563
0277-8033/01/1000-0563/0 © 2001 Plenum Publishing Corporation
of the lipocalin superfamily (Flower et al., 1991). The protein also shows a high degree of similarity to human neutrophil gelatinase-associated lipocalin (NGAL) (70% identity) (Chu et al., 1997; Kjeldsen et al., 1993). Our previous results support a hydrophobic pocket of this protein molecule, showing it to be suitable for fatty acid and retinoid binding (Chu et al., 1998). We also found that the epididymal 24p3 protein interacted predomi-nantly with the acrosome of spermatozoa (Chu et al., 2000). The biological function of this uterine protein re-mains unclear. This being evident, we are seeking further understanding of the function of the 24p3 protein.
Phosphorylation and dephosphorylation of protein is well-recognized as an important to regulate biological function: It may reflect a relationship between protein structure and biological activity. The phosphorylation or dephosphorylation of amino acid residue triggers struc-1. INTRODUCTION
A purified protein, derived from 24p3 cDNA, was origi-nally by Hraba-Renevey et al. (1989) cloned from mouse kidney culture cells infected with polyoma virus-40, from mouse uterine luminal fluid (Chu et al., 1996), and identified in the epididymis (Chu et al., 2000). We iden-tify this protein as 24p3, a 25 KDa glycoprotein, with a blocked N-terminus of pyroglutamate (Chu et al., 1997). This protein is also present in lipopolysaccharide-stimu-lated PU5.1.8 macrophage (Meheus et al., 1993) and bFGF-stimulated 3T3 cells (Davis et al., 1991). The re-sults of Liu and Nilson-Hamilton (1995) reveal it as an acute phase protein of liver. Based on a computer-assisted homologous search, it is classified as a member 1Institute of Biological Chemistry, Academia Sinica, Taiwan. 2Institute of Biochemical Science, College of Science, National
Tai-wan University, Taipei, 10617, TaiTai-wan.
3To whom correspondence should be addressed. Institute of Biological Chemistry, Academia Sinica, PO Box 23-106, Taipei, 10617, Taiwan; Tel: 886-2-23620261 (Ext. 4531); Fax: 886-2-23635038; e-mail: stc316@gate.sinica.edu.tw
4Abbreviations: CKII, casein kinase II; NC, nitrocellulose; NGAL, neutrophil gelatinase-associated lipocalin; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; PMSF, phenylmethyl sulfonyl; TFA, trifluoroacetic acid.
Phosphorylation of the 24p3 Protein Secreted from Mouse
Uterus in Vitro and in Vivo
Ying-Chu Lee,
1Shyh-Dyh Lin,
2Hui-Ming Yu,
1Shui-Tein Chen,
1and Sin-Tak Chu
1,3Received August 10, 2001
The 24p3 protein is a 25 KDa glycoprotein, having been purified from mouse uterine fluid. Thr54,
Ser88, and Thr128/Ser129on the protein molecule were predicted to be the phosphorylation site of
ca-sein kinase II, protein kinase C, and cAMP-dependent protein kinase, respectively. Incorporation of phosphate to this protein from [␥-32P]-ATP was tested in the solution suitable for the three kinases.
Neither casein kinase II nor cAMP-dependent protein kinase reacted to the 24p3 protein; however, protein kinase C demonstrated phosphorylation to this protein. This phosphorylation may be com-peting with a polypeptide segment: Arg79-Tyr-Trp-Ilu-Arg-Thr-Phe-Val-Pro-Ser88
-Ser-Arg-Ala-Gly-Gln-Phe-Thr-Leu-Gly97in the 24p3 protein molecule. To support this theory, Ser88is a
phos-phorylation site of protein kinase C on 24p3 protein. The enzyme kinetic parameter, based on the Michaelis-Menten equation, determined Km to be 2.96 M in the phosphorylation of 24p3 protein by the kinase. Both of the phosphorylated and dephosphorylated form of 24p3 protein can enhance the cAMP-dependent protein kinase activity in vitro. In addition, this experiment will show for the first time that serine-phosphorylated 24p3 protein exists in mouse uterine tissue.
tural change in the molecules, altering their biological function (Kurosawa, 1994). The analysis of the primary structure of 24p3 protein suggests the presence of poten-tial Ser/Thr sites for the phosphorylation by casein kinase II (CKII),4protein kinase C (PKC), or cAMP-dependent
protein kinase (PKA) (Fig. 1), thus further emphasizing the importance of verifying the phosphorylation in 24p3 protein. Accordingly, we investigated the phospho-rylation of 24p3 protein by these three types of kinases.
