 1
min,鍊合 1.5 min,溫度視引子序列而定,
72℃下聚合 2 min,最後一個循環之 72℃聚
合反應延長為
6 min。反應產物以 1.2%電泳
瓊 膠
(SeaKem, Agarose, Cambrex Bio
Science Rockland, Inc., Rockland, ME,
USA)進行分析。
(二)西方漬染法(Western blotting)
取
0.1 g 供試之病葉組織,以液態氮磨碎
後 , 加 入
200 μL 之 樣 品 處 理 液 (75 mM
Tris-HCl, 10 mM KCl, 10 mM MgCl2, 1mM
EDTA, 30% glycerol, 6% SDS, 9%
β-Mercaptoethanol, 0.015% Bromophenol
blue),研磨液加熱處理後經 SDS-PAGE 電泳,
膠體上之蛋白轉漬於
PVDF 膜(Millipore Co.,
MA, USA),再以病毒抗血清進行漬染反應。
甘藷病毒
G 抗血清稀釋 1000 倍進行反應,甘
藷黃矮病毒抗血清稀釋
250 倍進行反應。
三、資料統計與分析
本 試 驗 採 完 全 逢 機 設 計
(complete
randomized design; CRD),處理為 4 種不同
品種(系)及 2 種不同培養基,每一處理重複 3
次以上,每一重複至少培養
4 個培植體,所
獲得的資料進行每一品種(系)於兩種培養基
的 培 養 效 果 , 利 用 最 小 顯 著 性 差 異
(least
signification difference test; LSD)比較各處
理平均值間之差異。
Table 1. Sequences of primers used in this study and their amplicon size.
Name Polarity Nucleotide sequence Amplicon size SPFup Sense CCAAAYAT(A/C)AATGG(A/T)G(A/T)(T/G)TGGAC 200 bp SPFGdw Antisense GCATATCGCGCAA(T/G)ACTCAT(A/G)TC
SPGup Sense GA(T/C)TA(T/C)GG(A/T)GTGAA(T/C)GATGAGCA 280 bp SPFGdwZ Antisense GCATATCGCGCAA(T/G)ACTCAT(A/G)TC
SPCSup Sense AAATCT(T/C)GAGAAT(A/G)TGTT(T/C)ATGGT 350 bp SPCSdw Antisense TCAACAGTGAAGACC(A/T)GT(A/T)CC(A/G)G
結果與討論
去除植物病毒病的方法不外乎熱處理及
生長點培養等
(Cooper and Walkey 1978,
Walkey and Cooper 1975),近年來許多學者
發現利用植物莖頂組織經液態氮處理後存活
的植株,其獲得無病毒苗的比率較切取生長
點為高(Brison et al. 1997, Helliot et al . 2002,
Wang et al. 2003, 2006)。因此,本研究亦利
用莖頂組織超低溫冷凍法對甘藷病毒去除的
效果進行探討,以甘藷栽培種「台農
57 號」、
「台農
66 號」與野生種品系「WT248」及
「WT427」等 4 品種(系)病毒株的莖頂組織為
材料,經藻膠包埋及超低溫冷凍(-196℃)處理
後,回復生長培養基乃依據
Yu et al. (2012)
所發表即
MS 基本鹽類添加 1 μM NAA, 0.5
μM BA, 0.1 μM Kinetin, 30 g L
-1蔗糖及
9.4 g
L
-1Difco agar (A 培養基),另一 B 培養基即
MS 基本鹽類添加 1 μM IAA, 1 μM BA, 0.1
μM Kinetin, 30 g L
-1蔗糖及
9.4 g L
-1Difco
agar。以同一品種於 A 及 B 培養基分析結
果,如
Table 2 顯示,4 個品種(系)的莖頂組
織於
B 培養基所誘導的癒合組織形成率皆極
顯著高於
A 培養基,其中以兩個野生種的癒
合組織形成率更高達
93.5–94.8%;若將回復
生長培養基中以
1 μM IAA 替代 NAA,並且
將
BA 由 0.5 μM 提高為 1 μM (B medium),
則植株成活效果以「台農
