Increased Risk of Colorectal Cancer among Patients with Biliary Tract Inflammation: A Five-year Follow-Up Study
Hsiu-Li Lin, MD1,2 Hsiu-Chen Lin3, MD Ching-Chun Lin4, MA Herng-Ching Lin4, PhD
1
Department of Neurology, General Cathay Hospital, Sijhih Branch, Taipei, Taiwan 2
Graduate School of Medical Informatics, Taipei Medical University, Taipei, Taiwan 3
Department of Pediatric Infection, Taipei Medical University and Hospital, Taipei, Taiwan
4
School of Health Care Administration, Taipei Medical University, Taipei, Taiwan
Address for reprints: Herng-Ching Lin
School of Health Care Administration, Taipei Medical University,
250 Wu-Hsing St., Taipei 110, Taiwan, Tel: 886-2-2776-1661 ext. 3613;
Fax: 886-2-2378-9788;
E-mail: henry11111@tmu.edu.tw
Short title: Colorectal Cancer and Biliary Tract Inflammation No author has a conflict of interest to declare
No funding source
Novelty and impact of this paper: No population-based study has ever been conducted to explore the relationship between patients with biliary tract
inflammation (BTI) and the risk of colorectal cancer. We found that the adjusted hazard ratios for colorectal cancer for patients with BTI were 6.54-times as high as for those without BTI within a 1-year follow-up period.
Abstract
The purpose of the study was to investigate the risk of colorectal cancer
among patients with biliary tract inflammation (BTI) compared to non-BTI patients
during a 5-year follow-up period. The study group comprised 1613 patients with BTI,
among which 32 cases (1.98%) developed colorectal cancer. The comparison group
included 8065 randomly selected subjects (five for every patient with BTI), 74 of
whom contracted colorectal cancer (0.92%). Stratified Cox proportional hazard
regressions were calculated to estimate the adjusted hazard of colorectal cancer
between the study group and comparison group. The adjusted hazard ratio for
colorectal cancer for patients with BTI was 6.54-times (95% CI = 3.07-13.92) as high
as for those without BTI within a 1-year follow-up period, 3.20-times (95% CI =
1.93-5.30) as high within a 3-year follow-up period, and 2.21-times (95% CI = 1.45-3.37)
as high within a 5-year follow-up period. We also found that the adjusted hazard ratio
for colorectal cancer for those with bile duct inflammation was 3.30-times (95% CI =
1.87-5.84) as high as for those without BTI within the five-year follow-up period.
However, no increased hazard of colorectal cancer was observed for patients with
gallbladder inflammation. We concluded that patients with BTI had a significantly
higher risk of colorectal cancer compared to patients without BTI. Key words: biliary tract inflammation; colorectal cancer; epidemiology
Introduction
Biliary tract inflammation (BTI), including cholecystitis and cholangitis, both
infectious and non-infectious types, refers to an inflammation of the gallbladder and
bile duct. BTI, a common illness,1,2 is a significant cause of morbidity and mortality
worldwide, particularly in older patients with comorbid diseases.3-8 Although
endoscopic, percutaneous, and surgical treatments are commonly available for BTI in
recent years, BTI still carries a mortality rate of 10%-20%.9-11
On the other hand, colorectal cancer is a major global health problem and is the
third most common cancer in the US and Taiwan.12-15 Since exposure to risk factors
can increase the development of such neoplasms, modification of risk factors for
colorectal cancer to prevent its occurrence can significantly reduce societal impacts of
this neoplasm disorder.
During the past decade, plenty of studies have reported that associated
inflammatory processes can increase the risk of developing ovarian, oral, and
colorectal cancers.16-20 In particular, one study by Triantafillidis et al. documented that
the presence of inflammatory manifestations resulting from cholangitis might increase
the risk of colorectal cancer.21 In addition, BTI can increase serum bile acid
according to our knowledge, no population-based study has ever been conducted to
explore the relationship between BTI and the risk of colorectal cancer.
