www.elsevier.com / locate / chromb
Review
L
aser-induced fluorescence technique for DNA and proteins
separated by capillary electrophoresis
*
Yang-Wei Lin, Tai-Chia Chiu, Huan-Tsung Chang
Department of Chemistry, National Taiwan University, 1, Section 4, Roosevelt Road, Taipei, Taiwan
Abstract
Recent developments in capillary electrophoresis (CE) in conjunction with laser-induced fluorescence (LIF) using long-wavelength (maximum excitation wavelength.500 nm) dyes are reviewed. These dyes are particularly of interest when conducting the analyses of biopolymers by CE–LIF using He–Ne lasers. These systems are benefited from low background, low costs, easy maintenance, and compactness. Derivatizations of DNA and proteins with fluorescent or nonfluorescent chemicals can be carried out prior to, during, or after separations. With the advantages of sensitivity, rapidity, and high efficiency, the applications of CE–LIF to the analysis of polymerase chain reaction products, DNA sequencing, trace analysis of proteins, and single cell analysis have been presented.
2003 Elsevier B.V. All rights reserved. Keywords: Reviews; DNA; Proteins
Contents 1 . Introduction ... 38 2 . Analysis of DNA... 39 2 .1. Non-intercalating dyes... 40 2 .2. Intercalating dyes ... 41 2 .2.1. Mono-intercalating dyes ... 41 2 .2.2. Bis-intercalating dyes ... 43 2 .2.2.1. EthDs ... 43 2 .2.2.2. Cyanine family ... 43 3 . Analysis of proteins... 43 3
.1. Rhodamine and cyanine families ... 44 3
.2. Solvatochromic dyes ... 44 3
.3. Albumin blue series ... 45 3 .4. Phycobiliproteins ... 45 4 . Conclusions ... 46 Acknowledgements ... 46 References ... 47
*Corresponding author. Tel. / fax: 1886-2-2362-1963.
E-mail address: changht@ccms.ntu.edu.tw(H.-T. Chang).
1570-0232 / 03 / $ – see front matter 2003 Elsevier B.V. All rights reserved. doi:10.1016 / S1570-0232(03)00363-5
1
. Introduction needed and costly. Short lifetimes of He–Cd lasers are problematic. Thus, we do not doubt the
su-1
Capillary electrophoresis (CE) in conjunction with periority of Ar lasers at 488 nm and 514 nm and laser-induced fluorescence (LIF) provides utmost He–Ne lasers over the others for the analyses of speed, sensitivity and resolving power and has DNA and proteins, mainly because they are low documented for the analyses of a wide number of costly, stable, and compact. Although these two solutes. Most successful examples include single types of lasers provide the advantages of relatively molecule detection, DNA sequencing, analysis of low background from intrinsic fluorescence of the polymerase chain reaction (PCR) products, single matrix and scattered lights, they are not a suitable cell analysis, and analyses of proteins and small light source for exciting most proteins and DNA to solutes[1–9].For the past years, we have witnessed induce native fluorescence. To overcome this dis-a drdis-amdis-atic impdis-act of cdis-apilldis-ary dis-arrdis-ay electrophoresis advantage, derivatization of the analytes with a (CAE) on sequencing human genomes and drug suitable chemical is required. When derivatization is screening[10,11].It is thus our belief that techniques slow, a pre-column mode is commonly carried out to based on CE–LIF are likely to be suitable for achieve high yields in spite of the risk of contamina-sequencing further genomes for comparison, genetic tion and loss of dynamic information. If derivatiza-testing and diagnostic, as well as proteomics that tion is fast but could causes problems on separation, now represent major pressure for the development of a post-column mode is a better choice. However, an even higher throughput and more robust analytical extra connection is usually needed, leading to peak technologies. broadening and difficulty for automation. For a fast Lasers with distinct advantages of coherence, high reaction without causing loss of resolution, an on-intensity, and single wavelength have been found column mode is popular because derivatization sim-suitable for CE. To achieve low limits of detection ply takes place during separation. When the labeling (LODs), it is extremely important to minimize the agent is fluorescent, a plug of a solution containing stray light from optics and Rayleigh and Raman the agent is injected after or prior to sample in-scattering of the solvent molecules. Lasers with jection, depending on its relative mobility to that of small spots and easily focused are definitely much the analytes. For the sake of sensitivity, the fluores-better than conventional light sources in this aspect. cent complex must be stable during the separation. Several LIF designs, including cross-beam excitation When the mismatch of the system conductivities [12], epi-illumination [13], axial-beam excitation occurs, efforts must be made to minimize peak [14],and sheath-flow cuvette [15],have been tested broadening. Particular attentions must also be paid to and found effective to induce fluorescence, to collect the effect of plug length on sensitivity, speed, and fluorescence, and to minimize the background. Al- resolution. When the dye is nonfluorescent, it can be though the systems based on cross-beam excitation simply added to the background electrolyte. The dye are popular due to simplicity, CE–LIF using a concentration must be carefully evaluated in order to sheath-flow cuvette provides better sensitivity mainly optimize resolution and sensitivity because the because of a minimum Rayleigh scattering from the mobility and intensity of the fluorescent complex is capillary wall and Raman scattering from water. generally sensitive to the concentration ratio of dye / A variety of lasers have been employed for the analyte. It is important to point out that this mode is analyses of DNA and proteins by CE–LIF with suitable for both weak and strong complexes. varying degrees of success [16–19]. These include There are a number of fluorescent and nonfluores-KrF excimer laser at 248 nm, Nd:YAG laser at 266 cent reagents that are commercially available and
1
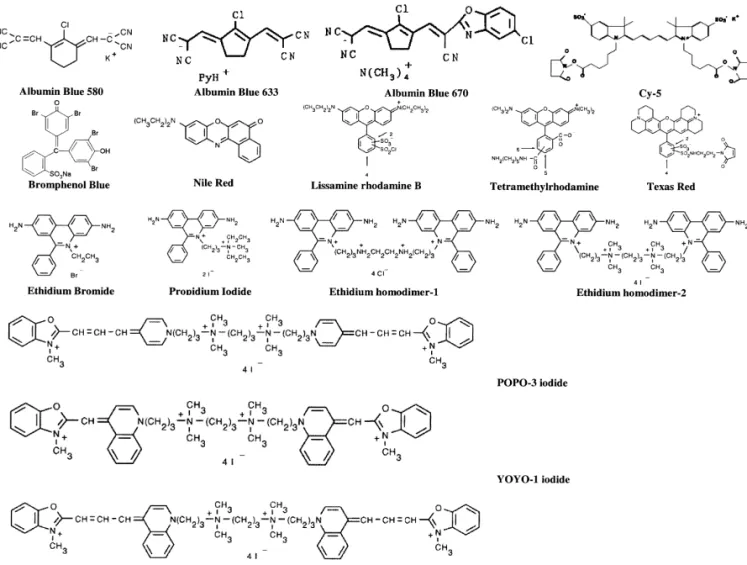
nm, Ar lasers at 275 nm, 488 nm, and / or 514 nm, conveniently used for labeling DNA and proteins. He–Cd lasers at 320 and 442 nm, and He–Ne lasers The handbook of fluorescent probes and research at 543.6, 592.6, and 633 nm. Because some proteins chemicals produced by Molecular Probes is a good have native fluorescence around 310 nm when source for the information of these reagents and excited by UV light, the first three lasers have been labeling conditions[20].Structures of some common used for trace analysis of proteins[16–18].However long-wavelength dyes for the analyses of DNA and the systems are expensive and / or maintenance is proteins are depicted inFig. 1.With respect to speed
Fig. 1. Structures of some common long-wavelength dyes used for the analyses of DNA and proteins..