57 號」及「台農 66
號」皆極顯著高於
A 培養基。其中,以「台
農
57 號」效果最佳達 44.6% (Fig. 1), 其次
為「台農
66 號」的 15.6%,而「WT427」及
「
W T 2 4 8 」 兩 品 系 的 植 株 形 成 率 僅
011.1%;相反地,兩野生種品系於 A 培養
基的植株形成率較
B 培養基達極顯著差異,
其中以「WT427」的植株形成率最高達 15.2%
(Fig. 2)。根據 Yu et al. (2012)以甘藷「台農
57 號」、「台農 66 號」、「台農 71 號」及「台
農
73 號」莖頂組織為材料,以藻膠包埋玻璃
質化法(Encapsulation–vitrification)進行超
低溫冷凍保存,結果
4 個品種的植株成活率
皆為
3.3%。本研究回復生長培養基先採用 Yu
et al. (2012)所發表者,結果顯示於 4 種不同
品種(系)經超低溫冷凍處後的植株成活率介
於
2.215.2%,但將回復生長培養基中 BA 濃
度提高
1 倍,除了野生種品系無效果外,極
顯著提高栽培種的植株成活率;尤其「台農
57 號」提高 19.3 倍之多,「台農 66 號」增加
0.19 倍。而莖頂組織切取的大小亦為關鍵,
Yu et al. (2012)所取的甘藷莖頂大小約 2–3
m m , 經 超 低 溫 冷 凍 後 植 株 成 活 率 僅 達
3.3%。Wang and Valkonen (2008)亦指出甘
藷莖頂組織切太小約
0.5 mm 或太大約 1.5
mm 以上,進行超低溫冷凍處理後不利於植
Table 2. Effects of induced callus and regrowth of survived shoot tips for different medium and varieties (lines) of sweet potato after cryopreservation by encapsulation–vitrification.
A medium z B medium y Genotype Explant No. Callus (%) Regrowth of survived shoot tips (%) Explant No. Callus (%) Regrowth of survived shoot tips (%) TNG57 104 70.0bx 2.2b 38 81.8a 44.6a TNG66 55 84.7b 13.1b 14 88.9a 15.6a WT248 104 89.7b 2.7a 46 93.5a 0.0b WT427 51 85.2b 15.2a 35 94.8a 11.1b
z A media : full strength MS basal medium with 1 μM NAA, 0.5 μM BA, 0.1 μM Kinetin, 30 g L-1 sucrose
and 9.4 g L-1 Difco agar at pH 5.7 ± 0.1. Data were collected after 90 d of culture.
y B media : full strength MS basal medium with 1 μM IAA, 1 μM BA, 30 g L-1 sucrose and 9.4 g L-1 Difco
agar at pH 5.7 ± 0.1. Data were collected after 90 d of culture.
x Means within each column followed by the same letter(s) are not significantly (p < 0.01) different
Fig. 1. The regrowth of survived shoot tips of ‘TNG57’ cultured on B medium after cryopreservation by encapsulation–vitrification for 60 d.
Fig. 2. The regrowth of survived shoot tips cultured on A medium after cryopreservation by encapsulation– vitrification on ‘WT427’ line for 60 d.