Therefore, the aim of this study was to investigate the risk for colorectal cancer
among BTI patients during a 5-year follow-up period after establishment of a
diagnosis of BTI, compared to non-BTI patients during the same period, while
Materials and Methods
Database
In this study, we used the “Longitudinal Health Insurance Database 2005
(LHID2005)” released by the Taiwan National Health Research Institute (NHRI) in
2006. Taiwan implemented its National Health Insurance (NHI) program in 1995 to
provide affordable health care for all the island’s residents. There are currently 25.68
million enrollees covered by the program, representing over 98% of the island’s
population. The LHID2005 contains all the medical claims data as well as a registry
of 1,000,000 persons, randomly sampled from the 25.68 million enrollees covered
by the NHI. The Taiwan NHRI reports that there were no statistically significant
differences in age, sex, or healthcare costs between the sample group and all
enrollees. Therefore, the LHID2005, a nationwide population-based dataset,
provides an excellent opportunity to examine the risk of colorectal cancer among
patients with BTI.
Since the dataset used in this study consisted of de-identified secondary data
released to the public for research purposes, this study was exempt from full review
by the Institutional Review Board.
We selected patients who visited ambulatory care centers or were hospitalized
with a principal diagnosis of BTI (ICD-9-CM codes 575.0, 575.1, 575.2, 576.1, or
576.2) between January 1, 1999 and December 31, 2001 (n=1,686) for the study
group. We excluded patients who had had any type of cancer (ICD-9-CM codes
140-239) or BTI diagnosis during the previous 3-year period (n=72). We also excluded
patients with comorbid inflammatory bowel disease (n=1). Ultimately, our study
cohort included 1,613 patients with BTI.
The comparison group was selected from the remaining persons in the
LHID2005. We randomly selected 8065 enrollees (five for every patient with BTI)
from the registry of persons, matched with the study group in terms of age (< 45,
45-64, 65-74 and > 74 years) and sex. We assigned their first ambulatory care visit
between January 1, 2001 and December 31, 2001 as the index ambulatory care visit.
Ultimately, 9,678 patients were included in our study. These patients had no history
of BTI or cholecystectomy during the period from 1996 to 2006. However, since the
NHI program in Taiwan was initiated in 1995, the dataset used in the present study
only allowed us to trace use of medical services from 1996 to 2006. Therefore, we
could not exclude patients who may have had BTI or cholecystectomy before 1996.
Each patient was individually tracked for 5 years from their index outpatient visit to
Since the National Health Insurance Research Database allows us to trace all
medical service utilization for all enrollees, it was possible to follow all sampled
patients throughout the study period.
Statistical Analysis
The SAS statistical package (SAS System for Windows, vers. 8.2) was used to
perform all statistical analyses in this study. We used Pearson χ2 tests to examine
differences in sociodemographic characteristics (age, sex, monthly income, level of
urbanization, and the geographic location of the community in which the patient
resided, i.e., northern, central, eastern, and southern Taiwan) between the study and
comparison groups. Monthly income was grouped into four categories: < NT$15,000,
NT$15,000-30,000, NT$30,001-50,000, ≥ NT$50,001 (US$1.00 = NT$33.00 in 2003).
Urbanization levels in Taiwan were divided into five strata, with level 1 referring to
the ‘most urbanized’ and level 5 referring to the ‘least urbanized’ communities based
on criteria used in prior studies. We calculated the 5-year colorectal cancer-free
survival rate and examined differences in the risk for colorectal cancer between the
two groups. Furthermore, Stratified Cox proportional hazard regressions (stratified by
age and sex) were calculated to estimate the hazard of colorectal cancer for the study
the geographic location of the sampled patients. Differences were considered
RESULTS
Table 1 presents a comparison of sociodemographic characteristics for patients with
and without BTI. The mean age of the study sample was 53.9 years, with a standard
deviation of 17.8 years. The majority of patients were between 45 and 64 years, and only
9.4% of the sampled patients were over 74 years old. After matching the patient age and
sex, we found no significant differences between these two groups in the level of
urbanization or geographic location of the community in which the patient resided (Table
1).
