Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in
low arsenic exposure area
Chi-Jung Chung
a, Chi-Jung Huang
a, Yeong-Shiau Pu
b, Chien-Tien Su
c, Yung-Kai Huang
d,
Ying-Ting Chen
d, Yu-Mei Hsueh
e,⁎
aGraduate Institute of Public Health, Taipei Medical University, Taipei, Taiwan bDepartment of Urology, National Taiwan University Hospital, Taipei, Taiwan cDepartment of Family Medicine, Taipei Medical University Hospital, Taipei, Taiwan
dGraduate Institute of Medical Sciences, Taipei Medical University, Taipei, Taiwan e
Department of Public Health, School of Medicine, Taipei Medical University, No. 250 Wu-Hsing Street, Taipei 110, Taiwan Received 27 March 2007; revised 23 August 2007; accepted 26 August 2007
Available online 31 August 2007
Abstract
Arsenic is a well-documented human carcinogen and is known to cause oxidative stress in cultured cells and animals. A hospital-based case–
control study was conducted to evaluate the relationship among the levels of urinary 8-hydroxydeoxyguanosine (8-OHdG), the arsenic profile, and
urothelial carcinoma (UC). Urinary 8-OHdG was measured by using high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits. The
urinary species of inorganic arsenic and their metabolites were analyzed by high-performance liquid chromatography (HPLC) and hydride
generator-atomic absorption spectrometry (HG-AAS). This study showed that the mean urinary concentration of total arsenics was significantly
higher, at 37.67 ± 2.98
μg/g creatinine, for UC patients than for healthy controls of 21.10±0.79 μg/g creatinine ( pb0.01). Urinary 8-OHdG levels
correlated with urinary total arsenic concentrations (r = 0.19, pb0.01). There were significantly higher 8-OHdG levels, of 7.48±0.97 ng/mg
creatinine in UC patients, compared to healthy controls of 5.95 ± 0.21 ng/mg creatinine. Furthermore, female UC patients had higher 8-OHdG
levels of 9.22 ± 0.75 than those of males at 5.76 ± 0.25 ng/mg creatinine ( p
b0.01). Multiple linear regression analyses revealed that high urinary
8-OHdG levels were associated with increased total arsenic concentrations, inorganic arsenite, monomethylarsonic acid (MMA), and
dime-thylarsenate (DMA) as well as the primary methylation index (PMI) even after adjusting for age, gender, and UC status. The results suggest that
oxidative DNA damage was associated with arsenic exposure, even at low urinary level of arsenic.
© 2007 Elsevier Inc. All rights reserved.
Keywords: Urothelial carcinoma; 8-Hydroxydeoxyguanosine; Urinary arsenic profile
Introduction
The occurrence of chronic arsenic poisoning is a worldwide
public health problem, and the current maximum contaminant
level of arsenic for safe drinking water is still being discussed.
Arsenic is a naturally occurring element, ubiquitous in the
environment in both organic and inorganic forms. Inorganic
arsenic is commonly found in groundwater, surface waters, and
only a very small percentage of arsenic found in many foods, such
as rice, grains, and fish (
Brown and Ross, 2002
). In addition,
humans also experience occupational exposure (
Brown and Ross,
2002
). Since 1987, the International Agency for Research on
Cancer (IARC) documented that arsenic in drinking water is
carcinogenic to humans (
IARC, 2004
). Many epidemiological
studies have reported that long-term exposure to inorganic arsenic
is associated with increased risks of skin, liver, lung, and bladder
cancers and several non-cancerous diseases (
Tapio and Grosche,
2006; Tseng, 2002; Yoshida et al., 2004
). The carcinogenic
mechanism of arsenic is still unclear but arsenic-induced
oxi-dative DNA damage has recently been proposed (
Pi et al., 2002;
Liu et al., 2003; Huang et al., 2004
).
Results from in vitro studies demonstrated a role of various
arsenic species for directly or indirectly generating oxidative
Toxicology and Applied Pharmacology 226 (2008) 14–21
www.elsevier.com/locate/ytaap
⁎ Corresponding author. Fax: +886 2 27384831. E-mail address:ymhsueh@tmu.edu.tw(Y.-M. Hsueh).
0041-008X/$ - see front matter © 2007 Elsevier Inc. All rights reserved. doi:10.1016/j.taap.2007.08.021
stress. Reactive oxygen species (ROS) can be formed during
arsenic methylation or by stimulating the NADP(H) oxidase
p22phox subunit which causes oxidative DNA damage (
Lynn
et al., 2000; Nishikawa et al., 2002; Wei et al., 2002
). The
presence of arsenic-induced oxidative damage is also evident
from some epidemiological studies. A study from Inner
Mon-golia reported that elevated serum lipid peroxide levels and
a decreased non-protein sulfhydryl concentration in a
high-arsenic exposure group were directly correlated with blood
levels of inorganic arsenic and its methylated metabolites (
Huang
et al., 2004
). And it has been shown that a strong inverse
cor-relation was evident among serum nitrite/nitrate levels and blood
inorganic arsenic, MMA and DMA (
Pi et al., 2000
). In Taiwan,
Wu et al. found that the arsenic concentration in whole blood
showed a positive association with the levels of reactive oxidants
in plasma and an inverse relationship with the level of plasma
antioxidant capacity (
Wu et al., 2001
). Recent reports have
pro-vided evidence that arsenic can cause cell damage, chromosome
instability, cell proliferation, and alter telomerase activity and
apoptosis. These alterations may be involved in tumor
progres-sion or tumorigenesis through activation of oxidative-sensitive
signaling pathways (
Kamat et al., 2005; Liu et al., 2003; Zhang
et al., 2003
).
