Isolation and Characterization of a Lectin from
Edible Mushroom, Volvariella volvacea
Jung-Yaw LIN and Tze-Bin CHOU Institute of Biochemistry, College of Medicine, National Taiwan University, Taipei, Taiwan
Received for publication, December 5, 1983
A lectin was purified from edible mushroom, Volvariella volvacea by extraction with 5% cold acetic acid in the presence of 0.1% 2-mercaptoethanol, followed by ammonium sulfate fractionation, and DEAE-C-52 and CM-C-52 column chro-matographies. The molecular weight was measured to be 26,000, and the lectin consisted of two non-identical subunits as demonstrated by gel filtration and poly-acrylamide gel electrophoresis.
The lectin does not contain half-cystine, methionine, or histidine. The LD50 of the lectin is 17.5 mg per kg body weight of mice. The lectin has a moderate inhibitory effect on the growth of tumor cells.
Lectins of plant origin are very useful in studying cell membrane structure, and they have been used for the synthesis of chimeric proteins with the A chain of ricin for inhibiting the growth of tumor cells (1). Both activities of lectins are due to their characteristic of specific affinities toward glycopro-teins or glycolipids.
In a previous investigation, a hemolytic pro-tein, volvatoxin A, was isolated from Volvariella
volvacea (2). This report describes the isolation
of a new antitumor protein, Volvariella volvacea lectin, and the chemical, physical, and biological properties of the lectin.
MATERIALS AND METHODS
The edible mushroom, Volvariella volvacea, was
Abbreviations: VAG, Volvariella volvacea lectin; SDS, sodium dodecyl sulfate; EDTA, ethylenediaminetetra-acetic acid; MSH, 2-mercaptoethanol.
purchased from a local market. TPCK-trypsin, chymotrypsinogen A and ovalbumin were obtained from Worthington Co., U.S.A. Bovine serum albumin and cytochrome c were products of Sigma Chemical Co.
Hemagglutination—Red blood cells from
hu-mans (O type) and various animals were washed with 0.01 M phosphate-buffered saline, pH 7.4. Various amounts of lectin were added to the washed red blood cells, and the reaction mixtures were incubated at 37°C for 2 h. The degree of hemagglutination was assessed according to the pattern formed by agglutinated cells on the bottom of tubes (5).
Determination of Protein—Quantitative
deter-mination of protein was performed according to the method of Lowry et al. (4).
Hemolysis—Degree of hemolysis was
mea-sured as described by Henden and Frankel-Conrat (.5).
mo-lecular weight of lectin was determined by SDS-polyacrylamide gel electrophoresis and by gel filtration with a Sephadex G-75 column. SDS-polyacrylamide gel electrophoresis was performed according to the method of Weber and Osborn
(6). A 10% separation gel was used for these
purposes. Staining was carried out with 0.15% Coomassie Brilliant Blue in 10% acetic acid. Gel filtration was carried out according to the method of Andrews (7).
Amino Acid Analysis—Protein was hydrolyzed
with constant-boiling HC1 in evacuated, sealed tubes at 105°C for 24 h. The amino acid com-positions in the hydrolysates were measured with a Beckman amino acid analyzer (model 120C)
(8). Tryptophan was determined by the
spectro-photometric method of Edelhoch (9).
Tryptic Map Analysis—VAG (20 mg) was
di-gested with trypsin by using a pH Stat (Radi-omer) at pH 8.6 and 37°C. The reaction was terminated by addition of 6 M acetic acid to pH 4. The digests were analyzed by paper electro-phoresis at pH 1.9 (formic acid : acetic acid : wa-ter, 1 : 4 : 45, v/v) followed by paper chromatog-raphy using BAWP as a solvent system (butanol : acetic acid : pyridine : water, 75 : 50 :15 : 60, v/v)
(10). The tryptic peptides were detected with
0.05 % ninhydrin in ethanol.
Disc Polyacrylamide Gel Electrophoresis—The disc polyacrylamide gel electrophoresis was per-formed by a modification of the method of Gabriel
(11). Beta-alanine buffer, pH 4.6, and Tris-glycine
buffer, pH 8.5, were used as tray buffers. In both cases, 6 % separation gel in 8 M urea was used.
Lethality—The lethality of lectin was
deter-mined according to the method of Reed and Mueuch (12).