Fig. 1. The potential phosphorylation sites of protein kinases on 24p3
protein and its predicted secondary structure. Based on our previous report (Chu et al., 1998), the predicted secondary structures, which are conserved in the lipocalin protein superfamily (Monaco and Zanotti, 1992), are shown at the top of the sequence (-strands A–I and helices in the hatch blocks). The three short motifs, which are highly conserved between members of the family (Flower et al., 1991) are denoted. The consensus sequences for the phosphorylation site of different protein kinases are underlined and denoted.
Our results support a PKC phosphorylation site at Ser88 but exclude both phosphorylation sites for CKII and PKA on the protein molecule. The Km value is comparable to that reported for the most effective protein substrates for protein kinase C (Abe et al., 1991). Use of the immuno-precipitation method to identify the serine-phosphory-lated 24p3 protein in uterine tissue indicated the phos-phorylation of 24p3 protein might be important and further established that the 24p3 protein enhanced the PKA activity in vitro.
2. MATERIALS AND METHODS 2.1. Materials
Outbred ICR mice were purchased from Charles River Laboratory (Wilmington, MA) and were bred in the animal center at the College of Medicine, National Taiwan University. All enzymes were purchased from Boehringer Mannheim G.m.b.H (Germany). Antiphos-phoserine antibody and HRP-conjugated anti-rabbit IgG were obtained from Zymed Laboratories, Inc. (Cat no. 61-8100, CA. USA) and Promega (cat no. W4011, WI, USA), respectively. The 24p3 protein-induced antibody was prepared and partially purified with a protein A col-umn, as per a previous method (Chu et al., 2000). The [␥-32P]-ATP was from Amersham (Searle, Arlington Height, IL). All other chemicals were reagent grade.
2.2. Prediction of Phosphorylation Sites
The method of matching consensus sequence pat-terns based on stepwise discrimination analysis was ap-plied to seek the potential phosphorylation sites on the 24p3 protein molecule from a primary structure of the 24p3 protein. The analysis was executed by the computer program package PROSITE 9.1, which is (technically) interrelated to SWISS-PROT protein sequence data bank (Bairoch and Apweiler, 1997, 1998).
2.3. Solid-Phase Peptide Synthesis
Peptide was synthesized, via 4-(2,4-dimethoxyphenyl-Fmoc-amino-methyl) phenoxyl resin (0.281g, 0.89 mEq/g) with Fmoc-amino acid derivatives using an automatic peptide synthesizer (Applied Biosystem Model 433A, USA). After completion of synthesis, the peptide on resins were incubated with a cleavage mixture contain-ing 0.75 g crystalline phenol, 0.25 mL 1,2-ethandithol, 0.5 mL thioanisole, 0.5 mL D.I. water, 10 mL
trifluoro-acetic acid, for 90 min at room temperature, and the sol-vent was completely evaporated. The dry resin was then washed five times with 20 mL of cold ether. Synthetic peptide was then extracted by washing five times with 20 mL of 5% acetic acid. All extracts were lyophilized to yield a crude peptide (0.506g, 93.5% yield).
2.4. In Vitro Phosphorylation of 24p3 Protein The [␥-32p]-ATP was diluted with unlabeled ATP,
yielding a final specific activity of 0.5 Ci /mmol. In a specified solution reported previously (Gonzalez et al., 1993; Hathaway and Traugh 1979; Glover et al., 1983), 24p3 protein (5.0 g/mL) was phosphorylated by CKII (20 mU/mL), PKA (20 U/mL), or PKC (2 U/mL) in the presence of 0.2 nM radial labeled ATP at 30°C for 5 min. The phosphorylation by PKC was processed in 20 mM HEPES containing 15.0 M phosphatidylserine, 1.3 mM CaCl2, 10 mM MgCl2, and 1.0 mM DTT at pH
7.4. The PKA phosphorylation was performed in 50 mM MES containing 10 mM MgCl2, 0.5 mM EDTA, and
1.0 mM DTT at pH 6.9. The CKII phosphorylation was carried out in 20 mM MES containing 130 mM KCl, 10 mM MgCl2, and 4.8 mM DTT at pH 6.9. The
reac-tion mixtures were subjected to SDS/PAGE on a 15% polyacrylamide slab gel (0.075 ⫻ 5.0 ⫻ 6.0 cm). The gel was dried by a gel-dryer, then autoradiographed on x-ray film.