ROS can interact with DNA to produce damage including
single- and double-stranded DNA breaks, deletions, and
nucleoside modifications (
Valko et al., 2006
). 8-OHdG, the
oxidized form of the nucleoside 2'-deoxyguanosine present in
DNA, is one of the most reliable and abundant markers of DNA
damage because it reflects extremely low levels of oxidative
damage (
Howard et al., 1998
). Previous studies demonstrated
that urinary 8-OHdG levels are higher in smokers, cancer
patients, chronic renal failure patients, and semiconductor
workers with greater urinary arsenic and chromium exposure
(
Akagi et al., 2003; Hu et al., 2006; Kimura et al., 2006; Mizoue
et al., 2006; Rozalski et al., 2002
). In addition, it was suggested
that 4 months of 4 cups/day of green tea consumption is
significantly associated with decreased urinary 8-OHdG levels
among heavy smokers (
Hakim et al., 2004
).
Our study aims to investigate the relationship between
uri-nary 8-OHdG levels and the development of arsenic-associated
urothelial carcinoma (UC) among subjects who even had low
urinary level of arsenic.
Materials and methods
Study population. This was a hospital-based case–control study. Study methods have been described in detail elsewhere (Pu et al., 2007). Briefly, the study population consisted of 170 UC cases and 402 healthy control participants from September 2002 to April 2006. All cases were diagnosed UC patients with histological confirmation. Pathological verification of UC was done by routine urological practice including endoscopic biopsy or surgical resection of urinary tract tumors followed by histopathological examination by board-certified pathologists. Cytological evidence alone was not accepted as an adequate diagnosis of UC. Bladder cancer was staged into three groups: superficial (Ta, T1, and Tis), locally advanced (T2-4N0M0), and metastatic (N+ or M+). Tumor grading was based on the WHO 1999 classification system (WHO, 1999).
Controls were frequency matched to UC cases in terms of age, ± 5 years, and gender. Healthy controls have no prior history of cancer. The majority of study population (N80%) lived in Taipei City, and recruited from the medical center
including National Taiwan University Hospital and Taipei Municipal Wan Fang Hospital. These hospitals are located in Taipei approximately 200 to 300 km away from the arsenic-contaminated areas in Taiwan. The study population mostly came from Taipei City and drank tap water. The average arsenic con-centration of tap water is 0.7μg/L with ranges from non-detectable to 4.0 μg/L examined from the Taipei Water Department of Taipei City Government. No case subjects or controls came from arsenic-contaminated areas in southwestern (Chen et al., 2003) or northeastern Taiwan (Chiou et al., 2001). The Research Ethics Committee of National Taiwan University Hospital, Taipei, Taiwan, approved the study.
All participants provided informed consent forms before sample and data collection. The study was consistent with the World Medical Association Dec-laration of Helsinki.
Questionnaire interview and participant specimen collection. Standardized personal interviews based on a structured questionnaire were carried out by a well-trained personnel. Information collected included: demographic and socio-economic characteristics; general potential risk factors for malignancies such as lifestyle, cigarette smoking, alcohol, tea, and coffee consumption; occupational history; as well as personal and family histories of disease. Status of cigarette smoking history was classified as never, former, or current at the time of diag-nosis. Spot urine samples were collected from all participants and immediately transferred to−20 °C freezer until further use for urinary arsenic and 8-OHdG levels analysis.
Measurements of urinary arsenic species. It has been shown that urinary arsenic species are stable for at least 6 months when preserved at−20 °C (Chen et al., 2002); therefore, the urine sample assay was performed within 6 months post-collection. Urinary arsenic species concentrations were determined using high-performance liquid chromatography (HPLC), linked on line a to hydride generator and atomic absorption spectrometric (HG-AAS) method (Hsueh et al., 1998). Briefly, an aliquot of 200μL was used for separation of arsenic species by HPLC (Waters 501, Waters Associates, Milford, MA, USA), and then the levels of the individual arsenic species including iAs3+, iAs5+, MMA5+, and DMA5+ were quantified by HG-AAS. Recovery rates for iAs3+, DMA5+, MMA5+, and iAs5+ranged from 93.8% to 102.2% with detection limits of 0.02, 0.08, 0.05, and 0.07μg/L, respectively. Freeze-dried SRM 2670 urine, which was obtained from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA) containing 480 ± 100μg/L arsenic, was analyzed together with urine samples of subjects as a quality control. A direct measurement of total arsenic (not sum of iAs3+, iAs5+, MMA5+, and DMA5+) in SRM 2670 was 507 ± 17μg/L (n = 4).
Determination of urinary 8-OHdG levels. Urinary specimens were centri-fuged at 1500 rpm for 10 min to remove particulates. The supernatants were used for the measurement of the 8-OHdG levels using a competitive in vitro enzyme-linked immunosorbent assay (ELISA) kit (Japan Institute for the Control of Aging, Fukuroi, Japan) (Saito et al., 2000). A 50μL urine sample and 50μL of reconstituted primary antibody were added into each well of a 8-OHdG coated microtiter plate and incubated at 37 °C for 1 h for the ELISA assay. The antibodies in the sample bound to the coated 8-OHdG were washed three times with phosphate-buffered saline. The horseradish peroxidase-conjugated sec-ondary antibody was added to the plate, followed by incubation at 37 °C for 1 h, and the unbound enzyme-labeled secondary antibody was removed and the plates again washed three times. The amount of antibody bound to the plate was determined by the development of color intensity after the addition of a substrate containing 3,3',5,5'-tetra-methyl-benzidine. The reaction was terminated by the addition of phosphoric acid, and the absorbance was measured using a computer-controlled spectrophotometric plate reader at a wavelength of 450 nm. The concentration of 8-OHdG of the urine samples was interpolated from a standard curve drawn with the assistance of logarithmic transformation. The detection range of the ELISA assay was 0.5 to 200 ng/mL. The intra-assay coefficient of variance (CV) was 9.8%, and the inter-assay CV was 6.7%. All of the 8-OHdG measurements were performed within 6 months post-collection.