<Insert Table 1 here>
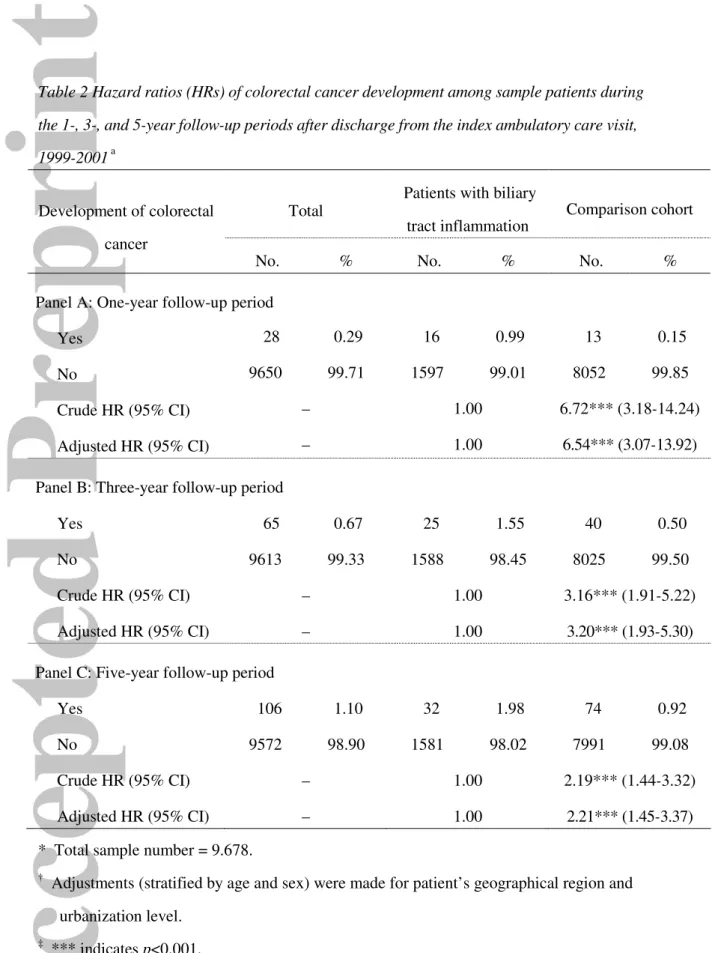
Of the total sample of 9678 patients, 106 patients (1.10%) developed colorectal
cancer during the 5-year follow-up period, 32 (1.98% of patients with BTI) from the study
group and 74 (0.92% of patients without BTI) from the comparison group (Table 2). The
results of the Kaplan-Meier survival analysis are displayed in Figure 1. The log-rank test
showed that patients with BTI had significantly lower 5-year colorectal cancer-free
survival rates than patients without BTI (p<0.001).
Table 2 also presents the percentages of colorectal cancer within the 1-, 3-, and
5-year follow-up periods after the index ambulatory care visit for patients in these two
groups. Compared to patients without BTI, patients with BTI had significantly higher
rates of colorectal cancer within the 1-year (0.99% vs. 0.15%), 3-year (1.55% vs.
0.50%), and 5-year (1.98% vs. 0.92%) periods after their index ambulatory care visit.
The crude and adjusted hazard ratios of colorectal cancer within the 1-, 3-, and
5-year follow-up periods after the index ambulatory care visits are presented in Table 2.
After adjusting for patient’s level of urbanization, and the geographical location of the
community in which the patient resided, compared to those patients without BTI, the
hazard ratio for colorectal cancer for those with BTI were 6.54-times (95% CI =
3.07-13.92) as high within the 1-year follow-up period, 3.20-times (95% CI = 1.93-5.30) as
high within the 3-year follow-up period, and 2.21-times (95% CI = 1.45-3.37) as high
within the 5-year follow-up period.
We further analyzed the risk of colorectal cancer between BTI patients who did
and did not undergo cholecystectomy during the follow-up period. We found that six
of the BTI patients who underwent cholecystectomy (1.7% of BTI patients who
underwent cholecystectomy) and 26 patients who did not undergo cholecystectomy
cancer during the five-year follow-up period. No significant difference in the risk of
colorectal cancer between BTI patients who did and did not undergo cholecystectomy
was observed.