2.5. The Phosphorylation Activity of PKC and PKA The PKC activity was assayed according to a phos-phocellulose technique (Casnellie, 1991). In the specified solution, 24p3 protein (0 –14 M), or synthetic polypep-tide (0 –2 M) comprising Arg79
-Tyr-Trp-Ilu-Arg-Thr-Phe-Val-Pro-Ser88
-Ser-Arg-Ala-Gly-Gln-Phe-Thr-Leu-Gly97, of the protein molecule was incubated in the
presence of PKC (2 U/mL) and 0.2 nM [␥-32P]-ATP at
30°C. The 10 L of aliquot was spotted onto a P81 phos-phocellulose paper for 5 min and then baked for 40 min under a lamp. The paper was washed five times with 2.0 mL of 150 mM phosphoric acid, rinsed with alcohol for 2 min, and baked dry. The radioactivity on the papers was counted by a -counter. The velocity of 32
P-incor-poration to the protein substrate was fitted to a double re-ciprocal plot constructed from the Michaelis-Menten equation.
The established method of Goueli et al. (1995), the cAMP-dependent protein kinase activity, was car-ried out in a 25 L reaction mixture containing 40 mM Tris-HCl, pH 7.4, 20 mM MgCl2, 100 M [␥-32p]ATP,
100 g/mL BSA, and 100 M biotinylated peptide sub-strate (Leu-Arg-Arg-Ala-Ser-leu-Gly) (V7480, Promega, WI, USA). The reaction mixture was preincubated at 30°C for 5 min, followed by 37°C for 5 min. At each treatment, a 10-L aliquot was spotted on a strepta-vidin-conjugated membrane and washed with 2 M NaCl. This washing process was repeated four times, followed by an additional four times washing with 1% H3PO4
containing 2M NaCl, and twice with distilled water to remove the free isotope impurities. The membranes were then dried and counted using a liquid scintillation counter.
2.6. Phosphorylation and Dephosphorylation of 24p3 Protein
The purified 24p3 protein of uterine luminal fluid was prepared according to our previous method (Chu et al., 1996). Fifty g of 24p3 protein was mixed with 300 U of bovine placenta alkaline phosphatase in 200L 0.1 M glycine, pH 10.4, containing 0.1 M NaCl, 50 mM MgCl2, 1.0 mM DTT, 2.0 mM EDTA, and 100
M phenylmethyl sulfonyl fluoride (PMSF). The mix-ture was incubated at 30°C for 4 h to remove the phos-phate from protein. After the dephosphorylation, 60 L 0.2 N HCl was added to neutralize and the mixture was fractionated by reversed-phase HPLC, with a 35% to 45% linear gradient of acetonitrile in 0.1% trifluoro-acetic acid (TFA), and a flow rate of 0.7 mL/min on a C18, 7 micron, 250 ⫻ 4.6 mm column (Macherey-Nagel, GmbH & Co., KG). The dephosphorylated 24p3 protein was collected manually and lyophilized.
To phosphorylate the protein, 30 g 24p3 protein was mixed with 50 ng PKC in 100 L of 20 mM Tris-HCl, pH 7.5, containing 10 mM MgCl2, 0.2 mM CaCl2,
3 g/L phosphatidylserine, 1 g/L 1,2-dioleoyl-sn-glycerol, 100 M Na3VO4, and 100 M PMSF. The
re-action mixture was incubated at 37°C for 1 h to ensure the phosphorylation reaction. After the reaction, the phosphorylated 24p3 protein was fractionated by re-versed-phase HPLC, as with the previous method. Phos-phorylated 24p3 protein was then collected manually and lyophilized. The dephosphorylation/phosphorylation 24p3 protein was analyzed by Western-blot analysis with an antiphosphoserine antibody.