Statistical analysis. Total arsenic concentration (μg/g creatinine) was the sum of urinary inorganic arsenic (iAs3+and iAs5+), and its metabolites such as MMA5+and DMA5+. The arsenic methylation capability was assessed by PMI,
defined as the ratio between the MMA5+and inorganic arsenic levels, and secondary methylation index (SMI), defined as the ratio between DMA5+and MMA5+(Tseng et al., 2005). A decrease of PMI and/or a decrease of SMI
reflected a decrease methylation capability. All significant analyses of difference between arsenic and 8-OHdG levels were based on logarithmic transformed value. Student's t-test was used to compare the differences of urinary arsenic Table 1
Urinary arsenic species concentrations in study subjects
Variable Total Mean (standard error) of arsenic concentrations in urine (μg/g creatinine)
iAs3+ iAs5+ MMA DMA iAs % MMA % DMA % PMI SMI
Total (n = 572) 26.02 (1.09) 0.61 (0.04) 0.91 (0.08) 2.50 (0.17) 22.00 (0.98) 7.01 (0.38) 9.21 (0.40) 83.77 (0.54) 3.17 (0.40) 18.09 (1.85) UC status Yes (n = 170) 37.67 (2.98) 0.86 (0.09) 1.40 (0.21) 4.53 (0.49) 30.87 (2.72) 7.18 (0.58) 13.19 (0.99) 79.63 (1.14) 4.26 (0.78) 11.57 (3.00) No (n = 402) 21.10 (0.79) 0.50 (0.05) 0.71 (0.07) 1.63 (0.11) 18.25 (0.71) 6.94 (0.48) 7.53 (0.36) 85.52 (0.58) 2.70 (0.46) 21.00 (2.30) p value b0.01 b0.01 b0.01 b0.01 b0.01 0.75 b0.01 b0.01 0.07 0.02 Healthy controls (n = 402) Age (years) b63 (n=204) 16.52 (0.94) 0.38 (0.05) 0.78 (0.10) 1.29 (0.13) 14.07 (0.83) 8.73 (0.83) 7.71 (0.55) 83.55 (0.94) 1.85 (0.21) 19.28 (2.50) ≥63 (n=198) 25.81 (1.18) 0.63 (0.08) 0.63 (0.09) 1.99 (0.18) 22.56 (1.06) 5.10 (0.41) 7.35 (0.47) 87.56 (0.63) 3.64 (0.93) 22.77 (3.88) p value b0.01 0.01 0.27 b0.01 b0.01 b0.01 0.61 b0.01 0.06 0.45 Gender Male (n = 277) 19.60 (0.85) 0.58 (0.07) 0.56 (0.06) 1.76 (0.15) 16.70 (0.73) 6.49 (0.46) 8.31 (0.46) 85.19 (0.64) 3.06 (0.64) 19.84 (2.92) Female (n = 125) 24.40 (1.65) 0.34 (0.05) 1.03 (0.18) 1.35 (0.15) 21.68 (1.54) 7.94 (1.15) 5.81 (0.50) 86.26 (1.21) 1.87 (0.27) 23.77 (3.47) p value 0.01 b0.01 0.01 0.05 b0.01 0.24 b0.01 0.43 0.09 0.39 UC patients (n = 170) Age (years) b63 (n=80) 38.09 (5.29) 0.89 (0.16) 1.47 (0.38) 4.66 (0.86) 31.08 (4.81) 7.89 (0.95) 13.93 (1.80) 78.18 (2.04) 3.52 (0.64) 8.05 (0.71) ≥63 (n=90) 37.29 (3.14) 0.83 (0.11) 1.35 (0.20) 4.42 (0.54) 30.69 (2.86) 6.55 (0.69) 12.53 (0.98) 80.92 (1.14) 4.91 (1.36) 14.72 (5.63) p value 0.90 0.76 0.79 0.81 0.95 0.26 0.50 0.24 0.36 0.24 Gender Male (n = 123) 36.05 (3.60) 0.93 (0.12) 1.30 (0.26) 4.65 (0.65) 29.17 (3.23) 7.01 (0.60) 13.94 (1.26) 79.04 (1.43) 4.31 (1.03) 11.49 (4.07) Female (n = 47) 41.89 (5.28) 0.66 (0.12) 1.69 (0.32) 4.22 (0.57) 35.32 (5.01) 7.61 (1.38) 11.22 (1.36) 81.17 (1.70) 4.14 (0.96) 11.79 (2.48) p value 0.38 0.11 0.34 0.62 0.31 0.69 0.14 0.34 0.90 0.95 Table 2
Associations between patient characteristics and urinary 8-OHdG levels Variables No. of
case/ controls
8-OHdG (ng/mg creatinine)
Total (n = 572) Healthy controls (n = 402) UC patients (n = 170) Mean (S.E.) p value Mean (S.E.) p value Mean (S.E.) p value 170/402 6.40 (0.32) 5.95 (0.21) 7.48 (0.97)⁎ Age (years) b63 80/204 6.10 (0.60) b0.01 5.46 (0.30) b0.01 7.71 (1.99)# 0.10 ≥63 90/198 6.71 (0.25) 6.45 (0.28) 7.27 (0.53) Gender Male 123/400 6.08 (0.44) b0.01 6.38 (0.35) 0.10 6.81 (1.31) b0.01 Female 47/172 7.15 (0.34) 5.76 (0.25) 9.22 (0.75)⁎ Total arsenic b12.15 13/147 5.44 (0.31) b0.01 5.50 (0.34) b0.01 4.87 (.71) 0.12 12.15–22.50 36/170 5.34 (0.25) 5.24 (0.27) 5.70 (0.60) N22.50 121/255 7.69 (0.68) 7.12 (0.42) 8.33 (1.37) Cigarette smoking Never 78/333 6.24 (0.22) 0.62 5.98 (0.24) 0.79 7.08 (0.49)⁎ 0.31 Former 66/143 6.83 (1.14) 5.84 (0.49) 7.98 (2.40) Current 26/94 6.41 (0.56) 6.05 (0.61) 7.37 (1.24) Stage Superficial 98/– 6.64 (0.48) 0.45 Locally advanced 37/– 11.17 (4.22) Metastatic 19/– 7.05 (1.19) Grade I 29/– 6.08 (0.58) 0.75 II 61/– 6.73 (0.66) III 70/– 9.06 (2.27)
All p values were tested by t-test or ANOVA to compare 8-OHdG levels stratified by age, gender, stage/grade, total arsenic, and cigarette smoking. ⁎pb0.05 and
#
profile and 8-OHdG levels between UC cases and healthy controls. ANOVA and Duncan test was used to evaluate the differences of urinary 8-OHdG levels between more than two strata of baseline characteristics. Pearson's correlation was used to assess the relationship between urinary 8-OHdG levels and the concentrations of various arsenic species. Subsequently, we developed a multiple logistic regression model to estimate the joint effects of various arsenic species and urinary 8-OHdG on UC risk, with adjustment for potential confounders. All data were analyzed using the SAS statistical package (SAS, version 8.0, Cary, NC). A p value ofb0.05 (two-sided) was considered significant.