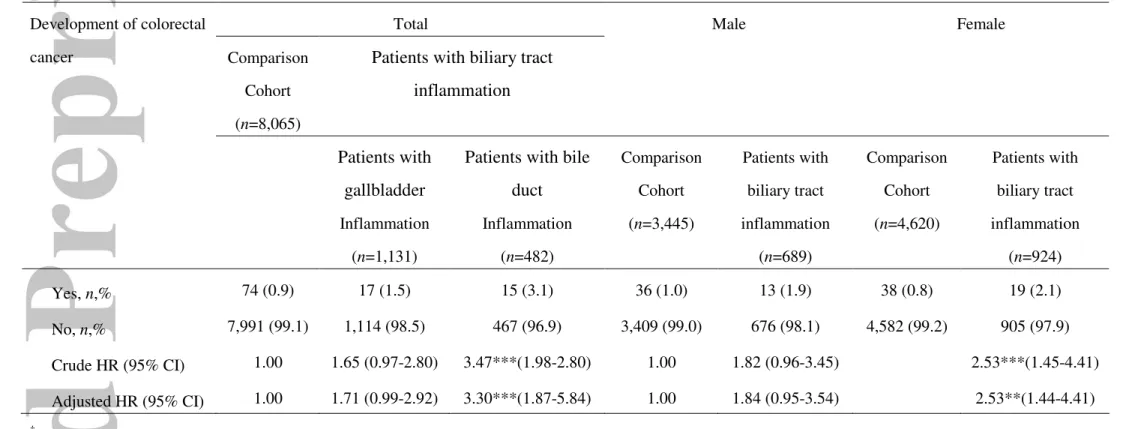
In addition, we examined crude and adjusted hazard ratios of colorectal cancer
within the five-year follow-up period after the index ambulatory care visit, stratified by
the type of BTI (gallbladder inflammation vs. bile duct inflammation) and gender.
Table 3 shows that the adjusted hazard ratio for colorectal cancer for those with bile
duct inflammation was 3.30-times (95% CI = 1.87-5.84) as high as those without BTI
over the five-year follow-up period. However, no increased hazard of colorectal cancer
was observed for patients with gallbladder inflammation. Among female patients, the
adjusted hazard of colorectal cancer during the five-year follow-up period was 2.53
(95% CI = 1.44-4.41) times greater for those with BTI than for those in the
comparison group.
Discussion
As far as we know, this is the first population-based follow-up study to examine
the risk for subsequent colorectal cancer among patients with BTI. Our results indicate
that patients with BTI had significantly higher risk of colorectal cancer compared to
patients without BTI; the risks of colorectal cancer for patients with BTI were 6.54-,
3.20-, and 2.21-times as high in the 1-, 3-, and 5-year follow-up periods, respectively,
as for patients without BTI, after adjusting for sociodemographic characteristics. Our
findings agree with a related study by Broome and Bergquist23 which concluded that
cholangitis appearing on the ground of ulcerative colitis increased the risk of colorectal
cancer by 4.8-times in patients with ulcerative colitis without cholangitis. In addition,
our study found that there was no significant difference in the risk of colorectal cancer
between BTI patients who did and did not undergo cholecystectomy. This finding is
also consistent with prior studies by Friedman et al. and Adami et al. which concluded
that cholecystectomy did not increase the subsequent risk of colorectal cancer.24,25
The mechanism underlying the association between BTI and colorectal cancer is
still unclear. It is possible that the excessive production of bile acid during the
inflammatory process in the gallbladder and bile duct might play an important role in
colorectal carcinogenesis. An inflammatory process in the biliary tract may cause more
higher unconjugated deoxycholic acid concentration in serum and a higher bile acid
level in feces were correlated with higher incidences of colorectal cancer.26 Bile acids
in the colon directly stimulate the colorectal mucosal epithelium and facilitate the
carcinogenesis process.27,28 It also explains why the risk of colorectal cancer decreases
with time since the BTI event, because the stimulating effect reaches a peak when BTI
occurs and then is ameliorated in later years.