2.7. In Vivo Phosphorylation Determination
Mature female mice (6 to 8 weeks) at proestrus phase were sacrificed by cervical dislocation and their uteri removed. The uteri were homogenized in a solution
containing 20 mM Tris-HCl, pH 7.4, 1 mM EDTA, 25 mM NaF, 0.1 M okadic acid, 2 mM Na3VO4, and
2.5 mM -glycerophosphate to extract the proteins, and then centrifuged at 360,000 ⫻ g for 40 min to remove the debris. The extraction mixture also contained 1X di-lution of protease inhibitor cocktail, (PI Cocktail, Cat No. 1697498, Roche Molecular Biochemicals, Ger-many). The supernatant was then obtained as a tissue ho-mogenate extract. Using the Dynabeads immunomagetic system to isolate the 24p3 protein from tissue extract, a target antigen-antibody complex can be isolated with an indirect technique. With the indirect technique, a sheep anti-rabbit IgG, specific for the IgG-antigen complex, is coupled to the Dynabeads M-280 (Dynal, cat No. 112.03, Dynal A. S, Oslo, Norway). The coated beads are then used to capture the desired target of protein complex. The homogenate extract containing 24p3 protein (3 mg) is incubated with the 24p3 protein-induced antibody (3g) for 2 h at 4°C, while gently agitating with a rota-tor. This procedure allows for the antigen-antibody com-plex formation to be completed. The antigen-antibody complex can now be isolated by incubation with 50 L Dynabeads M-280 sheep anti-rabbit IgG for 1 h at 4°C. After the incubation, a magnet is applied on the wall of the test tube for 1 min, isolating the target. A 10 L elec-trophoresis sample buffer is added and boiled for 5 min, and then centrifuged at 6000 ⫻ g for 1 min to remove the beads. The dissolved proteins in the sample buffer were resolved by SDS/PAGE [15% (w/v) acrylamide] on a gel slab. Proteins were transferred from gel to a nitrocellu-lose (NC) membrane in PBS at 4 °C for 32 h by the dif-fusion method (Bowen et al., 1980). The transferred pro-teins were detected with antiphosphoserine antibody (diluted to 1.25 g/10 mL), or 24p3 protein antibody (di-luted to 2 g/15 mL), followed by HRP-conjugated anti-rabbit IgG diluted to 1:12000 and fluorography. The re-active bands were visualized using an enhanced chemiluminescene (ECL) kit (RPN 2132, Amershan Pharmacia Biotech U.K. Ltd) and exposed on x-ray film.
3. RESULTS
3.1. Phosphorylation of 24p3 Protein by Kinases Fig. 1 shows the motif search for the consensus se-quences of protein kinase phosphorylation sites on the 24p3 protein molecule. Based on our previous report (Chu et al., 1998), both the predicted secondary struc-ture, including the three short motifs, are highly con-served in the lipocalin protein superfamily (Monaco and
Zanotti, 1992), and are deciphered in Fig. 1. The Thr54
-Ile-Tyr-Glu57, Ser88-Ser-Arg90, and Arg126
-Lys-Thr-Ser129 matched with the consensus sequences for the
phosphorylation site of CKII, PKC, and PKA, respec-tively. The three peptide segments are not included in the three short, highly conserved lipocalin family mo-tifs. The first one is inside -strand B, the second in a less rigid conformation and may be in a -turn or in un-ordered region between -strands D and E. Part of the third is overlapped with -strand G. The ability of these protein kinases to phosphorylate Thr54, Ser88, and
Thr128/Ser129 was examined. The progressive result of
the 32P-incorporation from [␥-32P]-ATP to the proteins
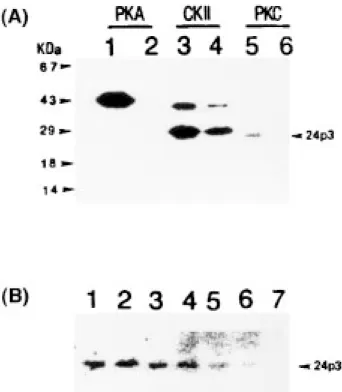
in the specified solution for each kinase at 30°C for 5 min is determined by -counter. Fig. 2A displays the
Fig. 2. In vitro phosphorylation of 24p3 protein by kinases. (A) Each
kinase was incubated alone (lanes 2, 4, 6) or in the presence of the 24p3 protein (lanes 1, 3, 5) and the 32P-incorporation to the protein (20 M)
was detected on the autoradiogram after the reaction mixture had been resolved by SDS/PAGE on a 15% polyacrylamide gel slab (see text for details). (B) 24p3 Protein (20 M) was incubated with PKC (2 mU/mL) in the presence of [␥-32P]-ATP (0.5 Ci/mmol) and a synthetic
polypeptide comparing Arg79-Tyr-Trp-Ilu-Arg-Thr-Phe-Val-Pro-Ser88
-Ser-Arg-Ala-Gly-Gln-Phe-Thr-Leu-Gly97 in the protein at 30°C for
5 min. The reaction mixture was resolved by SDS/PAGE and the extent of 32P-incorporation to the 24p3 protein was compared following
autoradiography. The molar ratio of the synthetic polypeptide to the 24p3 protein in the incubation was: 0 (lane 1), 1.0 (lane 2), 2.0 (lane 3), 5.0 (lane 4), 10.0 (lane 5), 20.0 (lane 6), 40.0 (lane 7).