株成活,因此切含
3–4 片葉原基(約 1 mm)較
有利於植株成活(Hirai and Sakai 2003)。本
研究所取的甘藷莖頂大小亦為
1 mm,經超低
溫冷凍處理後使栽培種的植株成活率大幅提
高,但不同品種(系)之間所適合的回復生長培
養基亦不同,並且可知癒合組織形成率的高
低與植株形成率無關。
將甘藷「台農
57 號」、「台農 66 號」、
「WT248」及「WT427」等 4 品種(系)有病
毒的莖頂組織,經藻膠包埋超低溫處理後所
存活的植株進行病毒病檢測,以
Table 1 的簡
併式引子進行
RT-PCR,感染甘藷羽狀斑駁
病毒增幅
200 bp 產物、感染甘藷病毒 G 增幅
280 bp 產物、感染甘藷黃矮病毒增幅 350 bp
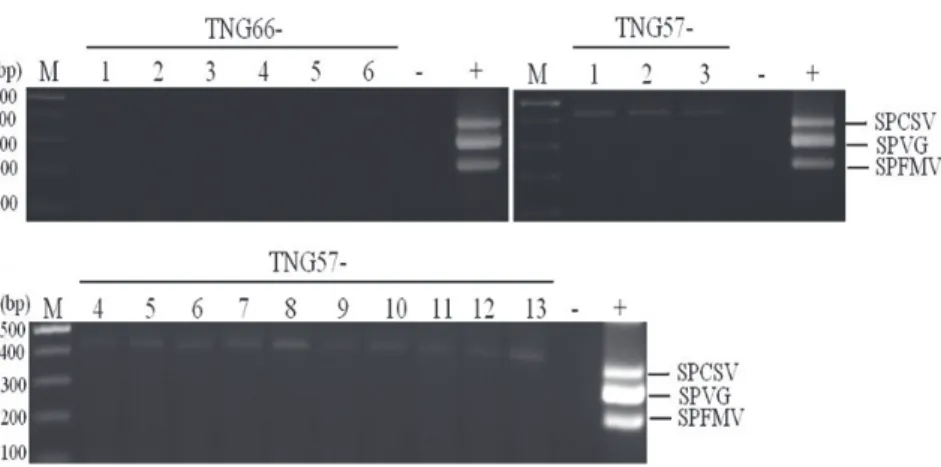
產物。檢測供試甘藷材料,測出栽培種「台
農
57 號」感染甘藷病毒 G 與甘藷黃矮病毒,
「台農
66 號」感染甘藷羽狀斑駁病毒、甘藷
病毒
G 與甘藷黃矮病毒,野生種「WT248」
感 染 甘 藷 羽 狀 斑 駁 病 毒 與 甘 藷 病 毒
G 、
「WT427」感染甘藷病毒 G (Fig. 3)。4 種供
試品種亦以甘藷潛隱病毒(SPLV)的簡併式引
子對進行
RT-PCR,但未測到此病毒(data not
shown),因此進行藻膠包埋超低溫處理後的
再生植株不再偵測甘藷潛隱病毒。進行超低
溫冷凍處理後所獲得的成活植株取下位葉進
行病毒病檢測,即栽培種「台農
57 號」測試
13 再生植株、
「台農
66 號」測試 6 再生植株,
以
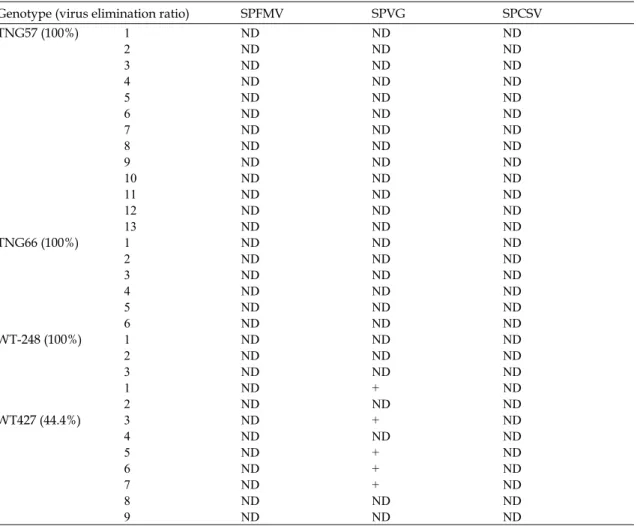
RT-PCR 均未測到上述三種病毒,去病毒
率為
100% (Fig. 4)。「WT248」測試的 3 個再
生 植 株 中 , 都 未 測 到 病 毒 , 去 病 毒 率 為
100%;野生種「WT427」測試的 9 個再生植
株中有
5 個測到甘藷病毒 G 與 4 個未測到病
毒,去病毒率為
44.4% (Fig. 5),進行病毒病
檢測之
4 品種再生植株的檢測結果如 Table
3。再生植株以西方漬染法偵測是否去病毒,
任選
4 個‘TNG57’再生植株(‘TNG57’-1~4),3
個‘TNG66’再生植株(‘TNG66’-1~3),1 個
‘T427’再生植株(‘WT427’-1),分別以甘藷病
毒
G 與甘藷黃矮病毒抗血清進行測試,結果
只於作為對照的去病毒試驗前的‘TNG57’供
Fig. 3. Agarose gel analysis showing the amplification products obtained by RT-PCR for detection viruses from 4 varieties used in cryopreservation. Total RNA of healthy and 3 viruses infected ‘Tainung66’ (Lane – , +) was used as negative and positive control. Lane M: molecular size markers.