Evaluation of Tumor Growth—Sarcoma 180
cells were used to determine the antitumor activity of lectin. Tumor inoculation was carried out by injection of 0.2 ml of ascitic fluid containing 2 x 10' cells. A single dose of lectin was injected ip 1 h after tumor transplantation. The growth of Sarcoma 180 tumor was evaluated by determining the increase of life span (13).
RESULTS
Purification of VAG—A mixture of 2 kg of
edible mushrooms (Volvariella volvacea) and 2 liters of cold 5% acetic acid containing 0.1% 2-mercaptoethanol was homogenized with a blender. The homogenate was kept at 4°C for 3 h, and then centrifuged at 10,000 rpm for 10 min with a
Sor- >-I— > LJ < 13 ID 1000 M) 60 60 FRACTION NO. 100 120
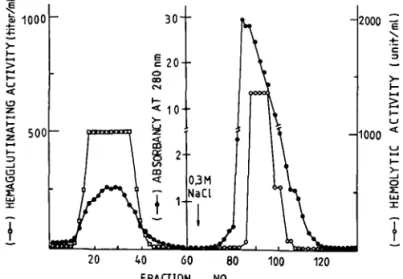
Fig. 1. Chromatography of crude extract on a DEAE-C-52 column. The super-natant of the dialysate from an ammonium sulfate fractionation was applied to a DEAE-C-52 column (4x25 cm) and eluted with 5mM Tris buffer, pH8.2 (60 frac-tions) followed by 0.3 M NaCl in 0.01 M Tris buffer, pH 8.2. The active fractions of hemagglutinating activity were pooled for ,the following experiments. Fractions of 15 ml each were collected.
vail RC-2B refrigerated centrifuge. Solid ammo-nium sulfate was added to the supernatant at 4°C to make 95% saturation. The precipitate was pelleted by centrifugation at 10,000 rpm for 10 min, and dialyzed against 5 mM sodium acetate containing 0.1 % 2-mercaptoethanol and 1 mvi EDTA at 4°C for 48 h with three changes of the buffer. The supernatant of the dialysate was sub-jected to fractionation by anion exchange column chromatography with a DEAE-C-52 column (4x 25 cm). The hemagglutinating activity was eluted with the equilibrium buffer, but it was found to be heterogeneous (Fig. 1). The peak containing hemagglutinating activity was further fractionated
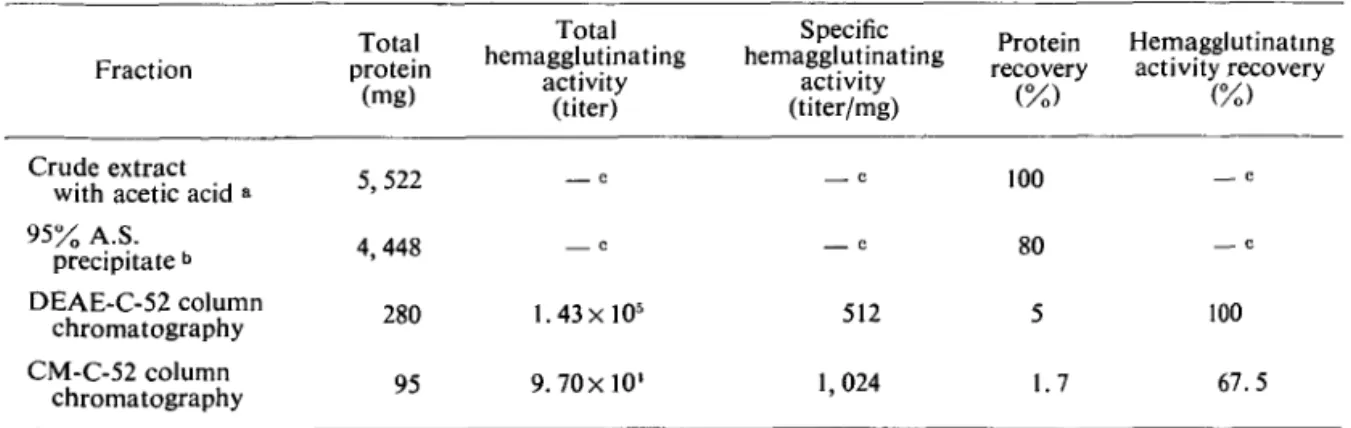
by cation exchange column chromatography with a CM-C-52 column (2x 1.2 cm). The results are shown in Fig. 2. The peak eluted with 0.2 M NaCl had hemagglutinating activity, and it was pooled, dialyzed against distilled water at 4°C and lyoph-ilized. The result of a typical purification of VAG is summarized in Table I. About 95 mg of puri-fied VAG was obtained from 2 kg of the edible mushrooms.