autoradiogram of 32P-labeled proteins after the reaction
mixtures were resolved by SDS/PAGE; these protein ki-nases could be distinguished by their migration on SDS/PAGE. PKA appeared as a 41 kDa band and PKC as a 82 kDa band. CKII was resolved into one 42 kDa ␣-subunit and one 26 kDa -subunit. Neither PKA nor PKC but both subunits of CKII became radiolabeled when each kinase was isolated and incubated (cf. lanes 2, 4, and 6, of Fig. 2A), indicating that the autophos-phorylation occurred only at both subunits of CKII among the three protein kinases. PKA showed no activ-ity to phosphorylate 24p3 protein but the kinase became phosphorylated by the presence of 24p3 protein during incubation (cf. lanes 1 and 2, of Fig. 2A). The au-tophosphorylation of both subunits of CKII was en-hanced by 24p3 protein, although the enzyme did not phosphorylate 24p3 protein (cf. lanes 3 and 4 of Fig. 2A). One radiolabeled protein band corresponding to 24p3 protein was detected in the incubation of PKC with 24p3 protein (cf. lanes 5 and 6 of Fig. 2A), indicating that PKC is able to phosphorylate 24p3 protein. Because Ser88is a predicted phosphorylation site of PKC, the
in-teraction between PKC and a synthetic polypeptide comprising Arg79
-Tyr-Trp-Ilu-Arg-Thr-Phe-Val-Pro-Ser88-Ser-Arg-Ala-Gly-Gln-Phe-Thr-Leu-Gly97 in the
24p3 protein molecule was further investigated. The
32P-incorporation of [␥-32P]-ATP to 24p3 protein is
sup-pressed by the presence of this polypeptide. The results of one representative experiment (shown in Fig. 2B) in-dicates a decrease 24p3 protein phosphorylation coin-cided with the increase in the molar ratio of the polypep-tide to 24p3 protein in the reaction. When the molar ratio was more than 20, the 24p3 protein was unable to be phosphorylated.
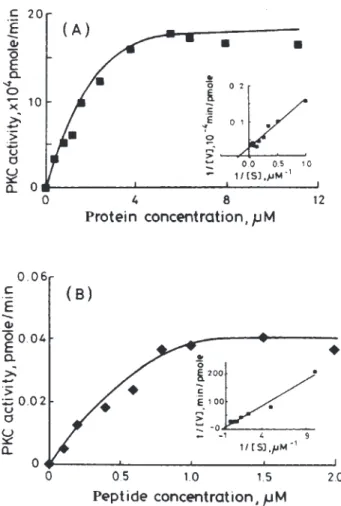
3.2. Phosphorylation Kinetic of PKC on the 24p3 Protein
The velocity of phosphate incorporation from ATP to the protein substrates by the PKC phosphorylation has been determined (Fig. 3A), and the kinetic parameters were obtained from the double reciprocal plot, based on Michaelis-Menten equation (Fig. 3A, inset). Km was es-timated at 2.96 and 0.42 M for phosphorylation of 24p3, or the synthetic polypeptide, respectively (Fig. 3B). It is surprising to note that the phosphorylation of the synthetic polypeptide occurs more rapidly than that of the 24p3 protein due to the phosphorylation site on the synthetic polypeptide being less rigid, and it becomes more susceptible to the enzyme catalysis.
3.3. The 24p3 Protein Enhances PKA Activity Fig. 4 shows the dose-relationship activity for PKA in the presence of the 24p3 protein. Comparing the PKA ac-tivity in the absence of 24p3 protein solution (Fig. 4 ⵧ) with the presence of phosphorylated (Fig. 4 䊏), or un-phosphorylated 24p3 protein (Fig. 4聺 ) in the incubation medium, sufficiently increased the PKA activity by 1.25-to 5-fold, because the 24p3 protein molar ratio was in-creased by 10- to 100-fold (Fig. 4A–E). Although the PKA activity in the presence of dephosphorylated 24p3 protein showed few enhancements than in the presence of phos-phorylated form, both forms enhanced he PKA activity. Fig. 3. The kinetics of PKC for the phosphorylation of 24p3 protein.