and sensitivity, derivatization should be fast as well lasers that are inexpensive. He–Ne lasers are attrac-as complete and thus formed complexes should be tive, especially for those who are looking for a stable and highly fluorescent. These requirements are cost-effective LIF detection system. The costs of extremely important when monitoring dynamic He–Ne lasers (2 mW) are around US $800, about
1
changes of proteins at the single cell level. When nine-times lower than that of Ar . In this article, we analyzing highly complicated samples like biological focus on approaches to the analyses of DNA and samples, the derivatization should be selective or proteins by CE–LIF using long-wavelength dyes. sometimes specific in order to reduce possible inter- Our reference list is certainly biased by our own ferences form unwanted signals in the electropherog- interests and much important work may not be
ram. included.
Driven by the human genome project and proteomics for many years, numerous papers dealing
with the analyses of biopolymers by CE–LIF have 2 . Analysis of DNA been published, including several review articles
[21–25].With the advantages addressed above, the CE has been applied to DNA sequencing, analysis two most popular types of lasers used for the of PCR products, as well as genotyping like single
1
several excellent reviews dealing with the theory of (-21) M13 universal primers from Applied Biosys-DNA separation by CE [26–28]. Ultraviolet ab- tems / Perkin-Elmer have been commonly used for sorbance detection mode has shown useful for the DNA sequencing by CE–LIF [35,36]. Another ex-analysis of DNA mainly because the system is ample is the use of FAM / TET (6-carboxy-4,7,29, simple and DNA has a great molar absorptivity at the 79-tetrachlorofluorescein) / HEX (6-carboxy-4,7,29, wavelength of 260 nm. On the other hand, LIF has 49,59,79-hexacholofluorescein) / TAMRA labeled established a sensitive detection mode for DNA DNA primers for DNA sequencing in a 91-capillary sequencing, single molecule detection, and trace array [37]. Although these techniques have demon-analysis of DNA. In addition, labeling of DNA with strated in DNA sequencing with varying degrees of different dyes based on Sanger reaction provides the success, the influence of fluorophor dye labels on the advantage of high throughput and has been applied migration behavior of DNA must be carefully con-to DNA sequencing in CAE systems [29,30]. These sidered in order to achieve reproducible and precise techniques have played a crucial role in completing short tandem repeat (STR) genotyping [38]. the human genome sequences. Fluorescence resonance energy transfer in
con-junction with CE or CAE using suitable primers 2
.1. Non-intercalating dyes labeled with dyes such as FAM and JOE at the 59 end as a donor and other dyes such as rhodamine 6G Covalent labeling of DNA with a highly fluores- and TAMRA attached to a modified thymidine cent and stable dye, such as Lissamine rhodamine-B residue within the primer sequence as acceptor has sulfonyl chloride (Lissamine 20), tetramethyl- been demonstrated for DNA sequencing[29,39].The rhodamine isothiocyanate (TRITC), sulfoin- higher intensity of the energy transfer primers allows docyanine succinimidyl ester (Cy5) is common for DNA sequencing with minute amount of DNA DNA analysis by CE–LIF. Examples include DNA templates and employment of a single laser. Alter-sequencing and single-strand conformation polymor- natively, CE in conjunction with time-resolved fluo-phisms (SSCPs)[29,31–34].Dyes may be coupled to rescence detection using multiplex dyes including DNA through sites present naturally such as amino rhodamine derivatives has shown promise for DNA groups on the bases, hydroxyl groups on the sugars sequencing[40–42].The advantage of these methods and phosphate groups, both terminal and internal, or is the possibility of using a single diode laser for through some other reactive linker groups, such as DNA sequencing, mainly due to the similar absorp-primary amines, thiols, or aldehydes. When working tion and emission characteristics but distinct fluores-in a pre-column mode, the mafluores-in advantages fluores-include: cence lifetimes of these so-called multiplexed dyes. (1) conditions for derivatization and separation can Using secondary anti-mouse antibody labeled with be independently chose for optimum sensitivity and rhodamine has demonstrated for the determination of separation efficiency; (2) conditions can be set to thymine glycol in DNA after irradiation of human allow complete reactions for numerous analytes cells with a clinical dose of 2 Gray (Gy) [43].This without quantitative bias; (3) the separation system method allows a specific detection of thymine glycol,
221
is relatively simple and flexible. However, attentions with an LOD of |10 mol. Texas Red, with broad sometimes must be paid to overcome problems emission above 600 nm, belongs to the rhodamine associated with poor labeling efficiency and multiple family and is relatively hydrophilic compared to labeling of the analytes. rhodamine. It has been used in the analysis of SSCP Although rhodamine dyes are less bright than of K-ras genes[44].Tseng et al. have demonstrated fluorescein, background noise source is easily avoid- on-line concentration and separation of GeneScan ed when operating at longer wavelengths. This 1000 ROX, fluorescently-labeled DNA ladders that advantage has been taken to perform DNA sequenc- allow determination of the fragment lengths between ing by CE and CAE. For example, conventional 100 and 900 base pairs under denaturing conditions FAM (5-carboxyfluorescein) / JOE (6-carboxyfluores- when loading in the same capillary injection as the cein) / TAMRA (6-carboxy-N,N,N9N9-tetramethyl- experimental samples [45]. The DNA fragments rhodamine) / ROX (6-carboxyrhodamine) labeled slow down due to increases in the viscosity as well