Fig. 4. Agarose gel analysis showing the amplification products obtained by RT-PCR for detection viruses in plantlets of ‘TNG57’ and ‘TNG66’ obtained from cryopreservation by encapsulation–vitrification. Total RNA of healthy and 3 viruses infected ‘TNG66’ (Lane – , +) was used as negative and positive control. Lane M: molecular size markers.
Fig. 5. Agarose gel analysis showing the amplification products obtained by RT-PCR for detection viruses in plantlets of ‘WT427’ and ‘WT248’ obtained from cryopreservation by encapsulation–vitrification. Total RNA of healthy and 3 viruses infected ‘TNG66’ (Lane – , +) was used as negative and positive control. Lane M: molecular size markers.
Table 3. Effects of virus elimination in plantlets of 4 varieties obtained from cryopreservation by encapsulation–vitrification.
Genotype (virus elimination ratio) SPFMV SPVG SPCSV TNG57 (100%) 1 ND ND ND 2 ND ND ND 3 ND ND ND 4 ND ND ND 5 ND ND ND 6 ND ND ND 7 ND ND ND 8 ND ND ND 9 ND ND ND 10 ND ND ND 11 ND ND ND 12 ND ND ND 13 ND ND ND TNG66 (100%) 1 ND ND ND 2 ND ND ND 3 ND ND ND 4 ND ND ND 5 ND ND ND 6 ND ND ND WT-248 (100%) 1 ND ND ND 2 ND ND ND 3 ND ND ND WT427 (44.4%) 1 ND + ND 2 ND ND ND 3 ND + ND 4 ND ND ND 5 ND + ND 6 ND + ND 7 ND + ND 8 ND ND ND 9 ND ND ND
試材料分別測到甘藷病毒
G 與甘藷黃矮病
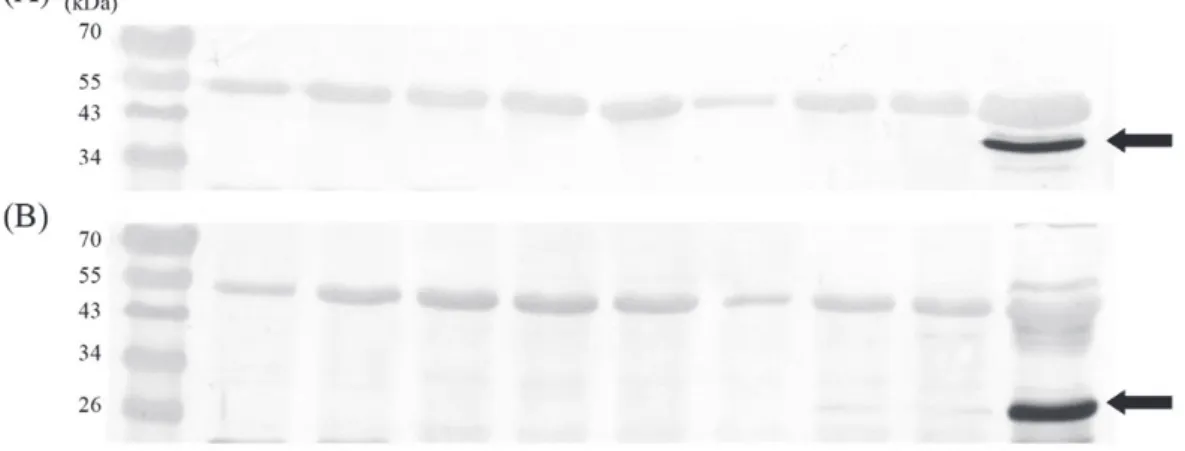
毒,‘TNG57’與‘TNG66’再生植株未測到 2 病
毒,‘WT427-1’雖於 RT-PCR 測到甘藷病毒
G,但在西方漬染法未測到此病毒(Fig. 6),
此結果與前人研究相符。甘藷羽狀斑駁病毒
與甘藷黃矮病毒複合感染時,
2 種病毒濃度都
增高,才易以抗血清偵測,單獨感染時病毒
濃 度 低 , 不 容 易 利 用 抗 血 清 偵 測 , 但 若 以
RT-PCR 偵測較無此限制。
利用藻膠包埋玻璃質化法進行超低溫冷
凍保存去病毒,已有香蕉等多個成功的例子
(Wang and Valkonen 2008b),甘藷亦有用此
方法成功去除甘藷黃矮病毒與甘藷羽狀斑駁
病毒(Wang and Valkonen 2008a)。本試驗以
此法在
4 個供試品種都成功地去除病毒。栽
