The Analysis of Eugenol from the essential oil of
Eugenia caryophyllata by HPLC and against the
proliferation of cervical cancer cells
Wei-Chun Chang1, Meen-Woon Hsiao2, Hsi-Chin Wu3, Yin-Yi Chang1, Yao-Ching Hung 1, Je-Chiuan Ye*4,5
1 Department of Obstetrics and Gynecology, China Medical University Hospital,
Taichung, Taiwan
2 School of Applied Chemistry, Chung Shan Medical University,
Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
3 School of Medicine , China Medical University, Taichung, Taiwan
4 Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan 5
Department of Nursing, Min-Hwei College of Health Care Management *To whom Corresponding should be addressed:
Je-Chiuan Ye,
Institute of Medicine, Chung Shan Medical University,
No.110,Sec.1,Jianguo N.Rd.,Taichung City 40201, Taichung, Taiwan Tel: 886-6-3133430
Abstract
Eugenia caryophyllata Thunb is a herbal medicine and eugenol is one of
its main components. Eugenol (4-allyl-2 methoxyphenol) has known to control cholesterol and triglycerides level, reducing the activity of cancer cell and enhancing immunity in the human body. Eugenol can also be found in the vegetable oil, such as soya been oil, peanut oil and so on. This paper presents the method by using high performance liquid chromatography (HPLC) with extracting method in the Eugenia caryophyllata Thunb and Syringa vulgaris L and using a time-dependent cell culture with Eugenol (50μM) from Eugenia
caryophyllata Thunb. It also shows the eugenol data of several kinds of
vegetable by using such method and examines the anti-proliferation effect of eugenol isolated from the herb of Eugenia caryophyllata Thunb on cervical cancer cell lines. This result was tested repeatedly. The analysis parameter was received and can be used to analyze the content of the eugenol in other vegetable' s oil. We found that eugenol can suppress the growth of cervical cancer cell lines (He-La).
Introduction
Eugenia caryophyllata Thunb grows in earth of tropical areas. In Taiwan, it
has survived in low latitude of elevation area. Commonly, Eugenia
caryophyllata Thunb is used as drug in the herbal medicine stores in Asia.
Their major ingredients are caffeic acid, Ursolic acid and eugenol etc. (Hsieh, 2007). The phenolic acid is one group of phytochemicals in the plant .Eugenol is known as simplephenol compounds of plants. It was also reported with a rich contents in Ocimum minimum L (Skalicka-Woźniak et al., 2009).
The efficacy of eugenol is reported as the following in the literature and the structures of eugenol (3,4-dihydroxycinnamic acid ) shown in Fig.1. It has been reported that eugenol of Ocimum sanctum L. (OS) leaves decrease serum lipid profile in normal and diabetic animals. It was conducted to investigate the anti-hyperlipidemic and antioxidative activities of essential oil extracted from OS leaves in rats fed with high cholesterol diet (Suanarunsawat et al., 2010). Eugenol is one of the simplephenol. It can fight against two breast cancer cell lines MDA-MB-231 and MCF-7 (Abdel Bar et al., 2010). Theantinociceptive effect of eugenol on formalin-induced hyperalgesia in mice (Yano et al., 2006) and the effect of eugenol in inhibiting flavus colonization and afla-toxin
2009). Furthermore, it was also observed that additional methyl group to eugenol increases its antifungal activity (Ahmad et al., 2010).
It could be concluded that eugenol markedly protects against chemically induced skin cancer and acts possibly by virtue of its antiproliferative, anti-inflammatory, and antioxidant activities (Kaur et al., 2010). Increased expression of COX-2 gene was also abolished by eugenol (4-allyl-2
methoxyphenol) (Yogalakshmi et al., 2010). The protective action of eugenol has been found due to interception of secondary radicals derived from ER lipids rather than interfering with primary radicals (Nagababu et al., 2010).