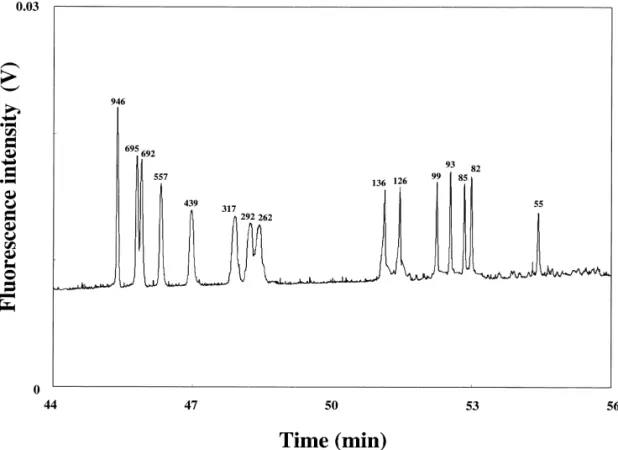
as sieving, and thus stack when migrating from the Common intercalating dyes include ethidium homo-sample zone to poly(ethylene oxide) (PEO) solution. dimer (EthD), benzoxazolium-4-pyridinium dimer Fig. 2 depicts the separation of 1.54 ml of 100-fold (POPO-3), benzoxazolium-4-quinolinium dimer diluted GeneScan 1000 ROX in 1.5% PEO, with a (YOYO-1), benzothiazolium-4-quinolinium dimer 265-fold sensitivity enhancement compared to that (TOTO-1), and ethidium bromide (EtBr), which all injected at 25 V/ cm for 10 s. It is noted that the have planar aromatic or hetero-aromatic rings that migration order is reversed to that using deactivated can be inserted between adjacent base pairs of capillary column because DNA migrates against the dsDNA. Once binding to DNA, free rotation of the electroosmotic flow (EOF). dye molecules reduces, thereby increasing fluores-cence intensity. The characteristics of intercalating 2
.2. Intercalating dyes dyes include high molar absorptivity, very low intrinsic fluorescence, large fluorescence enhance-Chemicals that are not or weakly fluorescent and ment upon binding to DNA, and moderate to high become strongly fluorescent upon forming complex- affinity for DNA.
es are excellent for CE–LIF because of low back-ground. This advantage has been well demonstrated
in the analysis of DNA, in which intercalating dyes 2 .2.1. Mono-intercalating dyes
in the background electrolytes (polymer solution) EtBr and propidium iodide (PI) are structurally form stable complexes with double-stranded (ds) similar and both have one phenanthridinium ring, DNA during electrophoretic separations [46–49]. while the last is more water soluble and less
mem-Fig. 2. Separation of a 100-fold diluted GeneScan 1000 ROX sample in the presence of EOF at 334 V/ cm using 1.5% PEO prepared in 200 mM Tris-borate (TB) buffers, pH 9.0. Reproduced with permission from Ref.[45].
brane permeant. They both bind with little or no PCR prior to performing CE–LIF for the analysis of sequence preference at a stoichiometry of one dye b-actin expression in single LNCaP (prostate cancer)
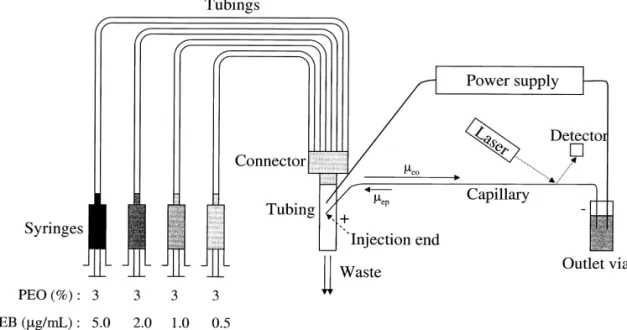
per 4–5 base pairs of DNA, with fluorescence cells [52].To improve sensitivity, Huang et al. have enhancements of 20- to 30-fold. Once binding to developed on-line concentration techniques for trace DNA, their excitation and emission wavelength analysis of DNA using PEO solution containing EtBr maxima shift about |30–40 nm to the red and |15 in the presence of EOF[53].This technique allows a nm to the blue, respectively. maximum injection volume of 5 ml and greater than Although EtBr does not bind DNA very spe- 400-fold improvements in the sensitivity compared cifically and strongly, it has been one of the most to that by conventional injections (ca. 36 nl). One popular dyes used in DNA analysis, mainly because feature of this method is no requirement for tedious it is low costly and the reaction is very fast. In CE, sample treatment prior to the analysis of large EtBr is generally added to the polymer solution and volumes of DNA prepared in high-conductivity forms fluorescent complexes with DNA immediately media. Importantly, this technique allows the analy-during separation [50,51].When binding to cationic sis of PCR products after only 17 cycles, with the EtBr, the mobility of DNA decreases and the struc- advantage of rapidity. The same group has also ture of DNA becomes stiffer, depending on the developed gradient techniques using different con-concentration of EtBr and DNA sizes as well as centrations of PEO and EtBr for separating a mixture conformation. It has been suggested that the mole of pBR322 /HaeIII, pBR328 /Bgll, and pBR 328 / ratio of EtBr / DNA 5:1 is suitable for dsDNA. CE– Hinf l digests, with optimum resolution, speed, and
LIF using EtBr has been applied to the separation of sensitivity[54,55]. Fig. 3represents a scheme show-different conformational DNA [51]. Supercoiled ing the use of a series of syringes to inject different plasmids migrate more slowly than linear dsDNA of PEO solutions to the tube, in which PEO solutions the same size using a deactivated capillary, pre- enter the capillary by EOF during separation. To sumably because plasmid migrates as an elastic rod, achieve optimized resolution and speed for the DNA while dsDNA migrates as a wormlike chain. Interest- with a wide size rang [e.g., 8 to 2176 base pairs ingly, the migration of supercoiled plasmids does not (bp)], the PEO and EtBr concentrations start from follow the elastic rod model in the presence of EOF. low (e.g., 0.5% PEO containing 0.5 mg / ml EtBr) to Zabzdyr et al. performed reverse transcriptase (RT) high (e.g., 2.0% PEO containing 5 mg / ml EtBr)[54].