培品種「台農
57」與「台農 66 號」供試材料
各感染
2 或 3 病毒,且甘藷黃矮病毒與甘藷
羽狀斑駁病毒或甘藷病毒
G 複合感染大幅提
高病毒濃度(Karyeija et al. 2000),但 2 栽培
品種再生株經檢測後去病毒率
100%,反而單
獨感染甘藷病毒
G 的野生種「WT427」去病
毒率只有
44.4%,是否因試驗操作誤差,需
要再重複試驗獲得更多再生株來釐清。Li et
al. (2012)利用複合式 RT-PCR 同時偵測甘藷
羽狀斑駁病毒、甘藷病毒
C、甘藷病毒 G 和
甘藷病毒
Y。本試驗中合併甘藷羽狀斑駁病
毒 、 甘 藷 病 毒
G 和 甘 藷 黃 矮 病 毒 於 同 一
RT-PCR,亦可大幅減少 RT-PCR 測試次數。
但在「台農
57 號」與野生種「WT248」的
產物電泳分析會有一
400 bp 微弱 DNA 條
帶,
RT-PCR 單獨檢測 3 病毒時未見此條
帶,經定序分析後為甘藷基因之非專一性反
應。
Benson (2008)指出植物超低溫冷凍時脫
水 及 玻 璃 質 化 處 理 , 使 植 物 對 低 溫 有 忍 受
性,可防止冰晶產生使器官及細胞膜減緩傷
害,因此提高細胞的存活。
Helliot et al. (2001)
說明植物無論在冷凍或解凍階段所產生的傷
害,最主要為細胞內產生的結晶水,所以可
以存活的小範圍細胞僅在葉原基及生長點的
位置(Haskins and Kartha 1980)。另外,超低
溫冷凍處理後快速的高溫處理,使病毒
RNA
的數量降低許多(Wang et al. 2008)。綜合以
上的結論,可知含有病毒的甘藷莖頂組織於
進 行 藻 膠 包 埋 時 會 經 脫 水 及 玻 璃 質 化 處 理
後,再放入液態氮(-196℃)處理 1h 後,以 40
℃水溫快速回溫
2 min,置入回復生長的培養
基,因此使成活的植株能獲得高比率的健康
株(Table 3),此結果使甘藷種原除了能長期
保存且為健康的種原具有深遠的意義。
Fig. 6. Western blotting analysis of SPVG (A) and SPCSV (B) from plantlets of obtained from cryopreservation by encapsulation–vitrification. Lanes 1-4: plantlets of ‘TNG57’-1~4; Lanes 5-7: plantlets of ‘TNG66’-1~3; Lane 8: plantlets of ‘WT427’-1; Lane +: virus infected ‘TNG57’; and Lane M: protein markers.
誌謝
本研究承蒙農業委員會科技計畫經費補
助(104 農科-9.7.1-農-C1),統計分析承農業試
驗所作物組呂副研究員椿棠及嘉義分所農藝
系廖大經助理研究員協助,並承嘉義農業試
驗 分 所 農 藝 系 賴 主 任 永 昌 博 士 協 助 英 文 斧
正,謹此一併誌謝。
引用文獻
Benson EE (2008) Cryopreservation of phyto-diversity: a critical appraisal of theory and practice. Crit. Rev. Plant Sci. 27:141–219. Brison M, MT de Boucaud, A Pierronnet, F Dosba(1997) Effect of cryopreservation on the sanitary state of a cv. Prunus rootstock experimentally contaminated with Plum Pox Potyvirus. Plant Sci. 123:189–196.