Amino Acid Composition—The amino acid
contents of a hydrolysate of VAG are summarized in Table II. No half-cystine, methionine, or his-tidine residue was detected in the hydrolysate. The lectin contained an unusually large amount
< 4 < 2 0,3M NaCl "2000 5 2 t— -1500 g~ IOOO t!f - 5 0 0 2>« 20 40 60 80 100 120 160 FRACTION NO.
Fig. 2. Chromatography of partially purified VAG on a CM-C-52 column. The active frac-tions obtained from the DEAE-C-52 column were applied to a CM-C-52 column (2x 1.2 cm). The column was eluted with 0.1 M, 0.2 M, 0.3 M NaCl in 0.01 M acetate buffer, pH 4.0. Fractions of 4 ml each were collected.
TABLE J. Purification of VAG from 2 kg of mushrooms {Voharieila volvacea).
Fraction
Total protein
(mg) dcuviiy(titer) y
(titer/mg)
with acetic acid a
95% A.S. precipitate b DEAE-C-52 column chromatography CM-C-52 column chromatography 5,522 4,448 280 95 c c 1.43X105 9.70x10' C c 512 1,024 100 80 5 1.7 — c c 100 67.5
a From 2 kg of mushrooms. b Ammonium sulfate. c The hemagglutinating activity could not be detected because
TABLE II. Amino acid composition of VAG. Values are the means of three different analyses.
Amino acid
Total
Residues per
mol of protein' Integer
Lys His Arg Asp Thr Ser Glu Pro Gly Ala 1/2 Cys Val Met He Leu T j r Phe Trp» 13.8 — 6.0 30.0 14.4 13.2 20.4 9.0 16.8 12.6 — 15.0 — 11.4 18.0 19.8 10.4 3.4 14 — 6 30 14
m
9 17 13 — 15 — 11 18 20 IS 213 1 The molecular weight of VAG was assumed to be26,000. b Measured spectrophotometrically.
of tyrosine residues.
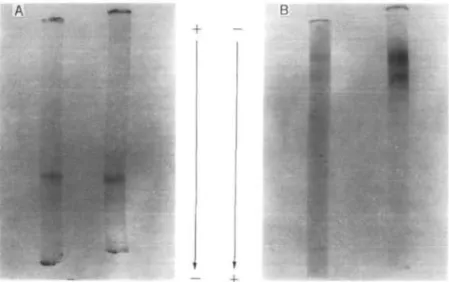
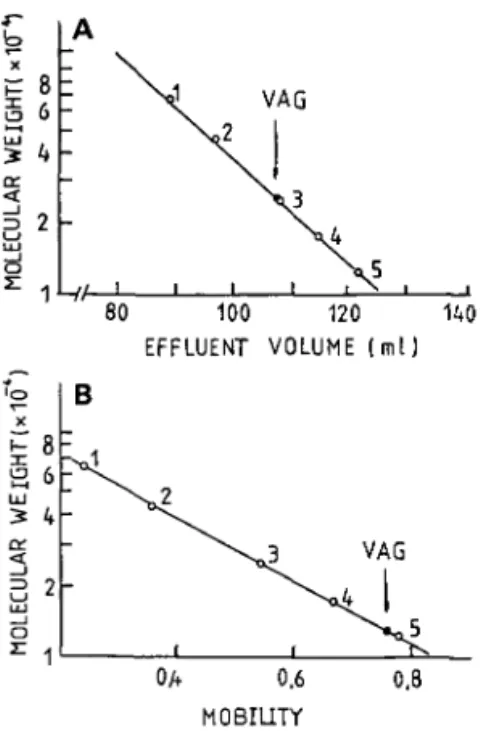
Molecular Weight—VAG gave a single band on disc polyacrylamide gel electrophoresis at pH 4.6, but it gave two bands at pH 8.5 (Fig. 3). The molecular weight of the lectin was determined to be 26,000 + 2,000 by gel filtration with a Sephadex G-75 column (Fig. 4A), but it was found to be 13,000± 1,000 by SDS-polyacrylamide gel electro-phoresis (Fig. 4B).