2
.2.2. Bis-intercalating dyes carbons linking the cyanide monomers) are longer Bis-intercalating dyes can be applied to the analy- compared to that of TOTO-1 series, and thus they sis of dsDNA, single-stranded (ss) DNA, as well as are more suitable for the DNA analysis when using a RNA and bind strongly to DNA through their two He–Ne laser. On the other hand, TOTO-1 series are planar rings. Thus, adding excess bis-intercalating suitable for DNA analysis when an argon ion laser at dyes in the background electrolyte is prevented, 488 nm is available. TOTO series work not only for leading to very low background. There are two dsDNA, but also for ssDNA and RNA. The forma-common groups of bis-intercalating dyes: EthD and tion constants of these dyes for dsDNA are larger
8
cyanine family. Compared to mono-intercalating than 10 and one dye molecule is incorporated with dyes, bis-intercalating dyes generally provide better 10 base pairs. With such high formation constants sensitivity for DNA analysis but are more expensive. and relatively high costs (compared to EtBr), small Sometimes, band broadening is found due to differ- amounts of these dyes are generally mixed with ent binding modes such as noncooperative binding DNA prior to separation. It is interesting to note that between the non-intercalated ring and the anionic the sequence of mixing the dyes and DNA samples phosphate ion of DNA, dye–dye, or cooperative affects the electropherogram patterns. The fluores-binding involving non-intercalated rings [48]. To cence enhancements upon complexation with DNA minimize the interference from side products, the are 1100- and 3200-fold for TOTO-1 and YOYO-1, concentration ratio between dye / DNA and other respectively, which are much greater than 40-fold of reaction conditions such as ionic strength and re- using EthD [56].
action times must be carefully controlled. SY-TO series are relatively low-affinity nucleic acid stains and have been used for labeling DNA and 2
.2.2.1. EthDs. The fluorescence enhancements of RNA. Free SYTO dyes are weakly fluorescent EthD-1 and EthD-2 upon complexation with dsDNA, (quantum yields,0.01), but become strongly fluores-ssDNA, RNA, and oligonucleotides are greater than cent (quantum yields.0.4) upon binding to DNA. In 40-fold, which is independent of base compositions this series, SYTOX Orange stain provides the best or sequences [56]. Although one molecule of both sensitivity for dsDNA, with a 500-fold lower LOD EthD-1 and EthD-2 binds per 4–5 bp in dsDNA, the compared to commonly used EtBr and PI [60]. affinity of EthD-2 for DNA is about twice that of However they provide less sensitivity for DNA when EthD-1. These dyes have been employed for the compared to TOTO series. As a result, their use for analysis of DNA restriction fragments and duck DNA analysis by CE is not as popular as the TOTO hepatitis B virus using a post-column sheath-flow series.
cuvette. Owing to a high signal and small noise, this
216
system allows a concentration LOD of 3.9?10 M
for the analyte[57]. 3 . Analysis of proteins 2
.2.2.2. Cyanine family. There are two series of The analysis of proteins is of importance because bis-intercalating dyes in the cyanine family, which there is always an urgent need to understand how are TOTO series, including TOTO, YOYO, and they are responsible for cellular structure and func-BOBO, as well as SY-TO series, including SYTOX tion. Proteomics is a large-scale protein analysis and Orange, SYTO 82, and SYTO 25. TOTO series are has emerged as an important field to study protein symmetric dimmers with four positive charges and function by examining the actions of proteins as part are among the most sensitive and highest affinity of a system or process. Although the study has been fluorescent probes available for nucleic staining heavily relied on two-dimensional gel electropho-[58,59]. It is interesting to note that the optical resis, the combination of high-performance liquid properties of TOTO series can be tuned by simply chromatography, CE, and mass spectrometry has changing the aromatic rings and the number of provided an alternative tool. In addition to the carbon atoms linking the cyanide monomers. The separation based on the sieving mechanism, several excitation wavelengths of TOTO-3 series (three different approaches in CE have been developed for
the separation of proteins, including capillary zone purpose. Tetramethylrhodamine (TMR) is a widely electrophoresis, capillary isoelectric focusing, and so used fluorescent dye because it is stable and highly
on [61–63]. fluorescent [66].The fluorescence quantum
efficien-The analysis of proteins by CE using UV absorp- cies almost double after TMR-labeled proteins are tion at 280 nm is common, but it suffers from low denatured by guanidine hydrochloride. Staphylococ-sensitivity (LODs only at the level of sub-mM to cal enterotoxin B (SEB) labeled with TRITC was
mM). Unlike DNA, proteins cannot be amplified by analyzed by CE using a competitive assay, with an
PCR, and thus highly sensitive detection systems are LOD of approximately 300 fg[67].Recently, Krylov more demanded. LIF is highly sensitive and is et al. demonstrated the use of disaccharide labeled certainly in the right place for trace analysis of with TMR for a metabolism study in single cells[75] proteins. Using an argon ion laser at 275 nm, the and the use of TMR for the analysis of HT 29 cell LODs for proteins are down to nM to sub-nM, which using a multipurpose single-cell injector [76]. Lis-allows the analyses of proteins such as hemoglobin samine 20 is water soluble and reacts rapidly (t |
1 / 2 and carbonic anhydrase in single cells [64]. The 10 min) with proteins at a ratio of 2:1 by acylation of detection of single enzyme molecules has been free amino acids at their N-termini or in amino acid achieved via a technique based on CE–LIF in residues such as lysine [77]. CE–LIF provides the
219