Furthermore, it was reported that approximately one-fifth of cancers worldwide
are caused by infection.29 Inflammation resulting from infection is considered to be an
important factor contributing to tumorigenesis and tumor progression.30 In addition,
many studies implicated inflammation as a cause of pancreatic, bladder, and colorectal
cancers.31-33
Because BTI brings patients to the attention of medical personnel, we also
examined the occurrence of renal tumors, another kind of abdominal tumor, in these
two groups to test this effect. We found there was no significant difference in renal
tumor incidences between these two groups (8 cases in the BTI group and 21 cases in
the comparison group, p=0.114). Accordingly, the possibility that receiving medical
attention for BTI contributes to the elevated odds of contracting colorectal cancer is
The strength of our study lies in its longitudinal database and large population
size. Nevertheless, the findings of this study need to be interpreted with awareness of
several limitations. First, prior studies demonstrated that a family history of colorectal
cancer increases the risk of colorectal cancer.21 However, the dataset used in this study
does not provide information which can be used to establish family histories of
colorectal cancer. Second, the dataset used in the study lacks information on body
mass index, obesity, smoking habits, alcohol use, the amount of daily fiber intake and
household monthly income. These factors were demonstrated to be associated with an
increased risk of colorectal cancer.34,35
This study found that patients with BTI had a significantly higher risk of
contracting colorectal cancer compared to patients without BTI, and the risk seemed to
come from bile acid stimulation, which is more highly produced during biliary tract
inflammation. Therefore, further studies are also recommended to test the ameliorating
References
1. Lee CC, Chang IJ, Lai YC, Chen SY, Chen SC. Epidemiology and prognostic
determinants of patients with bacteremic cholecystitis or cholangitis. Am J
Gastroenterol 2007;102: 563-9.
2. Gupta E, Chakravarti A. Viral infections of the biliary tract. Saudi J Gastroenterol
2008;14: 158-60.
3. Bornman PC, van Beljon JI, Krige JE. Management of cholangitis. J Hepatobil
Pancreat Surg 2003;10: 406-14.
4. What if it's acute cholangitis? Drug Ther Bull 2005;43: 62-4.
5. Bektaş M, Dökmeci A, Cinar K, Halici I, Oztas E, Karayalcin S, Idilman R,
Sarioglu M, Ustun Y, Nazligul Y, Ormeci N, Ozkan H, Bozkaya H, Yurdaydin C.
Endoscopic management of biliary parasitic diseases. Dig Dis Sci 2009.[please
update if possible]
6. Hanau LH, Steigbigel NH. Acute (ascending) cholangitis. Infect Dis Clin North
Am 2000;14: 521-46.
7. Stewart L, Griffiss JM, Way LW. Spectrum of gallstone disease in the veterans
population. Am J Surg 2005;190: 746-51.
9. Siegman-Igra Y, Schwartz D, Konforti N, Perluk C, Rozin RR. Septicemia from
biliary tract infection. Arch Surg 1988;123: 366-8.
10. Sugiyama M, Atomi Y. Treatment of acute cholangitis due to choledocholithiasis
in elderly and younger patients. Arch Surg 1997;132: 1129-33.
11. Kuo CH, Changchien CS, Chen JJ, Tai DI, Chiou SS, Lee CM. Septic acute
cholecystitis. Scand J Gastroenterol 1995;30: 272-5.
12. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden:
Globocan 2000. Int J Cancer 2001;94: 153-6.
13. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics,
2008. CA Cancer J Clin 2008;58: 71-96.
14. Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer
screening uptake among men and women in the United States. Cancer Epidemiol
Biomark Prev 2006;15: 389-94.
15. Taiwan Department of Health. Health Statistics in Taiwan 2008. Taipei, Taiwan:
Taiwan Department of Health, 2009.
16. Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of
inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer
17. Vairaktaris E, Serefoglou Z, Avgoustidis D, Yapijakis C, Critselis E, Vylliotis A,
Spyridonidou S, Derka S, Vassiliou S, Nkenke E, Patsouris E. Gene
polymorphisms related to angiogenesis, inflammation and thrombosis that
influence risk for oral cancer. Oral Oncol 2009;45: 247-53.
18. Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G, Caruso
C. Inflammation, ageing and cancer. Mech Ageing Dev 2009;130: 40-5.
19. Slattery ML, Wolff RK, Herrick J, Caan BJ, Samowitz W. Tumor markers and
rectal cancer: support for an inflammation-related pathway. Int J Cancer 2009.
20. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in
inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest
Liver Physiol 2004;287: G7-17.
21. Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and
inflammatory bowel disease: epidemiology, risk factors, mechanisms of
carcinogenesis and prevention strategies. Anticancer Res 2009;29: 2727-37.