Results
A total of 572 subjects, 170 UC patients and 402 healthy
controls, were included in this study. Their average age was
61.7 with a standard error of 0.6 years. The percentages of
former smokers and current smokers were 25.1% and 16.5%
respectively.
Concentrations of urinary arsenic profiles
As shown in
Table 1
, we found that the healthy controls age
≥63 years had significantly higher total arsenic, iAs
3+, MMA
5+,
DMA
5+, and DMA% than those in controls age
b63 years. In
addition, females had significantly lower concentrations of iAs
3+,
MMA
5+, and MMA% than males. UC patients had higher PMI
and lower SMI than healthy controls.
After adjusting for age, gender, and cigarette smoking, a strong
dose–response relationship was found between urinary total
arsenic concentrations and the risk of UC (trend analysis p
b0.01)
(data not shown). Subjects with urinary total arsenic
N22.10 μg/g
creatinine had a significantly higher risk of UC compared to those
with a urinary total arsenic
b0.15 μg/g creatinine (Odds ratio
(OR) = 12.60, 95% confidence interval (CI), 0.39 to 24.80) (data
not shown).
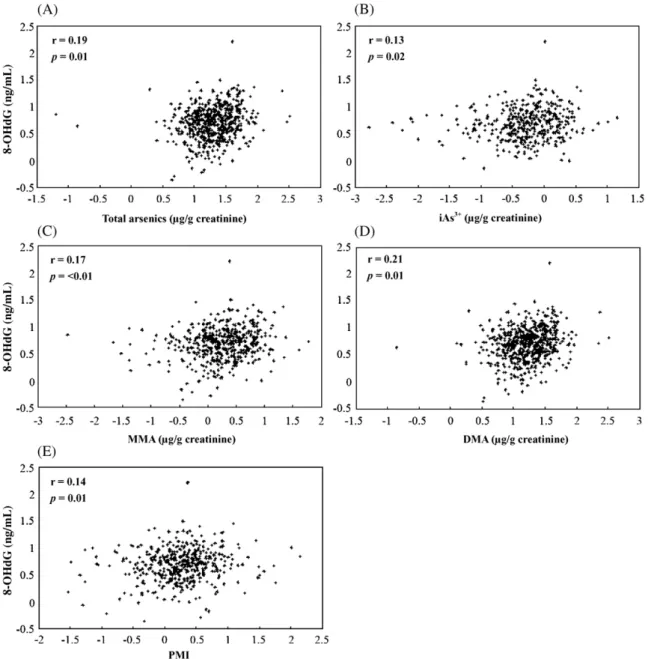
Fig. 1. Pearson's correlation between urinary 8-OHdG levels and urinary arsenic species concentrations in all study population (n = 572). (A) Total arsenics, (B) iAs3+,
Urinary 8-OHdG levels
The median urinary 8-OHdG levels for all study subjects
were 0.20 ng/mg creatinine (range, 0.43 to 160.90). UC subjects
had a significantly higher urinary 8-OHdG level than healthy
controls ( p
b0.05) (
Table 2
). Urinary 8-OHdG levels
signifi-cantly differ among different total arsenic strata. Notably,
urinary 8-OHdG levels did not increase with cigarette smoking
or with UC stage or grade.
Correlation between urinary 8-OHdG and arsenic profiles
After adjusting for age, gender, and UC status, log
10-transformed urinary 8-OHdG levels were found to be significantly
associated with the log
10-transformed concentrations of iAs
3+,
MMA
5+, DMA
5+, total arsenic, and PMI as shown in
Fig. 1
.
Joint effect of urinary 8-OHdG and arsenic profiles for UC risk
Our previous study found increase UC risk associated with
arsenic profiles (
Pu et al., 2007
). We further analyzed the
age-and gender-adjusted ORs of combination of arsenic profiles as
well as 8-OHdG for UC in
Table 3
. Significant dose–response
relationships were observed in most of the joint effects except
iAs %. In addition, elevated urinary 8-OHdG levels were
as-sociated with an increased UC risk by about 2-fold after
adjusting for age and gender ( p = 0.02). However, this
asso-ciation was not significant if further adjusted for urinary total
arsenic concentrations ( p = 0.28, data not shown).
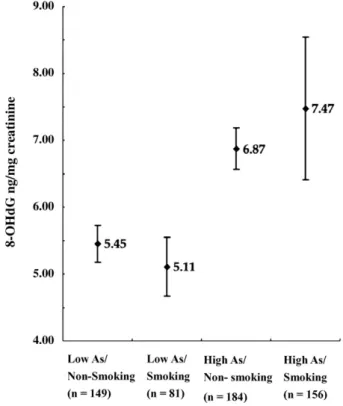
Effects of urinary total arsenic and cigarette smoking on
8-OHdG levels
Because it was found that cigarette smoking modified arsenic
methylation capacity-related UC risk (
Pu et al., 2007
), we
further evaluated whether cigarette smoking modified 8-OHdG
levels induced by arsenic or not. The urinary 8-OHdG levels
were corrected with a combination of urinary total arsenic
concentrations and cigarette smoking status of all study
pop-ulation (
Fig. 2
). The 8-OHdG levels of low arsenic and
non-smokers, low arsenic and non-smokers, high arsenic and non-smokers
as well as high arsenic and smokers were 5.45 ± 0.28, 5.11 ± 0.44,
6.87 ± 0.31, and 7.47 ± 1.07 (ANOVA test, p = 0.01). Subjects
with high arsenic whether smoking or not had higher 8-OHdG
levels than with low arsenic (Duncan test, p
b0.05). Similar
results were also observed in controls (data not shown).