conjunction with on-line enzyme reaction using an LOD of 3.9?10 mol for insulin labeled with argon UV laser at 360 nm[65].However the costs of Lissamine 20, which allows the investigation of the the lasers and maintenance remain a big issue for proteolysis kinetic of insulin by trypsin[78]. most of labs. In addition, these techniques can only A number of Cy dyes have been tested for the work for some proteins that have native fluorescence analysis of proteins, including Cy3, Cy3.5, Cy5, and and / or substrates that react with enzymes to produce Cy7, which have maximum excitation wavelengths fluorescent products or to use up fluorescent sub- at 550, 581, 650, and 678, respectively [68]. Cy5, stances. with a very reactive succinimidyl ester group, quick-When using a He–Ne laser, most proteins have to ly interacts with an aliphatic primary amine or be labeled with highly fluorescent substances such as hydroxyl group at neutral or alkaline pH and has rhodamine and cyanine families or non-fluorescent been used for the analysis of proteins[79–81].This molecules such as SYPRO Red, 9-diethylamino-5H- advantage allows one to perform immunoassay when benzo[a]phenoxazine-5-one (Nile Red), and albumin using antibodies or antigens labeled with Cy5 or to blue 580 (AB 580) that form stable and highly conduct enzymatic assay using substrates labeled fluorescent complexes with proteins[66–71].Gener- with Cy5. Together with high resolving power of CE ally, the reactions between these non-fluorescent and high specificity of molecular recognition, these dyes and proteins are faster and mostly the fluores- methods are highly specific and suitable for explor-cence intensities of thus formed complexes are pH ing protein interactions in very complicated bio-independent [72]. More recently, Molecular Probes logical systems. Attiya et al. developed a CE im-has commercialized Alexa Fluor family, which is munoassay method for the determination of the highly photostable, bright, and water soluble, for the concentration of ovalbumin (Ov), with an LOD of analysis of proteins[73,74]. 173 nM [82].Wu and Tsai demonstrated the use of a synthetic peptide (substrate) labeled with Cy5 for the 3
.1. Rhodamine and cyanine families measurement of the activity of kinase C and Src kinase [83].
Prior to separation, these dyes are attached to
proteins through covalent bonding. Attempts have 3 .2. Solvatochromic dyes made to accelerate the reaction speed, to increase
product yield as well as quantum efficiency, and to Solvatochromic dyes change color according to prevent loss of the activity of proteins after de- the polarity of the liquid in which they are dissolved. rivatization. As a result, there have been so many With such a characteristic, a number of solvato-derivatives of these dyes in the market for different chromic dyes including SYPRO Red and Nile Red
have been applied to the analysis of proteins in the simply adding MC 540 in the buffer electrolyte, they presence of sodium dodecyl sulfate (SDS) analyzed several proteins including hemoglobin, [69,70,84].The interactions of proteins with SYPRO conalbumin, bovine serum albumin, ovalbumin with Red and Nile Red are fast and the fluorescence the LODs at the level of nM. Please note that MC intensities of the complexes are much stronger 540 is very unstable at high pH (.9.0) or at high compared to the free dyes. Although the fluorescence concentrations of Tris buffer (.200 mM ).
intensity of the protein complexes increases in the
presence of SDS, a dramatic increase in the fluores- 3 .3. Albumin blue series cence background when the free dyes are distributed
in SDS micelles. Thus to minimize the fluorescent Albumin blue (AB) dyes, including AB 580, AB background, the concentration of SDS in the back- 633 and AB 670, are non-fluorescent and highly ground electrolyte must be kept lower than its critical specific for albumins. They quickly bind to albumins micelle concentration[84].Furthermore, the effect of in a specific way to undergo dramatic fluorescence SDS on changing the mobility of proteins must be enhancement [71].AB 633 and AB 670 provide the considered in order to achieve better resolution. LOD at the level of nM for albumins and have been Using 8% linear polyacrylamide containing 0.05% used to determine the concentration of human serum SDS, the LOD values for the standard proteins albumin (HSA) in urine samples[86].However, the labeled with SYPRO Red are at the pmol level[69]. limit stability of AB 633 and AB 670 solutions From our own experience, the sensitivity for proteins obviates their routine application. In order to over-can be improved by several-fold by adding suitable come this disadvantage, AB 580 has been developed amounts of salts like 5 mM NaCl in the background and become more popular for the analysis of al-electrolyte [85]. bumins [87,88]. The maximum excitation wave-Nile Red is highly insoluble in aqueous solution length and emission wavelength for albumin–AB and generally prepared in organic solvents such as 580 complexes are 593 and 608 nm, respectively. dimethyl sulfoxide. It has been used for protein Tseng et al. developed a method for the analysis of analyses by slab gel electrophoresis and CE, with HSA by CE–LIF using AB 580, with an LOD of LODs down to nM[84].Although Nile Red provides 11.1 nM [88].Using this method, the concentrations excellent sensitivity, problems such as loss of res- of HSA in urine and blood cells from a normal male olution and irreproducibility were found due to were determined without any pretreatment process, adsorption of dyes on the capillary wall and photo- with the results of 5.260.2 mg / l and about 8.260.2 bleaching. The use of relatively high amount of zmol / cell. Owing to highly specificity of the al-organic solvent also causes poor sensitivity. Com- bumin blue series to albumins, their use has been pared to Nile Red, merocyanine 540 (MC 540) is limited.