Cheng YH, LY Wang, ST Tsai, JP Wang, CC Chen, TC Deng (2012) Molecular characterization of
Sweet potato chlorotic stunt virus. (Abstract)
Plant Path. Bull. 22:183.
Chung ML, YH Hsu, MJ Chen, RJ Chiu (1986) Virus diseases of sweet potato in Taiwan. p.84-90. In: Plant Virus Diseases of Horticultural Crops in the Tropics and Subtropics. FFTC Book series33. Food and Fertilizer Technology Center for the Asian and Pacific Region. Taipei, Taiwan, ROC.
Chung ML, CH Liao, MJ Chen, RJ Chiu (1985) The isolation, transmission and host range of sweet potato leaf curl disease agent in Taiwan. (in Chinese with English abstract) Plant Prot. Bull. 27:333–342.
Chung ML, CH Liao, L Lee (1981) Effect of virus infection on the yield and quality of sweet potatoes. (in Chinese with English abstract) Plant Prot. Bull. 23: 137–141.
Clark CA, JA Davis, JA Abad, WJ Cuellar, S Fuentes, JF Kreuze, RW Gibson, SB Mukasa, AK Tugume, F Tairo, JPT Valkonen (2012) Sweetpotato viruses: 15 years of progress on understanding and managing complex diseases. Plant Dis. 96:168–185.
Clark CA, MW Hoy (2006) Effects of common viruses on yield and quality of beauregard sweet potato in Louisiana. Plant Dis. 90:83–88. Cooper VC, DGA Walkey (1978) Thermal
inactivation of cherry leaf roll virus in tissue
cultures of Nicotiana rustica raised from seeds and meristem tips. Ann. Appl. Biol. 88: 273– 278.
Green SK, YH Hsu, DE Lesemann (1989) Sweet potato leaf curl disease-Further investigation and association of a geminitype virus particle with the disease. (Abstract). Plant Prot. Bull. 31:410.
Haskins RH, KK Kartha (1980) Freeze preservation of pea meristems: cell survival. Can. J. Bot. 58:833–840.
Helliot B, B Panis, A Locicero, K Reyniers, H Muylle, M Vandewalle, C Michel, R Swennen, P Lepoivre (2001) Development of in vitro culture techniques for virus diseases elimination from Musa. In: 4th Intl. Symp. on
In Vitro Culture for Horticulture Breeding.
Acta Hortic. 560:535–538.
Helliot B, B Panis , Y Poumay, R Swennen, P Lepoivre, E Frison (2002) Cryopreservation for the elimination of cucumber mosaic and banana streak viruses from banana (Musa spp.) Plant Cell Rep. 20:1117–1122.
Hirai D, A Sakai (2003) Simplified cryo-preservation of sweet potato [Ipomoea
batatas (L.) Lam.] by optimizing conditions for
osmoprotection. Plant Cell Rep. 21:961–966. Karyeija RF, JF Kreuze, RW Gibson, JPT Valkonen
(2000) Synergistic interactions of a potyvirus and a phloem-limited crinivirus in sweet potato plants. Virology 269:26–36.
Li F, R Zuob, J Abad, D Xua, G Bao, R Li (2012) Simultaneous detection and differentiation of four closely related sweet potato potyviruses by a multiplex one-step RT-PCR. J. Virol. Meth. 186:161–166.
Liao CH, IC Chien, ML Chung, RJ Chiu, YH Han (1979) A study of sweet potato virus disease in Taiwan I: Sweet potato yellow spot virus disease. (in Chinese with English abstract) J. Agric. Res. China 28:127–138.
Li F, R Zuo, J Abad, D Xu, G Bao, R Li (2012) Simultaneous detection and differentiation of four closely related sweet potato potyviruses by a multiplex one-step RT-PCR. J. Virol Methods 186:161–166.
Liao CH, IC Chien, ML Chung, RJ Chiu, YH Han (1979) A study of sweet potato virus disease in Taiwan. I. Yellow spot virus. (in Chinese with English abstract) J. Agric. Res China 28:127– 138.