Discussion
Our study evaluated the oxidative stress in UC patients and
healthy controls by measuring urinary 8-OHdG levels, which
was found to be correlated with the levels of individual urinary
arsenic species. Lower percentages of ever smokers was 41.6%
Fig. 2. Associations between urinary 8-OHdG levels and total urine arsenic concentrations and cigarette smoking status among all study population (n = 572). The cutoff of total arsenic concentration was the mean value of 16.6μg/g creatinine. High As was defined as ≥16.6 μg/g creatinine. Table 3
Age- and gender-adjusted odds ratios for UC risk with regard to urinary arsenic profile and 8-OHdG levels
Urinary arsenic profile 8-OHdG levels (ng/mg creatinine) No. of case/controls OR (95% CI)
Total arsenic (μg/g creatinine)
b16.60 b5.20 19/114 1.00⁎ ≥5.20 11/87 0.91 (0.40, 2.05) ≥16.60 b5.20 66/87 5.43 (2.92, 10.08) ≥5.20 74/114 5.05 (2.73, 9.35) iAs % b4.32 b5.20 35/99 1.00 ≥5.20 38/102 1.11 (0.64, 1.91) ≥4.32 b5.20 50/102 1.42 (0.84, 2.40) ≥5.20 47/99 1.41 (0.83, 2.39) MMA % b6.10 b5.20 17/99 1.00⁎ ≥5.20 29/102 1.68 (0.86, 3.27) ≥6.10 b5.20 68/102 3.74 (2.05, 6.82) ≥5.20 56/99 3.29 (1.77, 6.08) DMA % ≥88.00 b5.20 13/100 1.00⁎ ≥5.20 29/101 2.19 (1.07, 4.47) b88.00 b5.20 72/101 5.43 (2.82, 10.47) ≥5.20 56/100 4.32 (2.22, 8.42) PMI b1.31 b5.20 28/113 1.00⁎ ≥5.20 38/108 1.51 (0.85, 2.68) ≥1.31 b5.20 57/88 2.64 (1.54, 4.53) ≥5.20 47/93 2.14 (1.23, 3.74) ⁎Trend test, p valueb0.05.
The cutoff values were the mean values of urinary arsenic metabolites and 8-OHdG.
in this study compared to 53.6% of the official statistical survey
from Taiwanese age
N18 years old. Hence, in our study we did
not observe the effect of cigarettes smoking on oxidative stress,
which was the same as
Wen et al.'s (2005)
study. The effects of
alcohol, tea, coffee, hair dyes, and analgesic medicines were
eliminated from having had any effects on urinary 8-OHdG
levels, because there were no significant associations between
these variables and urinary 8-OHdG levels in our study.
There-fore, we might accept that urinary arsenic species were the main
effect on evaluated 8-OHdG levels.
Recently, the risk of low doses arsenic has been a questioned in
the US, European Union, and other countries. The European
Union adopted a new drinking water standard of 10
μg/L for
arsenic in 2003 while the US Environmental Protection Agency
had not adopted the new standard of 10
μg/L until 2006. Some
developing countries such as Bangladesh have kept their arsenic
standard at 50
μg/L (
Tapio and Grosche, 2006
). In Taiwan, the
standard of arsenic concentration in drinking water was decreased
from 50 to 10
μg/L in 2000. There may be minor differences in
arsenic levels between various regions in Taiwan. However,
majority of our study population (
N80%) lived in Taipei city. All
subjects recruited in this study had a urinary total arsenic
concentration of 20 to 40
μg/L even though they had consumed
drinking water containing low arsenic concentration for many
years. Besides, we found that subjects who have an unfavorable
urinary arsenic profile have an increased UC risk even at low
exposure levels recently (
Pu et al., 2007
). The exact origin of any
other possible environmental sources of inorganic arsenic in these
subjects is unknown. Our study subjects had significantly lower
urinary total arsenic concentrations than the residents of the
Blackfoot disease endemic area whose urinary total arsenic
ranged from 60 to 90
μg/L (
Tseng et al., 2005
). But our results still
showed that UC patients had a significantly high urinary arsenic
profile compared to healthy controls. The evidence for
arsenic-associated bladder cancer was previously shown with animal
models and human studies primarily through measuring
environ-mental arsenic concentrations in drinking water (
Chiou et al.,
2001; Karagas et al., 2004; Su et al., 2006
). In addition, in a study
by
Steinmaus et al. (2005)
, the mean urinary arsenic concentration
was 27.8
μg/L among metabolic products measured in urine
repeatedly collected over nearly 1 year from 81 individuals, while
the adjusted urinary total arsenic concentrations in individuals
remained constant over time (
Steinmaus et al., 2005
). In the
following year, Steinmaus et al. studied 137 patients with bladder
cancer and 163 controls from Argentina and the US. They
measured the individual urinary arsenic species and found that
individuals who excreted an increased proportion of the MMA
species were more susceptible to arsenic-related bladder cancer
(
Steinmaus et al., 2006
). However, two other studies have
demonstrated that the association of low arsenic and UC risk only
existed among smokers (
Bates et al., 2004; Steinmaus et al.,
2003
).
Conflicting data have existed for the relationship between
8-OHdG production and age, gender, cigarette smoking, and
alcohol consumption (
Irie et al., 2005; Proteggente et al., 2002;
Yamauchi et al., 2004
). We found an age-related increase in
urinary 8-OHdG levels, which supports the results of
Dhawan
and Jain (2005)
. They showed that 8-OHdG levels were
positively correlated with age in patients with essential
hyper-tension (
Dhawan and Jain, 2005
). In a Japanese study based on
372 healthy workers, Irie et al. showed that males had higher
urinary 8-OHdG levels than females (mean ± standard error,
4.17 ± 0.10 vs. 3.20 ± 0.20, p
b0.01, respectively). In addition,
smokers and alcohol consumers were reported to have higher
urinary 8-OHdG levels than non-smokers, and those not
consuming alcohol (
Irie et al., 2005; Kimura et al., 2006
).