more suitable for the analysis of proteins by CE–
LIF, simply because of its better aqueous solubility. 3 .4. Phycobiliproteins MC 540 belongs to the family of benzoxazol
merocyanine dyes with heterocyclic aromatic groups Phycobiliproteins are water soluble and highly linked by a polymethine chain. It has been known fluorescent proteins over a wide pH range and are that its optical characteristics are very sensitive to the produced by cyanobacteria (blue–green algae) and changes in environmental factors, such as viscosity, red algae [89]. Three major phycobiliproteins are temperature, and polarity, and the quantum efficiency allophycocyanins (APCs), phycocyanins (PCs), and of MC 540 in aprotic solvent is greater because MC phycoerythrins (PEs), which have excitation and 540 tends to form dimers and undergoes a faster emission maxima at 652 / 660, 615 / 647, and photoisomerization in protic solvent. MC 540 is a 565(494) / 575 nm, respectively. Viskari and co-weak fluorescent dye in aqueous solution and forms workers showed that the LODs for three highly fluorescent complexes with proteins. Recent- phycobiliproteins are down to pM using a He–Ne ly, Chiu et al. have taken these advantages to laser [90,91].With high quantum efficiency (|1.0), perform the analysis of proteins by CE–LIF[85].By these proteins labeled with biotin, avidin, antibodies
or antigens are commonly used for the analysis of electrophoresis on a chip with varying degree of important solutes in CE[92].One main drawback of success [93–96].With the capability of integration using these proteins compared to small organic dyes and rapidity, we are sure that the so-called lab-on-a is that the steric effect might occur, leading to loss of chip techniques will soon become popular in the activity or recognition of biomolecules. scientific community. Compared to CE, highly sensi-tive detection modes, such as LIF and absorption detection methods based on thermal lens effect, and / 4
. Conclusions or approaches like on-line concentration techniques are highly demanded for chip electrophoresis due to CE–LIF has emerged as a premier technique for small sample volumes (fl) and short optical lengths. rapid, high-resolution, and sensitive analysis of Efforts to synthesize dyes that have high quantum solutes of biological interests. To achieve better efficiencies at long wavelengths and can quickly, sensitivity, the wavelength of the laser used should efficiently, and / or selectively react with biopolymers match the peak of absorbance of the analyte. Lasers will still be made. In the end, we should mention that
1
with different wavelengths, such as Ar , Nd:YAG, diode lasers or light emitting diodes will be more He–Cd, and He–Ne lasers, have been employed in widely accepted in CE and electrophoresis on a chip CE for the analyses of numerous analytes or analytes because of their low costs, long lifetime, and com-derivatized with different dyes. With the advantages pact [97–100].
of high-throughput and sensitivity, it is our belief that CE and CAE in conjunction with LIF will still play an important role in the analysis of
biopoly-mers. Although we only reviewed the analysis of A cknowledgements DNA and proteins by CE–LIF and briefly
summa-rized some results in Table 1, some formats ad- This work was supported by the National Science dressed in this article have also been tested in Council of the Republic of China under contract
T able 1
Summary of the long-wavelength dyes used for DNA and proteins
21
Reagent Molecular formula Molecular mass l /lex em(nm) Compound K (Mb ) Quantum yield LOD Ref. 7 AB 580 C H ClKN14 8 4 306.8 593 / 608 HSA 4.2?10 NG 11.1 nM [88] 7 AB 633 C H N Cl18 12 5 333.7 620 / 635 HSA 1.3?10 NG 0.2 mg / l [71] 7 AB 670 C H N OCl23 21 5 2 454.4 669 / 687 HSA 1.8?10 NG 0.2 mg / l [71] 6 BPB C H Br NaO S19 9 4 5 692.0 592 / 625 HSA 1.5?10 NG 89.2 nM [88] Cy5 C H KN O S45 54 4 14 2 978.1 649 / 670 Ov NG .0.3 173 nM [82] 5
EtBr C H BrN21 20 3 394.3 518 / 605 DNA fragments 1.5?10 .0.2 9.05 ng / ml [53] 8
EthD-1 C H Cl N46 50 4 8 856.7 528 / 580 DNA fragments 2.0?10 .0.6 NG [58]
9 216
EthD-2 C H I N51 60 4 8 1292.7 535 / 624 DNA fragments 1.0?10 .0.6 3.9?10 M [57]
Lissamine 20 C H ClN O S27 29 2 6 2 577.1 556 / 576 Insulin NG NG 35 pM [78]
Nile Red C H N O20 18 2 2 318.3 550 / 609 BSA NG NG NG [84]
8
PI C H I N27 34 2 4 668.4 535 / 617 DNA fragments 1.0?10 NG 37.4 pg / ml [60]
8 221
POPO-3 C H I N O45 58 4 6 2 1222.6 534 / 570 DNA fragments 1.5?10 .0.9 1.1?10 mol [47] 221
Rhodamine C H N O30 34 4 4 514.6 555 / 580 DNA fragments NG NG 1.0?10 mol [43] a
SYPRO Red NG 650 547 / 631 BSA NG .0.4 73 pM [69]
7
SYTO 25 NG 450 521 / 556 DNA fragments 5.0?10 .0.4 2.6 pg / ml [60] 7
SYTO 82 NG 350 541 / 560 DNA fragments 2.5?10 .0.4 1.4 pg / ml [60] 8
SYTOX Orange NG 500 547 / 570 DNA fragments 5.8?10 .0.4 0.072 pg / ml [60]
Texas Red C H N O S37 36 4 8 2 728.8 595 / 615 DNA fragments NG NG NG [44]
8 221
YOYO-1 C H I N O49 58 4 6 2 1270.6 491 / 509 DNA fragments 6.0?10 .0.9 5.0?10 mol [47]
8 221
YOYO-3 C H I N O53 62 4 6 2 1322.7 612 / 631 DNA fragments 1.5?10 .0.9 1.9?10 mol [47] a
[31] H . Zhou, A.W. Miller, Z. Sosic, B. Buchholz, A.E. Barron, L. Nos. NSC M002-058 and NSC
90-2113-Kolter, B.L. Karger, Anal. Chem. 72 (2000) 1045. M002-052.
[32] E .S. Mansfield, M. Vainer, D.W. Harris, P. Gasparini, X. Estivill, S. Surrey, P. Fortina, J. Chromatogr. A 781 (1997) 295.
´ ´
[33] G . Matyas, C. Giunta, B. Steinmann, J.P. Hossle, R. Hellwig, R
eferences
Hum. Mutat. 19 (2002) 58.
[34] W .N. Vreeland, R.J. Meagher, A.E. Barron, Anal. Chem. 74 [1] D .B. Craig, E. Arriaga, J.C.Y. Wong, H. Lu, N.J. Dovichi,
(2002) 4328. Anal. Chem. 70 (1998) 39A.
[35] O . Salas-Solano, E. Carrilho, L. Kotler, A.W. Miller, W. [2] H . Li, G. Xue, E.S. Yeung, Anal. Chem. 73 (2001) 1537.
Goetzinger, Z. Sosic, B.L. Karger, Anal. Chem. 70 (1998)
´ ´ ´ ˇ
[3] K . Kleparnık, Z. Mala, P. Bocek, Electrophoresis 22 (2001)
3996. 783.