Eugenia caryophyllata Thunb was called Din Sian in the herbal medicine
stores but Ze Din Sian is ineffective in clinic. However, they are easier to be mixed in the market. This paper adopts the high performance liquid
chromatography (HPLC) to analyze the eugenol in Din Sian, and the vegetable oils. It also adopts the method to determine the eugenol between Din Sian and Ze Din Sian. In cancer cells culture, we examined the eugenol from the herb of
Eugenia caryophyllata Thunb which significantly reduced the proliferation of
Materials and Methods I. Apparatus and Reagents:
The chromatographic system includes a HITACHI D-6500 MODEL
gradient pump (Japan), a stainless steel injector (5 μL loop), a UV-VIS detector (Jasco, Tokyo, Japan) operated at 280 nm for caffeic acid , wheat germ oil , peanut oil, potato plants, curly kale and Ocimum gratissimum extracted. A Chromolith RP-18 column (Inertsil 7 ODS-3 4.6 mm i.d., 250 mm, Merck) was used as analytical column. The optimal compositions of the mobile phase is 80﹪solution A(Diluted water with 1﹪acetic acid) and 20﹪Acetonitrile. The flow rate of the mobile phase was 1 ml/min and the column temperature was kept at 25°C. The sample solution and reagent solution were degassed before each run. The Soxhlet extracting method was used for extracting the eugenol from the desire samples. UV-Visible Spectra was taken from the spectrometer (DU-800 Beckman coulter, U.S.A). All reagents, such as methanol, ethanol, acetonitrile, acetic acid, etc, are HPLC grade from Merck (Darmstadt,
Germany). Petroleum ether from BDH (Poole, UK). Reagents was degassed in an ultrasonic bath as required before injecting into HPLC.
II. Sample Preparation:
the plant body. This dried plant was broken into pieces to become powders which are of coffee color. A sample which contains 100 grams dried powder was placed in the soxhlet extraction setup to extract eugenol for 48 hours. The dark green oil was obtained after storing the extracting oil for 7 days. This green oil was separated and as it went through glass tube column which filled the amberlite, ethanol solution was added. The liquid was obtained as HPLC sample. The sample of Syringa vulgaris L., Ocimum minimum L, Ocimum
gratissimum was obtained with the same process described as above. Soya
bean oil and peanut oil were got from supermarket in Taichung area and the HPLC samples were obtained after adding ethanol process. All samples were filtered as required before injecting into HPLC. The chemical of eugenol was purchased from Sigma-Aldrich Co. (U.S.A) and a concentration of 1mg/1ml was prepared by dissolving in methanol.
III. He-La cells experiment:
Human cervical cancer cells, HeLa cells, were obtained from ATCC. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies Inc., Grand Island, NY, USA), supplemented with 10% FBS (Life Technologies Inc.), 1% penicillin, and 100 μg/mL streptomycin (Life
containing 5% CO2 in the air. Then we collected eugenol at time that we analyzed from Eugenia caryophyllata Thunb. Taken in medium, the concentration of medium is 50μM.
IV.MTT Assay
Cells were seeded in a 96-well plate at 1000 cells per well and cultured for 24 hours. Cells were then incubated with different concentrations of
eugenol (50μM) for 48 hours. For the time course assay, the incubation times were 12, 24, and 48 hours.
After incubation, MTT was dissolved in PBS at 5 mg/mL and then added to culture medium at a final concentration of 0.5%. After incubation at 37°C for 4 hours, the medium was gently aspirated and 150 μL DMSO was added to each well to dissolve any formazan crystal. The plate was shaken for 10 minutes to allow complete solubilization. Cell viability was determined spectrophotometrically by measuring the absorbance at 570 nm using a 96-well plate reader.
Results
Figure 2a is the HPLC chromatogram of eugenol in the mobile phase 100 % solution A(Diluted water with 1%acetic acid) at pH 6.5 and the UV detector was set at 280 nm, optimized by the UV-Visible spectrometer. The retention time of eugenol in Eugenia caryophyllata Thunb is shown at 63.13 minutes
(figure2b) and it is confirmed by the standard solution in the chromatogram at the same conditions in figure 2a.
The selectivity factor and the retention time can be adjusted by varying the compositions of the mobile phase. Therefore, 95% solution A(Diluted water with 1%acetic acid) and 5% acetonitrile as mobile phase were adopted to run the samples in figure 3. 80% solution A (Diluted water with 1%acetic acid) and 20% acetonitrile as mobile phase were adopted to run the samples in figure 4a. The retention time of caffeic acid is shown at 32.15 minutes (figure 3) and 21.73 minutes (figure 4a) respectively. The retention time is reduced from 63.13 minutes to 21.25 minutes apparently. However, it can not be adjusted further to reduce the retention time with the volume ratio between solution A(Diluted water with 1%acetic acid) and acetonitrile due to the solubility problem. The optimizing conditions for analyzing eugenol in Eugenia
caryophyllata Thunb by HPLC are 80% solution A and 20% acetonitrile as
mobile phase in which the chromotogram is shown in figure 4b. This HPLC method was applied to analyze the sample obtained from Eugenia
caryophyllata Thunb. It was also applied to analyze the samples obtained from
Ze Din Sian, but no peak was found for eugenol in its chromatogram. Thus, we may suggest this HPLC method is an excellent tool to identify eugenol of
Eugenia caryophyllata Thunb scientifically.