However,
Kimura et al. (2006)
studied 248 healthy Japanese
and found that the mean urinary 8-OHdG levels did not
significantly differ among groups based upon ages (b45 and
≥45 years), gender, cigarette smoking status, or alcohol
consumption (
Kimura et al., 2006
). In the present study, females
were found to have significantly higher urinary 8-OHdG levels
than males. The reason remains to be investigated. Until now,
little information is available on the effects of other oxidative
stress sources such as coffee and tea consumption, hair dyes,
and medicines. A randomized controlled study in 2003 revealed
that regular green tea consumption might protect smokers from
oxidative damage and that drinking decaffeinated green tea for
4 months was associated with a significant decrease in urinary
8-OHdG levels (
Hakim et al., 2003
). The present study did not
find a significant association between urinary 8-OHdG levels and
UC-related risk factors such as cigarette smoking, tea and alcohol
consumption, hair dyes, and clinical stage or grade. This may be
related to small numbers of subjects with these risk factors.
Although arsenic is a human carcinogen, the mechanism of
arsenic carcinogenesis is largely unknown. Recent advances
from in vivo studies have provided strong evidence for
arsenic-induced ROS generation. It has been shown that inorganic
arsenic induced concentration-dependent and time-dependent
superoxide generation in a human keratinocyte cell line (
Shi
et al., 2004
). Dimethylated arsenic peroxide was produced by
the reaction of trivalent dimethylated arsenic with molecular
oxygen (
Yamanaka et al., 2004
). Therefore, trivalent
dimethy-lated arsenic might be more genotoxic than inorganic arsenic.
Furthermore, Wu et al. recruited 64 residents of the Lanyang
Basin in northeastern Taiwan and measured their reactive
oxi-dants and antioxidant capacity in plasma. A positive association
was found between the blood arsenic concentrations and levels
of reactive oxidants and an inverse relationship was found
between blood arsenic concentrations and levels of plasma
antioxidant capacity (
Wu et al., 2001
). Mesencephalic cells
treated with low concentrations of sodium arsenate resulted in
the activation of early transcription factors such as nuclear
factor-κB (NF-κB) and activator protein-1 (AP-1), which regulate
the expression of a variety of downstream target genes, such as
proinflammatory genes that are known to be involved in
car-cinogenesis (
Felix et al., 2005
). Oxidative stress can act in all
stages of cancer development. A non-lethal mutation in DNA
(e.g. 8-OHdG) that produces an altered cell during the initiation
followed by interrupting their cell cycle, repairing the damage,
and resuming division. The level of 8-OHdG may determine
the transformation from benign to malignant tumor (
Loft and
Poulsen, 1996
). Elevated levels of 8-OHdG have also been linked
to increased risk of cancers in breast, bladder, hepatocellular
carcinoma, non-small-cell lung cancer, etc. (
Malins et al., 2006;
Akcay et al., 2003; Ichiba et al., 2003; Shen et al., 2007
).
Our results showed that an increase in urinary 8-OHdG levels
was related with increased iAs
3+, MMA, DMA, total arsenics,
and PMI. These results are compatible with the association of
urine creatinine-adjusted 8-oxo-7,8-dihydro-2'-deoxyguanosine
(-oxodGuo) with MMA and PMI, with correlation coefficients
of 0.44 and 0.40 (p
b0.005), respectively, among semiconductor
workers with arsenic exposure as suggested by
Hu et al. (2006)
.
Because the workers had been exposed to arsenic, the total
arsenic concentrations and urinary 8-OHdG were higher than the
participants in our study. Even with low urinary total arsenic
concentrations, a clear association was observed between
uri-nary total arsenic concentrations and 8-OHdG levels.
Our study has several limitations that need to be considered
when interpreting our results. In the current study, selection bias
was minimized even through cases and controls recruited from
two different hospitals, because these hospitals both belonged to
medical centers and located in southern Taipei. Furthermore, the
majority of cases and controls lived in Taipei and were similar to
each other in socioeconomic characteristics. The UC patients
were prevalence cases and some individuals might have changed
their diet habit or increased vitamins consumption to such an
extent that their measured levels of urinary 8-OHdG were lower
compared to those of other studies (
Chiou et al., 2003; Miyake et
al., 2004; Yamauchi et al., 2004
). In addition, we only collected
tap water from 37 subjects and the mean (standard error) of total
arsenic level was 0.14 (0.55)
μg/L. Nevertheless we did not
collect the quantity of drinking water and could not explore their
historical arsenic exposure. Finally, the accuracy of one spot
evaluation of urinary arsenic and 8-OHdG may be in doubt.
However, the values might be reliable under no change of life
style in all subjects. Future studies should evaluate in more detail
exposure to arsenic and 8-OHdG levels to elucidate the
mechanisms of oxidative stress in arsenic carcinogenesis.
Conclusions
To our knowledge, this is the first study showing that urinary
8-OHdG levels are correlated with individual urinary arsenic
profiles in a human population with low arsenic exposure. Our
data provide evidence that chronic low arsenic exposure from
drinking water in humans may be related to the induction of
oxidative stress as indicated by the increase in urinary 8-OHdG
levels. Arsenic-induced oxidative stress was associated with
high levels of iAs
3+, MMA
5+, DMA
5+, and PMI. Moreover,
high levels of 8-OHdG might be predictors of arsenic-related
UC risk.
Acknowledgments
The study was supported by grants (NSC91-3112-B-038-0019,
NSC92-3112-B-038-001, NSC93-3112-B-038-001,
NSC94-2314-B-038-023, and NSC-95-2314-B-038-007) from the
Nation-al Science Council of the ROC. We thank Dr. Ying-Chin Lin of the
Health Management Center, Taipei Medical University Municipal
Wan Fang Hospital for recruitment of the healthy controls.
References
Akagi, S., Nagake, Y., Kasahara, J., Sarai, A., Kihara, T., Morimoto, H., Yano, A., Nakao, K., Nanba, K., Ichikawa, H., Makino, H., 2003. Significance of 8-hydroxy-2'-deoxyguanosine levels in patients with chronic renal failure. Nephrology 8, 192–195.