[36] H . He, B.A. Buchholz, L. Kolter, A.W. Miller, A.E. Barron, [4] D .T. Chiu, S.J. Lillard, R.H. Scheller, R.N. Zare, S.E.
B.L. Karger, Electrophoresis 23 (2002) 1421. Rodriguez-Cruz, E.R. Williams, O. Orwar, M. Sandberg, J.A.
[37] A . Hanning, J. Westberg, J. Roeraade, Electrophoresis 21 Lundqvist, Science 279 (1998) 1190.
(2000) 3290. [5] R .T. Kennedy, J.E. Thompson, T.W. Vickroy, J. Neurosci.
[38] M . Hahn, J. Wilhelm, A. Pingoud, Electrophoresis 22 (2001) Methods 114 (2002) 39.
2691. [6] C .E. MacTaylor, A.G. Ewing, Electrophoresis 19 (1998)
[39] L . Berti, I.L. Medintz, J. Tom, R.A. Mathies, Bioconjug. 1234.
Chem. 12 (2001) 493. [7] W .-L. Tseng, H.-T. Chang, J. Chromatogr. A 924 (2001) 93.
[40] U . Lieberwirth, J. Arden-Jacob, K.H. Drexhage, D.P. Herten, [8] M .T. Bowser, R.T. Kennedy, Electrophoresis 22 (2001)
¨
R. Muller, M. Neumann, A. Schulz, S. Siebert, G. Sagner, S. 3668.
Klingel, M. Sauer, J. Wolfrum, Anal. Chem. 70 (1998) 4771. [9] M . Girod, D.W. Armstrong, Electrophoresis 23 (2002) 2048.
[41] H . He, L.B. McGown, Anal. Chem. 72 (2000) 5865. [10] L . Berti, I.L. Medintz, J. Tom, R.A. Mathies, Bioconjug.
[42] B .K. Nunnally, H. He, L.-C. Li, S.A. Tucker, L.B. McGown, Chem. 12 (2001) 493.
Anal. Chem. 69 (1997) 2392. [11] N . Zhang, H. Tan, E.S. Yeung, Anal. Chem. 71 (1999) 1138.
[43] J .Z. Xing, J. Lee, S.A. Leadon, M. Weinfeld, X.C. Le, [12] E .S. Yeung, P. Wang, W. Li, R.W. Giese, J. Chromatogr. 608
Methods 22 (2000) 157. (1992) 73.
[44] H . Arakawa, A. Tsuji, M. Maeda, M. Kamahori, H. Kam-[13] L . Hernandez, J. Escalona, N. Joshi, N. Guzman, J.
Chroma-bara, J. Pharm. Biomed. Anal. 15 (1997) 1537. togr. 559 (1991) 183.
[45] W .-L. Tseng, M.-M. Hsieh, S.-J. Wang, C.-C. Huang, Y.-C. [14] J .A. Taylor, E.S. Yeung, Anal. Chem. 64 (1992) 1741.
Lin, P.-L. Chang, H.-T. Chang, J. Chromatogr. A 927 (2001) [15] D .C. Nguyen, R.A. Keller, J.H. Jett, J.C. Martin, Anal.
179. Chem. 59 (1987) 2158.
[46] H . Zhu, S.M. Clark, S.C. Benson, H.S. Rye, A.N. Glazer, [16] H .J. Issaq, K.C. Chan, Electrophoresis 16 (1995) 467.
R.A. Mathies, Anal. Chem. 66 (1994) 1941. [17] W .-L. Tseng, H.-T. Chang, Anal. Chem. 72 (2000) 4805.
[47] D . Figeys, E.A. Arriaga, A. Renborg, N.J. Dovichi, J. [18] E .S. Yeung, J. Chromatogr. A 830 (1999) 243.
Chromatogr. A 669 (1994) 205. [19] L .J. Jin, B.C. Giordano, J.P. Landers, Anal. Chem. 73 (2001)
[48] Y . Kim, M.D. Morris, Anal. Chem. 66 (1994) 1168. 4994.
[49] J . Skeidsvoll, P.M. Ueland, Anal. Biochem. 231 (1995) 359. [20] R .P. Haugland, Handbook of Fluorescent Probes and
Re-[50] W .-L. Tseng, M.-M. Hsieh, S.-J. Wang, H.-T. Chang, J. search Chemicals, 6th ed., Molecular Probes, Eugene, OR,
Chromatogr. A 894 (2000) 219. 1996.
[51] O . de Carmejane, J.J. Schwinefus, S.-C. Wang, M.D. Morris, [21] J .C.M. Waterval, H. Lingeman, A. Bult, W.J.M. Underberg,
J. Chromatogr. A 849 (1999) 267. Electrophoresis 21 (2000) 4029.
[52] J .L. Zabzdyr, S.J. Lillard, Anal. Chem. 73 (2001) 5771.
´ ´
[22] X . Paez, L. Hernandez, Biopharm. Drug Dispos. 22 (2001)
[53] C .-C. Huang, M.-M. Hsieh, T.-C. Chiu, Y.-C. Lin, H.-T. 273.
Chang, Electrophoresis 22 (2001) 4328. [23] L . Mitnik, M. Novotny, C. Felten, S. Buonocore, L. Koutny,
[54] H .-S. Chen, H.-T. Chang, J. Chromatogr. A 853 (1999) 337. D. Schmalzing, Electrophoresis 22 (2001) 4104.
[55] M .-F. Huang, C.-E. Hsu, W.-L. Tseng, Y.-C. Lin, H.-T. [24] S . Hu, N.J. Dovichi, Anal. Chem. 74 (2002) 2833.
Chang, Electrophoresis 22 (2001) 2281. [25] W .J.M. Underberg, J.C.M. Waterval, Electrophoresis 23
[56] A .N. Glazer, H.S. Rye, Nature 359 (1992) 859. (2002) 3922.
[57] W .G. Tan, D.L.J. Tyrrell, N.J. Dovichi, J. Chromatogr. A 853 [26] G .W. Slater, C. Desruisseaux, S.J. Hubert, J.-F. Mercier, J.
(1999) 309. ´
Labrie, J. Boileau, F. Tessier, M.P. Pepin, Electrophoresis 21
[58] H .S. Rye, S. Yue, D.E. Wemmer, M.A. Quesada, R.P. (2000) 3873.