Tryptic Peptide Map Analysis—The results of tryptic peptide map analysis are shown in Fig. 5. There are fourteen lysine and six arginine residues in one molecule of VAG with a molecular weight of 26,000, but only ten tryptic peptides were de-tected. This suggests that VAG consists of two non-identical subunits each with a molecular weight of 13,000 as demonstrated by disc gel elec-trophoresis at pH 8.5, and SDS-polyacrylamide gel electrophoresis.
Hemagglutinating Activity—The hemaggluti-nating activities of VAG were tested with red blood cells from different animals. The results are summarized in Table 111. The red blood cells from cat or dog have the highest titer (8,192 titer/ mg) of hemagglutinating activity for VAG while those from duck or chick have the lowest titer (32 titer/mg) or no hemagglutinating activity at all.
Fig. 3. Disc polyacrylamide gel electrophoresis of purified VAG. The purified VAG (about 20/<g after the step of CM-C-52 column chromatography) was applied to 6% separation gel in 8 M urea in 10% /?-alanine buffer, pH4.6, (A) or in 0.1 M Tris-glycine buffer, pH 8.5, (B). The patterns shown on the left and right sides in (A) and (B) are for VAG after and before MSH reduction, respectively. MSH reduction was carried out by treating VAG (1 mg/ml) with 5% MSH at 37°C for 2h.
FIRST DIMENSION VAG 80 100 120 140 EFFLUENT VOLUME ( m l ) VAG 0A 0,6 MOBIUTY 0,8
Fig. 4. Estimation of the molecular weight of VAG by gel filtration on Sephadex G-75 and by SDS-poly-acrylamide gel electrophoresis. (A) Gel filtration: The Sephadex G-75 column (1.5x93 cm) was equilibrated with 0.01 M phosphate buffer, pH 7.4 and eluted with the same buffer. The flow rate was 30 ml/h, and frac-tions of 2 ml each were collected. Molecular weight markers used were; 1, bovine serum albumin (mol. wt. 67,000); 2, ovalbumin (mol. wt. 45,000); 3, chymotryp-sinogen A (mol. wt. 25,000); 4, myoglobin (mol. wt. 17,800); 5, cytochrome c (mol. wt. 12,400). (B) SDS-polyacrylamide gel electrophoresis: The experiment was carried out as described in " MATERIALS AND METHODS." Molecular weight markers used were the same as those in gel filtration.
The titer for VAG to agglutinate Sarcoma 180 cells was 128 per mg. The hemagglutinating ac-tivity of VAG was quite stable to high temper-ature. At 1 mg/ml, VAG is stable up to 80°C (2h incubation). However, 25% of the hemag-glutinating activity was lost when VAG was treated at 90°C for 30 min. The hemagglutinating activity of VAG (1 mg/ml) was also quite stable at extreme pH. It was fully active at pH 2 or 11 or 24 h. The hemagglutinating activity of VAG (1 mg/ml) was not inhibited by any of the following sugars (0.3 M) at room temperature: D-glucose, D-galac-tose, D-mannose, L-fucose, D-xylose, D-arabinose, lactose, maltose, raffinose, sucrose,
N-acetyl-D-o
oo
CO
Fig. 5. Tryptic map analysis was carried out accord-ing to the method described in " MATERIALS AND METHODS." The first dimension is paper electro-phoresis at pH1.9 followed by a second dimension of paper chromatography using BAWP (butanol : acetic acid : pyridine : water, 75 : 50 : 15 : 60, v/v) as the solvent system.
TABLE III. Hemagglutinating activity of VAG.
RBC species Human A type Btype O type AB type Albino rat Swiss mouse Guinea pig Long Evans rat Chicken Duck Cat D o g Frog Specific hemagglutinating activity (titer/mg) 64 64 64 128 1,024 1,024 512 2,048 0 32 8,192 8,192 64
glucosamine, and JV-acetyl-D-galactosamine. It is likely that VAG has affinity for a complex car-bohydrate structure on the cell surface (14).
Lethality—The LD50 of VAG was determined to be 17.5 mg per kg body weight of mice. The lethality of VAG is much lower than that of volvatoxin A, which causes hemolysis (2).