Akcay, T., Saygili, I., Andican, G., Yalcin, V., 2003. Increased formation of 8-hydroxy-2'-deoxyguanosine in peripheral blood leukocytes in bladder cancer. Urol. Int. 71, 271–274.
Bates, M.N., Rey, O.A., Biggs, M.L., Hopenhayn, C., Moore, L.E., Kalman, D., Steinmaus, C., Smith, A.H., 2004. Case–control study of bladder cancer and exposure to arsenic in Argentina. Am. J. Epidemiol. 159, 381–389. Brown, K.G., Ross, G.L., 2002. Arsenic, drinking water, and health: a position paper
of the American Council on Science and Health. Regul. Toxicol. Pharmacol. 36, 162–174.
Chen, Y.C., Amarasiriwardena, C.J., Hsueh, Y.M., Christiani, D.C., 2002. Stability of arsenic species and insoluble arsenic in human urine. Cancer Epidemiol. Biomark. Prev. 11, 1427–1433.
Chen, Y.C., Su, H.J., Guo, Y.L., Hsueh, Y.M., Smith, T.J., Ryan, L.M., Lee, M.S., Christiani, D.C., 2003. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control 14, 303–310.
Chiou, H.Y., Chiou, S.T., Hsu, Y.H., Chou, Y.L., Tseng, C.H., Wei, M.L., Chen, C.J., 2001. Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am. J. Epidemiol. 153, 411–418.
Chiou, C.C., Chang, P.Y., Chan, E.C., Wu, T.L., Tsao, K.C., Wu, J.T., 2003. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin. Chim. Acta 334, 87–94.
Dhawan, V., Jain, S., 2005. Garlic supplementation prevents oxidative DNA damage in essential hypertension. Mol. Cell. Biochem. 275, 85–94. Felix, K., Manna, S.K., Wise, K., Barr, J., Ramesh, G.T., 2005. Low levels of
arsenite activates nuclear factor-kappaB and activator protein-1 in immor-talized mesencephalic cells. J. Biochem. Mol. Toxicol. 19, 67–77. Hakim, I.A., Harris, R.B., Brown, S., Chow, H.H., Wiseman, S., Agarwal, S.,
Talbot, W., 2003. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J. Nutr. 133, 3303S–3309S.
Hakim, I.A., Harris, R.B., Chow, H.H., Dean, M., Brown, S., Ali, I.U., 2004. Effect of a 4-month tea intervention on oxidative DNA damage among heavy smokers: role of glutathione S-transferase genotypes. Cancer Epidemiol. Biomark. Prev. 13, 242–249.
Howard, D.J., Ota, R.B., Briggs, L.A., Hampton, M., Pritsos, C.A., 1998. Environmental tobacco smoke in the workplace induces oxidative stress in employees, including increased production of 8-hydroxy-2'-deoxyguano-sine. Cancer Epidemiol. Biomark. Prev. 7, 141–146.
Hsueh, Y.M., Huang, Y.L., Huang, C.C., Wu, W.L., Chen, H.M., Yang, M.H., Lue, L.C., Chen, C.J., 1998. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J. Toxicol. Environ. Health 54, 431–444.
Hu, C.W., Pan, C.H., Huang, Y.L., Wu, M.T., Chang, L.W., Wang, C.J., Chao, M.R., 2006. Effects of arsenic exposure among semiconductor workers: a cautionary note on urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine. Free Radic. Biol. Med. 40, 1273–1278.
Huang, C., Ke, Q., Costa, M., Shi, X., 2004. Molecular mechanisms of arsenic carcinogenesis. Mol. Cell. Biochem. 255, 57–66.
IARC, 2004. Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 84, 1–477. Ichiba, M., Maeta, Y., Mukoyama, T., Saeki, T., Yasui, S., Kanbe, T., Okano, J.,
Tanabe, Y., Hirooka, Y., Yamada, S., Kurimasa, A., Murawaki, Y., Shiota, G., 2003. Expression of 8-hydroxy-2'-deoxyguanosine in chronic liver disease and hepatocellular carcinoma. Liver Int. 23, 338–345.
Irie, M., Tamae, K., Iwamoto-Tanaka, N., Kasai, H., 2005. Occupational and lifestyle factors and urinary 8-hydroxydeoxyguanosine. Cancer Sci. 96, 600–606.
Kamat, C.D., Green, D.E., Curilla, S., Warnke, L., Hamilton, J.W., Sturup, S., Clark, C., Ihnat, M.A., 2005. Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol. Sci. 86, 248–257.
Karagas, M.R., Tosteson, T.D., Morris, J.S., Demidenko, E., Mott, L.A., Heaney, J., Schned, A., 2004. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control 15, 465–472.
Kimura, S., Yamauchi, H., Hibino, Y., Iwamoto, M., Sera, K., Ogino, K., 2006. Evaluation of urinary 8-hydroxydeoxyguanine in healthy Japanese people. Basic Clin. Pharmacol. Toxicol. 98, 496–502.
Liu, L., Trimarchi, J.R., Navarro, P., Blasco, M.A., Keefe, D.L., 2003. Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. J. Biol. Chem. 278, 31998–32004.
Loft, S., Poulsen, H.E., 1996. Cancer risk and oxidative DNA damage in man. J. Mol. Med. 74, 297–312.
Lynn, S., Gurr, J.R., Lai, H.T., Jan, K.Y., 2000. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ. Res. 86, 514–519.
Malins, D.C., Anderson, K.M., Jaruga, P., Ramsey, C.R., Gilman, N.K., Green, V.M., Rostad, S.W., Emerman, J.T., Dizdaroglu, M., 2006. Oxidative changes in the DNA of stroma and epithelium from the female breast: potential implications for breast cancer. Cell Cycle 5, 1629–1632.
Miyake, H., Hara, I., Kamidono, S., Eto, H., 2004. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J. Urol. 171, 1533–1536.