22. Ochsenkühn T, Bayerdörffer E, Meining A, Schinkel M, Thiede C, Nüssler V,
Sackmann M, Hatz R, Neubauer A, Paumgartner G. Colonic mucosal
proliferation is related to serum deoxycholic acid levels. Cancer 1999;85: 1664-9.
24. Friedman GD, Goldhaber MK, Quesenberry CP Jr. Cholecystectomy and large
bowel cancer. Lancet 1987;1:906-8.
25. Adami HO, Meirik O, Gustavsson S, Nyrén O, Krusemo UB. Colorectal cancer
after cholecystectomy: absence of risk increase within 11-14 years.
Gastroenterology 1983;85:859-65.
26. van Faassen A, Ochsenkühn T, Houterman S, van der Ploeg EM,
Bueno-de-Mesquita BH, Ocké MC, Bayerdörffer E, Janknegt RA. Plasma deoxycholic acid
is related to deoxycholic acid in faecal water. Cancer Lett 1997;114: 293-4.
27. Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous
etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:
3329-40
28. Pearson JR, Gill CI, Rowland IR. Diet, fecal water, and colon
cancer-development of a biomarker. Nutr Rev. 2009;67:509-26.
29. American Cancer Society. Cancer facts and figures 2005. city?, ST?: American
Cancer Society, 2005.
30. Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ.
Demonstration of inflammation-induced cancer and cancer immunoediting during
31. McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer.
Best Pract Res Clin Gastroenterol 2008;22: 65-73.
32. Michaud DS. Chronic inflammation and bladder cancer. Urol Oncol 2007;25:
260-8.
33. Roxburgh CS, Crozier JE, Maxwell F, Foulis AK, Brown J, McKee RF,
Anderson JH, Horgan PG, McMillan DC. Comparison of tumour-based (Petersen
index) and inflammation-based (Glasgow prognostic score) scoring systems in
patients undergoing curative resection for colon cancer. Br J Cancer 2009;100:
701-6.
34. Breynaert C, Vermeire S, Rutgeerts P, Van Assche G.. Dysplasia and colorectal
cancer in inflammatory bowel disease: a result of inflammation or an intrinsic risk?
Acta Gastroenterol Belg 2008;71: 367-72.
35. Thomsen RW, Thomsen HF, Nørgaard M, Cetin K, McLaughlin JK, Tarone RE,
Fryzek JP, Sørensen HT. Risk of cholecystitis in patients with cancer: a
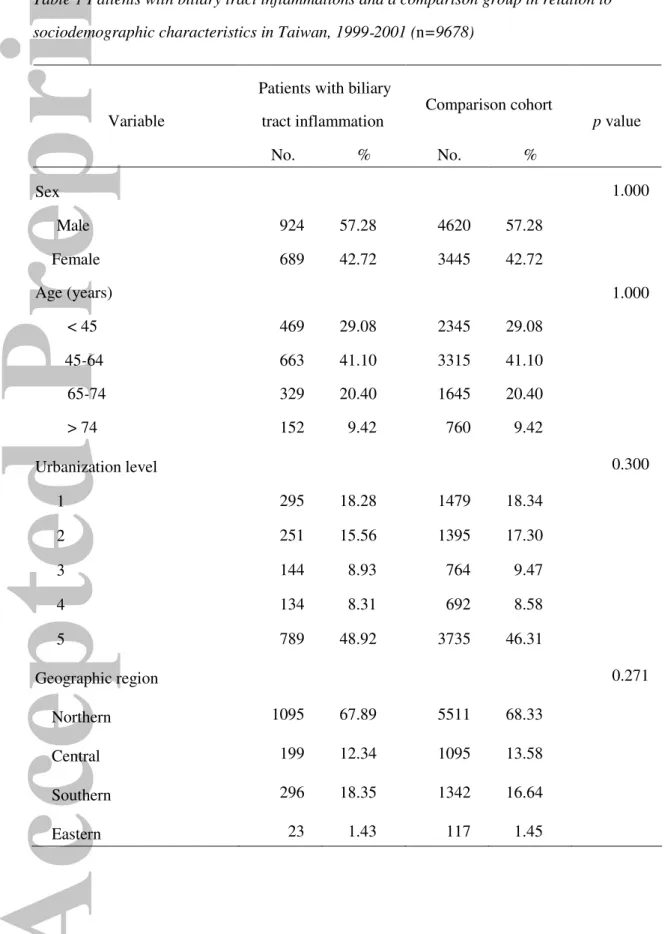
Table 1 Patients with biliary tract inflammations and a comparison group in relation to sociodemographic characteristics in Taiwan, 1999-2001 (n=9678)
Patients with biliary
tract inflammation Comparison cohort