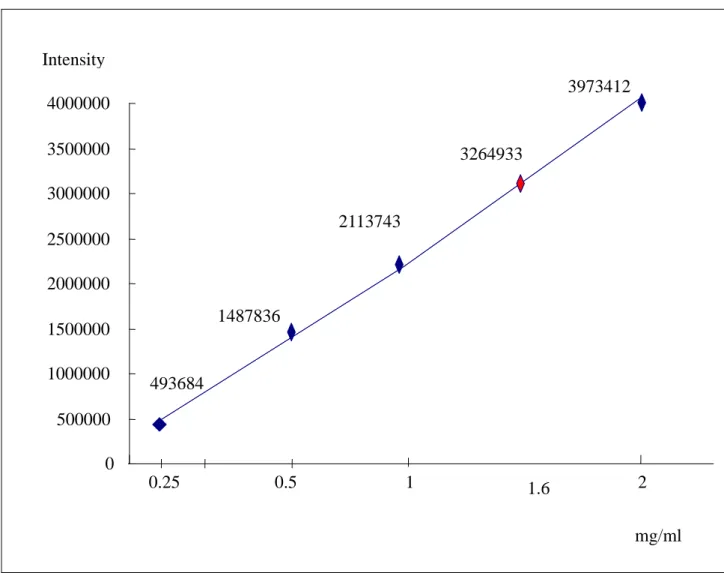
Figure 5 is a linear plot of the eugenol between 0.25 mg/ml and 2.0 mg/ml. Table I shows the concentrations of eugenol, obtained from the Taichung area in Taiwan, in the vegetable oils. The concentration of eugenol in Eugenia
caryophyllata obtained in Taiwan is 1.6mg/ml. The concentrations of eugenol in
the soya bean oil, peanut oil, Ocimum gratissimum, Ocimum minimum were 0.6 mg/ml, 0.37 mg/ml, 0.82 mg/ml and 1.41 mg/ml (Figure 6).
Table I shows the concentrations of eugenol in the vegetable oils. The data were obtained by the HPLC method and sample treatments were described in the experiment section. The activity of eugenol from Eugenia
caryophyllata Thunb to treat He-La cancer cells acts as reacting agent in
present study. We also found that the cervical cancer cell lines (He-La) are reduced and concentrated in shape obviously. (Fig.7-a) Because of MTT quantitative analysis, eugenol significantly inhibited the proliferation of HeLa cells in a time-dependent manner (see Figure 7-b). Therefore, eugenol can inhibit the proliferation of HeLa cells.
Discussion
Eugenol is one kind of phenol, and analysis phenol method has many kinds. Previous research discovery, analyse phenolic compounds in plants, already used High-Performance Liquid Chromatography-PAD -Atmospheric Pressure Chemical Ionization-Mass Spectroscopy(HPLC-PAD-APCI/ MS ) (Harris et al., 2007)and liquid chromatography method to analysis phenol (Islam et al., 2009). Fecka I. has used Thin layer chromatography (TLC) Method to analyze polyphenols (Fecka, 2009). Wang X has used validated liquid chromatography tandem mass spectrometric (LC-MS)method analysis phenethyl ester in plasma of rats (Wang et al., 2009); Mäder J has analyzed phenolic compounds in the potato during commercial potato processing with HPLC method. Point out in 2009. (Mäder et al., 2009); DellaGreca M. has used capillary gas chromatography-mass spectrometry (GC-MS) method to analyze aromatic compounds of olive oil (DellaGreca et al., 2004); Gursale A has used
reversed-phase high performance liquid chromatographic method to analyze eugenol of Cinnamomum zeylanicum Blume (Gursale et al., 2010) and Reiner GN has analyzed Lipophilicity of some GABAergic phenols and related
compounds determined by HPLC and partition coefficients in different systems (Reiner et al., 2009).