Haugland, R.A. Mathies, A.N. Glazer, Nucleic Acids Res. 20 [27] C . Heller, Electrophoresis 22 (2001) 629.
(1992) 2803. [28] S .N. Krylov, N.J. Dovichi, Anal. Chem. 72 (2000) 111R.
[59] X . Yan, W.K. Grace, T.M. Yoshida, R.C. Habbersett, N. [29] J .R. Scherer, I. Kheterpal, A. Radhakrishnan, W.W. Ja, R.A.
Velappan, J.H. Jett, R.A. Keller, B.L. Marrone, Anal. Chem. Mathies, Electrophoresis 20 (1999) 1508.
71 (1999) 5470. [30] Y . Zhang, H. Tan, E.S. Yeung, Anal. Chem. 71 (1999) 5018.
[60] X . Yan, W. Hang, V. Majidi, B.L. Marrone, T.M. Yoshida, J. [81] P . Jing, T. Kaneta, T. Imasaka, Electrophoresis 23 (2002)
Chromatogr. A 943 (2002) 275. 550.
[61] Y . Shen, R.D. Smith, J. Microcol. Sep. 12 (2000) 135. [82] S . Attiya, T. Dickinson-Laing, J. Cesarz, R.D. Giese, W.E. ´
[62] V . Dolnık, Electrophoresis 20 (1999) 3106. Lee, D. Mah, D.J. Harrison, Electrophoresis 23 (2002) 750. [63] S .H. Kang, X. Gong, E.S. Yeung, Anal. Chem. 72 (2000) [83] W .-S. Wu, J.-L. Tsai, Anal. Biochem. 269 (1999) 423.
3014. [84] J .-R. Daban, S. Bartolome, M. Samso, Anal. Biochem. 199´ ´ [64] S .J. Lillard, E.S. Yeung, R.M.A. Lautamo, D.T. Mao, J. (1991) 169.
Chromatogr. A 718 (1995) 397. [85] T .-C. Chiu, Y.-W. Lin, C.-C. Huang, A. Chrambach, H.-T. [65] M .J. Eggertson, D.B. Craig, Biomed. Chromatogr. 13 (1999) Chang, Electrophoresis 24 (2003) in press.
516. [86] M .A. Kessler, M.R. Hubmann, B.A. Dremel, O.S. Wolfbeis, [66] K . Shimura, K. Kamiya, H. Matsumoto, K. Kasai, Anal. Clin. Chem. 38 (1992) 2089.
Chem. 74 (2002) 1046. [87] M .A. Kessler, A. Meinitzer, O.S. Wolfbeis, Anal. Biochem. [67] M .T. Lam, C.A. Boulet, X.C. Le, Anal. Chim. Acta 457 248 (1997) 180.
(2002) 21. [88] W .-L. Tseng, T.-C. Chiu, J.-M. Weng, H.-T. Chang, J. Liq. [68] H .J. Gruber, C.D. Hahn, G. Kada, C.K. Riener, G.S. Harms, Chromatogr. Rel. Technol. 24 (2001) 2971.
W. Ahrer, T.G. Dax, H.-G. Knaus, Bioconjug. Chem. 11 [89] A .N. Glazer, J. Appl. Phycol. 6 (1994) 105.
(2000) 696. [90] P .J. Viskari, C.S. Kinkade, C.L. Colyer, Electrophoresis 22 [69] M .D. Harvey, D. Bandilla, P.R. Banks, Electrophoresis 19 (2001) 2327.
(1998) 2169. [91] P .J. Viskari, C.L. Colyer, J. Chromatogr. A 972 (2002) 269. ´
[70] F .J. Alba, A. Bermudez, J.-R. Daban, Electrophoresis 22 [92] K . Peck, L. Stryer, A.N. Glazer, R.A. Mathies, Proc. Natl.
(2001) 399. Acad. Sci. USA 86 (1989) 4087.
[71] M .A. Kessler, O.S. Wolfbeis, Anal. Biochem. 200 (1992) [93] J . Ferrance, J.P. Landers, Luminescence 16 (2001) 79. 254. [94] J . Khandurina, A. Guttman, J. Chromatogr. A 943 (2002) [72] E .D. Moody, P.J. Viskari, C.L. Colyer, J. Chromatogr. B 729 159.
(1999) 55. [95] P .-A. Auroux, D. Iossifidis, D.R. Reyes, A. Manz, Anal. [73] I . Biran, D.R. Walt, Anal. Chem. 74 (2002) 3046. Chem. 74 (2002) 2637.
´
[74] K . Macounova, C.R. Cabrera, P. Yager, Anal. Chem. 73 [96] I .L. Medintz, B.M. Paegel, R.G. Blazej, C.A. Emrich, L. (2001) 1627. Berti, J.R. Scherer, R.A. Mathies, Electrophoresis 22 (2001) [75] S .N. Krylov, E.A. Arriaga, Z. Zhang, N.W.C. Chan, M.M. 3845.
Palcic, N.J. Dovichi, J. Chromatogr. B 741 (2000) 31. [97] G . Jiang, S. Attiya, G. Ocvirk, W.E. Lee, D.J. Harrison, [76] S .N. Krylov, D.A. Starke, E.A. Arriaga, Z. Zhang, N.W.C. Biosens. Bioelectron. 14 (2000) 861.
Chan, M.M. Palcic, N.J. Dovichi, Anal. Chem. 72 (2000)
[98] J .E. Melanson, C.A. Boulet, C.A. Lucy, Anal. Chem. 73 872.
(2001) 1809. [77] S .N. Smith, R.P. Steer, J. Photochem. Photobiol. A 139
[99] E .M. McCorquodale, C.L. Colyer, Electrophoresis 22 (2001) (2001) 151.
2403. [78] H .B. Lim, J.J. Lee, K.-J. Lee, Electrophoresis 16 (1995) 674.
[100] M .L. Chabinyc, D.T. Chiu, J.C. McDonald, A.D. Stroock, [79] R .A. Evangelista, F.-T.A. Chen, J. Chromatogr. A 680
J.F. Christian, A.M. Karger, G.M. Whitesides, Anal. Chem. (1994) 587.
73 (2001) 4491. [80] R .B. Mujumdar, L.A. Ernst, S.R. Mujumdar, C.J. Lewis,