Liao CH, IC Chien, ML Chung, YH Han, RJ Chiu (1979) Two sap transmissible sweet potatoes virus occurring in Taiwan. A. Identification and some in vitro properties. In: U.S.-China Joint Seminar on Virus Diseases of Fruit and Vegetable Crops. August 15–17, 1979. Homestead, Florida, USA.
Loebenstein G, S Fuentes, J Cohen, LF Salazar (2003) Sweet potato. p.223–248. In: Virus and Virus-like Diseases of Major Crops in Developing Countries. G Loebenstein, G Thottappilly (Eds.) Kluwer Academic Publishers, Dordrecht, The Netherlands.
Loebenstein G, G Thottappilly, S Fuentes, J Cohen (2009) Virus and phytoplasma diseases. p.105-134. In: The Sweet Potato. G Loebenstein and G Totthappilly (Eds.) Springer Sciences Business Media BV, Dordrecht, The Netherlands.
Mukasa SB, PR Rubaihayo, JPT Valkonen (2006). Interactions between a crinivirus, an impomovirus and a potyvirus in co-infection sweet potato plants. Plant Pathol. 53:458–467. Rossel HW, G Thottappilly (1987) Complex virus
diseases of sweet potato. p.291-302. In: Exploration, Maintenance, and Utilization of Sweet Potato Genetic Resources. Report of first Sweet Potato Planning Conference. International Potato Centre, Lima, Peru.
Sakai A, S Kobayashi, I Oiyama (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 9:30– 33.
Schaefers GA, ER Terry (1976) Insect transmission of sweet potato disease agents in Nigeria. Phytopathology 66:642–645.
Tairo F, SB Mukasa, RAC Jones, A Kullaya, PR Rubaihayo, JPT Valkonen (2005) Unravelling the genetic diversity of the three main viruses involved in Sweet Potato Virus Disease (SPVD), and its practical implications. Mol. Plant Pathol. 6:199–211.
Walkey DGA, VC Cooper (1975) Effect of temperature on virus eradication and growth of infected tissue cultures. Ann. Appl. Biol. 80:185–190.
Wang QC, M Mawassi, P Li, R Gafny, I Sela, E Tanne (2003) Elimination of grapevine virus A (GVA) by cryopreservation of in vitro grown shoot tips of Vitis vinifera L. Plant Sci. 165:321– 327.
Wang QC, Y Liu, W He, YH Xie , MS You (2006) Cryotherapy of potato shoot tips for efficient elimination of Potato leaf roll virus (PLRV) and Potato virus Y (PVY). Potato Res. 49:119–129. Wang LY, KC Chen, TC Chen, SD Yeh (2007)
Nucleotide sequence analysis and detection application of the coat protein gene of Sweet potato latent virus isolated from central Taiwan. (in Chinese with English abstract) Plant Pathol. Bull. 16:141–148.
Wang LY, KC Chen, TC Chen, SD Yeh (2007) Nucleotide sequence analysis of the coat protein genes of two Taiwan isolates of Sweet potato feathery mottle virus from central Taiwan. Plant Pathol. Bull. 16:203–213.
Wang QC, JPT Valkonen (2008) Efficient elimination of sweet potato little leaf phytoplasma from sweet potato by cryotherapy of shoot tips. Plant Pathol. 57:338– 347.
Wang QC, JPT Valkonen (2008) Cryotherapy of shoot tips: novel pathogen eradication method. Trends Plant Sci. 14:119–122.
Wang QC, WJ Cuellar, ML Rajamakl, YM Hirata, JPT Valkonen (2008) Combined thermotherapy and cryotherapy for virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips to efficient production of virus-free plants. Mol. Plant Pathol. 9:237–250.
Wang LY, YH Cheng, NY Wang, KC Chen, SD Yeh (2013). First Report of Sweet potato virus G Infecting Sweet Potato in Taiwan. Plant Dis. 97: 260.
Yang IL (1972) Transmission of the feathery mottle complex of sweet potato in Taiwan. (in Chinese with English abstract) Taiwan Agric. Quartly 8:123–134.
Yu PC, CH Hung, SF Lo (2012) Study on Cryopreservation for Shoot Tip of Sweet Potato. (in Chinese with English abstract) Crop, Environ. Bioinform. 9:209–219.