Variable No. % No. % p value Sex 1.000 Male 924 57.28 4620 57.28 Female 689 42.72 3445 42.72 Age (years) 1.000 < 45 469 29.08 2345 29.08 45-64 663 41.10 3315 41.10 65-74 329 20.40 1645 20.40 > 74 152 9.42 760 9.42 Urbanization level 0.300 1 295 18.28 1479 18.34 2 251 15.56 1395 17.30 3 144 8.93 764 9.47 4 134 8.31 692 8.58 5 789 48.92 3735 46.31 Geographic region 0.271 Northern 1095 67.89 5511 68.33 Central 199 12.34 1095 13.58 Southern 296 18.35 1342 16.64 Eastern 23 1.43 117 1.45
Table 2 Hazard ratios (HRs) of colorectal cancer development among sample patients during the 1-, 3-, and 5-year follow-up periods after discharge from the index ambulatory care visit, 1999-2001 a
Total
Patients with biliary
tract inflammation Comparison cohort
Development of colorectal cancer
No. % No. % No. %
Panel A: One-year follow-up period
Yes 28 0.29 16 0.99 13 0.15
No 9650 99.71 1597 99.01 8052 99.85
Crude HR (95% CI) – 1.00 6.72*** (3.18-14.24)
Adjusted HR (95% CI) – 1.00 6.54*** (3.07-13.92)
Panel B: Three-year follow-up period
Yes 65 0.67 25 1.55 40 0.50
No 9613 99.33 1588 98.45 8025 99.50
Crude HR (95% CI) – 1.00 3.16*** (1.91-5.22)
Adjusted HR (95% CI) – 1.00 3.20*** (1.93-5.30)
Panel C: Five-year follow-up period
Yes 106 1.10 32 1.98 74 0.92
No 9572 98.90 1581 98.02 7991 99.08
Crude HR (95% CI) – 1.00 2.19*** (1.44-3.32)
Adjusted HR (95% CI) – 1.00 2.21*** (1.45-3.37)
* Total sample number = 9.678.
†
Adjustments (stratified by age and sex) were made for patient’s geographical region and urbanization level.
‡
*** indicates p<0.001. CI, confidence interval.
22
Table 3 Hazard ratios (HRs) of colorectal cancer development among sample patients during the 5-year follow-up periods after discharge from the index ambulatory care visit, 1999-2001 by type of biliary tract inflammation and by gender
Total Comparison
Cohort (n=8,065)
Patients with biliary tract inflammation Male Female Development of colorectal cancer Patients with gallbladder Inflammation (n=1,131)
Patients with bile duct Inflammation (n=482) Comparison Cohort (n=3,445) Patients with biliary tract inflammation (n=689) Comparison Cohort (n=4,620) Patients with biliary tract inflammation (n=924) Yes, n,% 74 (0.9) 17 (1.5) 15 (3.1) 36 (1.0) 13 (1.9) 38 (0.8) 19 (2.1) No, n,% 7,991 (99.1) 1,114 (98.5) 467 (96.9) 3,409 (99.0) 676 (98.1) 4,582 (99.2) 905 (97.9) Crude HR (95% CI) 1.00 1.65 (0.97-2.80) 3.47***(1.98-2.80) 1.00 1.82 (0.96-3.45) 2.53***(1.45-4.41) Adjusted HR (95% CI) 1.00 1.71 (0.99-2.92) 3.30***(1.87-5.84) 1.00 1.84 (0.95-3.54) 2.53**(1.44-4.41) ‡ ** indicates p<0.01*** indicates p<0.001.
John Wiley & Sons, Inc.
48 49 50 51 52 53 54 55
Caption
Figure 1. Colorectal cancer-free survival rates of patients with biliary tract inflammation and a comparison group in Taiwan, 1999-2001.