Antitumor Activity—In these experiments, 12
mice were used in each group, and the survival time was expressed as mean±S.D. The life span
- 2 - 3
2 8 10 12
OAYS
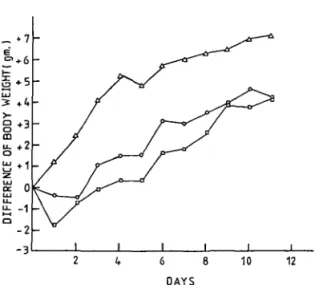
Fig. 6. Effect of VAG on the growth of experimental tumor. The experiments were carried out as described in "MATERIALS AND METHODS." A, control group; O, 85/j.g VAG/mouse; • , 175/*g VAG/mouse.
of the control group after inoculation of Sarcoma 180 cells was 12.5 ± 5 days. The test group given 85 fig of VAG showed a 63.2% increase of life span, while the group inoculated with 175 /xg of VAG showed a 109.7% increase. The body weight changes of the control and test groups are shown in Fig. 6. VAG retarded the growth of Sarcoma 180 cells.
DISCUSSION
In this work, 2-mercaptoethanol was used during the preparation of VAG to inactivate peroxidases which were very active in the aqueous homogenate of Volvariella volvacea and affected the yield of VAG.
Several lectins have been purified from mush-rooms. That from Flammulina veltipes has a mo-lecular weight of 20,000, and can be dissociated into two non-identical subunits with molecular weights of 12,000 and 8,000 (14). The lectin from
Agricus campestris has a molecular weight of
64,000 and a four-chain structure of the A2B2 type was proposed (75). Two lectins have been par-tially purified from Agricus bisporus, and one of them was found to have a molecular weight of 58,500 (16). The molecular weight of VAG was
found to 26,000 by gel nitration at pH 7.4, but on SDS-polyacrylamide gel electrophoresis, the molecular weight of VAG decreased to 13,000. The probability that VAG consists of two non-identical subunits was raised by the amino acid composition data coupled with tryptic peptide map analysis and the result of disc gel electrophoresis at pH 8.5.
The hemagglutinating activity of VAG is simi-lar to that of lectin isolated from Flammulina
veltipes but quite different from those of lectins
from Agricus campestris and Agricus bisporus, be-cause no simple specific sugar can inhibit the hemagglutination caused by VAG or the Flammu-lina lectin. Though the lethality of VAG to mice is very low in comparison with that of other toxic proteins (17), VAG has a moderate inhibitory effect on the growth of Sarcoma 180 cells.
REFERENCE
1. Olsnes.S. & Pihl.A. (1982) Pharmacol. Ther. 15, 355-381
2. Lin, J.Y., Jeng, T.W., Chen, C.C., Shi, G.Y., & Tung, T.C. (1973) Nature 245, 324-325
3. Stavitsdey, A.B. (1954) / . Immunol. 72, 360-367 4. Lowry, O.H., Rosebrough, N.J., Farr, A.L., & Randall, R.J. (1951) / . Biol. Chem. 183, 265-275 5. Henden, H.A. & Frankel-Conrat, H. (1971) Proc.
Natl. Acad. Set. U.S. 68, 1560-1563
6. Weber, K. & Osborn, M. (1969) / . Biol. Chem.
244, 4406-4412
7. Andrews, P. (1964) Biochem. J. 91, 222-233 8. Spackman, D.H., Stein, W.H., & Moore, S. (1958)
Anal. Chem. 30, 1190-1206
9. Edelhoch, H. (1967) Biochemistry 6, 1948-1954 10. Chernoff, A.I. & Liu, J.C. (1961) Blood 17, 54-70 11. Gabriel, O. (1971) Methods Enzymol. 22, 564-578 12. Reed, L.J. & Mueuch, H. (1938) Am. J. Hyg. 27,
493-497
13. Ghose, T., Guclu, T., & Tai, J. (1975) / . Natl. Cancer Inst. 55, 1353-1357
14. Tsuda, M. (1979) / . Biochem. 86, 1463-1468 15. Sage, H.J. & Connett, S.J. (1969) / . Biol. Chem.
244, 4713^79
16. Presant, C.A. & Karnfeld, S. (1972) / . Biol. Chem.
247, 6937-6945
17. Lin, J.Y., Lee, T.C, & Tung, T.C. (1982) Cancer Res. 42, 276-279