High-performance liquid chromatography (HPLC) method application has been used far and wide recently; The study indicated that this simple and efficient method can be used for quality assessment of complex matrices such us cosmetic scented products by HPLC (Villa et al., 2007) and the developed HPLC method which was used to identify and quantify cleomiscosin A,
cleomiscosin and cleomiscosin C in different extracts of seed of Cleome viscosa. (Kaur et al., 2010) Ogawa S has applied the method of HPLC to the analysis of phytochelatin synthase activity specific for soft metal ion chelators (Ogawa et al., 2010).
This is a fast method to analyze eugenol. We also found that eugenol in other vegetable's oil mainly equals to Eugenia caryophyllata Thunb, just like
Ocimum gratissimum. Because of ordinary take-in food, we may achieve the
prevention and the treatment effect. The molecular mechanism of eugenol to treat cancer cell as reacting agent is under investigation in our laboratory. It will be worth studying in the future.
Conclusion: This result was tested repeatedly. The analysis parameter
received can apply to detect eugenol content and different areas, besides Ze Din Sian, as well as other species. Thus, it can be used to analyze the content of the eugenol in other vegetable's oil. We found that eugenol can suppress
the growth of cervical cancer cell lines(He-La) and may act as an anti-cervical cancer reagent in the future.
References
Abdel Bar F.M., Khanfar M.A., Elnagar A.Y., Badria F.A., Zaghloul A.M., Ahmad K.F., Sylvester P.W., El Sayed K.A. (2010). Design and pharmacophore modeling of biaryl methyl eugenol analogs as breast cancer invasion inhibitors. Bioorg Med Chem. 18: 496-507.
Ahmad A., Khan A., Manzoor N., Khan L.A. (2010). Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb Pathog. 48: 35-41.
DellaGreca M., Previtera L., Temussi F., Zarrelli A. (2004).
Low-molecular-weight components of olive oil mill waste-waters. Phytochem Anal. 15: 184-188.
Fecka I. (2009). Development of chromatographic methods for determination of agrimoniin and related polyphenols in pharmaceutical products. J AOAC Int. 92: 410-18.
Gursale A., Dighe V., Parekh G. (2010). Simultaneous quantitative
determination of cinnamaldehyde and methyl eugenol from stem bark of Cinnamomum zeylanicum Blume using RP-HPLC. J Chromatogr Sci. 48: 59-62.
Harris C.S., Burt A.J., Saleem A., Le P.M., Martineau L.C., Haddad P.S., Bennett S.A., Arnason J.T. (2007). A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem Anal. 18: 161-69.
Islam M.N., Yoo H.H., Sung C.K., Dong M.S., Park Y.I., Jin C., Kim D.H. (2009). Simultaneous determination of phenolic acids and phthalide compounds by liquid chromatography for quality assessment of Rhizoma cnidii. J AOAC Int. 92: 375-81.
Kaur G., Athar M., Alam M.S. (2010). Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol Carcinog. 49: 290-301.
Kaur R., Kumar S., Chatterjee A., Chattopadhyay S.K. (2010).
High-performance liquid chromatographic method for identification and quantification of three potent liver protective
coumarinolignoids-cleomiscosin A, cleomiscosin B and cleomiscosin C-in extracts of Cleome viscosa. Biomed Chromatogr. 1: 32-38.
Mäder J., Rawel H., Kroh L.W. (2009). Composition of phenolic compounds and glycoalkaloids alpha-solanine and alpha-chaconine during commercial potato processing. J Agric Food Chem. 57: 6292-97.
Nagababu E., Rifkind J.M., Boindala S., Nakka L. (2010). Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol Biol. 610: 165-80.
Ogawa S., Yoshidomi T., Shirabe T. (2010). Yoshimura E. HPLC method for the determination of phytochelatin synthase activity specific for soft metal ion chelators.J Inorg Biochem. 104: 442-5.
Reiner G.N., Labuckas D.O., García D.A. (2009). Lipophilicity of some GABAergic phenols and related compounds determined by HPLC and partition coefficients in different systems. J Pharm Biomed Anal. 49: 686-91.
Skalicka-Woźniak K., Ludwiczuk A., Widelski J., Filipe J.J., Asakawa Y., Głowniak K. (2009). Volatile constituents of Ocimum minimum herb cultivated in Portugal. Nat Prod Commun. 4: 1383-86.