Mizoue, T., Kasai, H., Kubo, T., Tokunaga, S., 2006. Leanness, smoking, and enhanced oxidative DNA damage. Cancer Epidemiol. Biomark. Prev. 15, 582–585.
Nishikawa, T., Wanibuchi, H., Ogawa, M., Kinoshita, A., Morimura, K., Hiroi, T., Funae, Y., Kishida, H., Nakae, D., Fukushima, S., 2002. Promoting effects of monomethylarsonic acid, dimethylarsinic acid and trimethylarsine oxide on induction of rat liver preneoplastic glutathione S-transferase placental form positive foci: a possible reactive oxygen species mechanism. Int. J. Cancer 100, 136–139.
Pi, J., Kumagai, Y., Sun, G., Yamauchi, H., Yoshida, T., Iso, H., Endo, A., Yu, L., Yuki, K., Miyauchi, T., Shimojo, N., 2000. Decreased serum concen-trations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic. Biol. Med. 28, 1137–1142.
Pi, J., Yamauchi, H., Kumagai, Y., Sun, G., Yoshida, T., Aikawa, H., Hopen-hayn-Rich, C., Shimojo, N., 2002. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ. Health Perspect. 110, 331–336.
Proteggente, A.R., England, T.G., Rehman, A., Rice-Evans, C.A., Halliwell, B., 2002. Gender differences in steady-state levels of oxidative damage to DNA in healthy individuals. Free Radic. Res. 36, 157–162.
Pu, Y.S., Yang, S.M., Huang, Y.K., Chung, C.J., Hunag, S.K., Chiu, W.H., Yang, M.H., Chen, C.J., Hsueh, Y.M., 2007. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol. Appl. Pharmacol. 218, 99–106.
Rozalski, R., Gackowski, D., Roszkowski, K., Foksinski, M., Olinski, R., 2002. The level of 8-hydroxyguanine, a possible repair product of oxidative DNA damage, is higher in urine of cancer patients than in control subjects. Cancer Epidemiol. Biomark. Prev. 11, 1072–1075.
Saito, S., Yamauchi, H., Hasui, Y., Kurashige, J., Ochi, H., Yoshida, K., 2000. Quantitative determination of urinary 8-hydroxydeoxyguanosine (8-OH-dg) by using ELISA. Res. Commun. Mol. Pathol. Pharmacol. 107, 39–44.
Shen, J., Deininger, P., Hunt, J.D., Zhao, H., 2007. 8-Hydroxy-2'-deoxygua-nosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer 109, 574–580.
Shi, H., Hudson, L.G., Ding, W., Wang, S., Cooper, K.L., Liu, S., Chen, Y., Shi, X., Liu, K.J., 2004. Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem. Res. Toxicol. 17, 871–878. Steinmaus, C., Yuan, Y., Bates, M.N., Smith, A.H., 2003. Case–control study of
bladder cancer and drinking water arsenic in the western United States. Am. J. Epidemiol. 158, 1193–1201.
Steinmaus, C., Yuan, Y., Kalman, D., Atallah, R., Smith, A.H., 2005. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol. Biomark. Prev. 14, 919–924.
Steinmaus, C., Bates, M.N., Yuan, Y., Kalman, D., Atallah, R., Rey, O.A., Biggs, M.L., Hopenhayn, C., Moore, L.E., Hoang, B.K., Smith, A.H., 2006. Arsenic methylation and bladder cancer risk in case–control studies in Argentina and the United States. J. Occup. Environ. Med. 48, 478–488. Su, P.F., Hu, Y.J., Ho, I.C., Cheng, Y.M., Lee, T.C., 2006. Distinct gene
expression profiles in immortalized human urothelial cells exposed to inorganic arsenite and its methylated trivalent metabolites. Environ. Health Perspect. 114, 394–403.
Tapio, S., Grosche, B., 2006. Arsenic in the aetiology of cancer. Mutat. Res. 612, 215–246.
Tseng, C.H., 2002. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology 53, 529–537. Tseng, C.H., Huang, Y.K., Huang, Y.L., Chung, C.J., Yang, M.H., Chen, C.J.,
Hsueh, Y.M., 2005. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol. 206, 299–308.
Valko, M., Rhodes, C.J., Moncol, J., Izakovic, M., Mazur, M., 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40.
Wei, M., Wanibuchi, H., Morimura, K., Iwai, S., Yoshida, K., Endo, G., Nakae, D., Fukushima, S., 2002. Carcinogenicity of dimethylarsinic acid in male F344 rats and genetic alterations in induced urinary bladder tumors. Carcinogenesis 23, 1387–1397.
Wen, C.P., Levy, D.T., Cheng, T.Y., Hsu, C.C., Tsai, S.P., 2005. Smoking behaviour in Taiwan, 2001. Tob. Control 14, i51–i55.
WHO, 1999. Histological Typing of Urinary Bladder Tumours. International Classification of Tumours. World Health Organization, Geneva.
Wu, M.M., Chiou, H.Y., Wang, T.W., Hsueh, Y.M., Wang, I.H., Chen, C.J., Lee, T.C., 2001. Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environ. Health Perspect. 109, 1011–1017. Yamanaka, K., Kato, K., Mizoi, M., An, Y., Takabayashi, F., Nakano, M.,
Hoshino, M., Okada, S., 2004. The role of active arsenic species produced by metabolic reduction of dimethylarsinic acid in genotoxicity and tumorigenesis. Toxicol. Appl. Pharmacol. 198, 385–393.
Yamauchi, H., Aminaka, Y., Yoshida, K., Sun, G., Pi, J., Waalkes, M.P., 2004. Evaluation of DNA damage in patients with arsenic poisoning: urinary 8-hydroxydeoxyguanine. Toxicol. Appl. Pharmacol. 198, 291–296. Yoshida, T., Yamauchi, H., Fan, S.G., 2004. Chronic health effects in people
exposed to arsenic via the drinking water: dose–response relationships in review. Toxicol. Appl. Pharmacol. 198, 243–252.
Zhang, T.C., Schmitt, M.T., Mumford, J.L., 2003. Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis 24, 1811–1817.