Suanarunsawat T., Devakul Na Ayutthaya W., Songsak T., Thirawarapan S., Poungshompoo S. (2010). Antioxidant Activity and Lipid-Lowering Effect of Essential Oils Extracted from Ocimum sanctum L. Leaves in Rats Fed with a High Cholesterol Diet. J Clin Biochem Nutr. 46: 52-59.
Sudhakar P., Latha P., Sreenivasulu Y., Reddy B.V., Hemalatha T.M., Balakrishna M., Reddy K.R. (2009). Inhibition of A spergillus flavus
colonization and aflatoxin (AfB1) in peanut by methyleugenol. Indian J Exp Biol. 47: 63-67.
Villa C., Gambaro R., Mariani E., Dorato S. (2007). High-performance liquid chromatographic method for the simultaneous determination of 24
fragrance allergens to study scented products. J Pharm Biomed Anal. 44: 755-62.
Wang X., Pang J., Maffucci J.A., Pade D.S., Newman R.A., Kerwin S.M., Bowman P.D., Stavchansky S (2009). Pharmacokinetics of caffeic acid phenethyl ester and its catechol-ring fluorinated derivative following intravenous administration to rats. Biopharm Drug Dispos. 30: 221-228. Hsieh WC(2007). Map of used Chinese medicine commonly. Committee on
Chinese Medicine and Pharmacy, Taipei Publishers, Taiwan, p.108-9. Yogalakshmi B., Viswanathan P., Anuradha C.V. (2010). Investigation of
antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology. 268: 204-12.
Yano S., Suzuki Y., Yuzurihara M., Kase Y., Takeda S., Watanabe S., Aburada M., Miyamoto K. (2006). Antinociceptive effect of methyleugenol on
Table 1 The amounts of eugenol of the vegetable oils detected by HPLC. Fig. 1 Chemical structure of eugenol
Fig. 2 Chromatogram of (a) eugenol and (b) Eugenia caryophyllata Thunb
Mobile phase was 100% Diluted water( with 1% acetic acid) at pH 6.5;
analytical column: Inertsil 7 ODS-3 4.6 mm i.d. x 250 mm; flow rate;1ml/min; 25°C; injection volume: 5 μL; detection at 280 nm.
Fig. 3 Chromatogram of eugenol
Mobile phase was 95% Diluted water (with 1% acetic acid) and 5%
Acetonitrile at pH 6.5; analytical column: Inertsil 7 ODS-3 4.6 mm i.d. x 250 mm; 1ml/min; 25°C; injection volume: 5 μL; detection at 280 nm
Fig. 4 Chromatogram of (a) eugenol and(b) Eugenia caryophyllata
Thunb
Mobile phase was 80% Diluted water( with 1% acetic acid) and 20%
Acetonitrile at pH 6.5; analytical column: Inertsil 7 ODS-3 4.6 mm i.d. x 250 mm; 1ml/min; 25°C; injection volume: 5 μL; detection at 280 nm.
Fig. 5 The concentration of eugenol in Eugenia caryophyllata Thunb caried standard concentration from 0.25mg/ml to 2mg/ml.
Fig. 6 The chart of the detected concentration of caffeic acid in Eugenia
caryophyllata Thunb and the vegetable oils.
Fig. 7 Effects of eugenol on cell morphology of He-La cells exposed to solvent, (a) 50μM eugenol for 12 hr, 24hr, 48 hr were directly observed under
microscope with 400× magnification. Eugenol can suppress the growth of cervical cancer cell lines(He-La). (b) The effects of 50μM caffeic acid were time-dependent . Values are mean
±
S.D. *P < 0.05, **P < 0.01.Table 1
No Retention
Time
Concentration
Soya been oil
21.63 0.6
mg/ml
Peanut oil
21.47 0.37
mg/ml
Ocimum gratissimum
21.36 0.82
mg/ml
Ocimum minimum
21.79 1.41
mg/ml
Fig 1 OH H2C H3CO C H CH2
Eugenol
Fig.2
63.13 63.32
Fig. 3
Fig. 4
21.25 21.73
Fig 5 0 500000 1000000 1500000 2000000 2500000 3000000 3500000 4000000 0.25 0.5 1 2 mg/ml 3973412 1.6 2113743 1487836 493684 3264933 Intensity
Fig.7(a)