The 24p3 Protein (0–14 M) or the synthetic polypeptide (0–2 M) was incubated with PKC (2 U/mL) in the presence of [␥-32P]-ATP at
30°C. The rate of 32P-incorporation to 24p3 protein (A) or the synthetic
polypeptide (B) was measured. The double reciprocal plot based on the Michaelis-Menten equation is given in the inset.
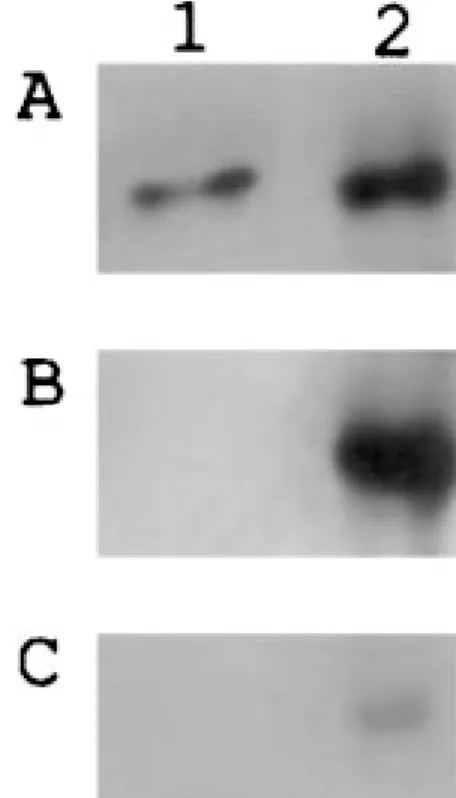
3.4. Detection of Phosphoserine in 24p3 Protein from Mouse Uteri
The 24p3 protein can be phosphorylated in vitro by PKC at the same site as observed in vivo, as judged by Dynabeads immunoprecipitation. The serinephosphate on the NC membrane has shown that the 24p3 protein in the immunoprecipitate is phosphorylated in vivo (lane 2, Fig. 5B). As shown in Fig. 5 (panel A), the precipitated 24p3 protein was verified by 24p3 protein induced anti-body at uterine tissue (lane 2) and purified 24p3 protein as a control (lane 1). Comparing the result of lane 1 with lane 2 in Fig. 5B, the phosphorylated-serine is absent in the purified-24p3 protein; dephosphorylation might occur in uterine luminal fluid or during purified process. The weakly fluorescent band of anti-rabbit IgG, shown in panel C (Fig. 5B), indicates the antiphosphoserine anti-body reacted specifically to phosphoserine of the 24p3 protein. Using alkaline phosphatase to dephosphorylate the precipitate taken from uterine tissue with an antibody for the 24p3 protein showed only a dim fluorescent band by the antiphosphoserine antibody, which supports the existence of in vivo phosphorylation of the 24p3 protein (data not shown).
4. DISCUSSION
Our observations have shown that an apparent Km in the PKC phosphorylation of the 24p3 protein (2.96M) is comparable to that hitherto reported as the most effective protein substrates for PKC (0.3–9.7 M) (Abe et al., 1991). The likelihood of phosphorylating 24p3 protein by PKC in vivo is conceivable. Our study of in vitro and
in vivo phosphorylation suggests PKC phosphorylation suggests PKC phosphorylation on phosphoserine of 24p3 protein does exist in this uterine protein. The result shown in Fig. 2A, lane 1; suggests that the 24p3 protein induces the conformational change of PKA and facilitates the autophosphorylation of the PKA. The phenomenon of autophosphorylation has been noted in many protein ki-nases, such as cAMP-dependent protein kinase II (Rubin and Rosen, 1975). The potential role of such autophos-phorylation to alter activity of the kinase has been sug-gested in many reports. The enhancement of enzymatic activity may result from the 24p3 protein association, thus causing the conformational change of PKA. Based on the results (Fig. 2, lane 1; Fig. 4), it is reasonable to speculate that the 24p3 protein may have induced the conforma-tional change of PKA to facilitate the autophosphorylation and then induced the enzymatic activity. The phenomenon of phosphorylation on 24p3 protein may not correlate to PKA activity directly. In the present study, the CKII ac-tivity was not affected by 24p3 protein (data not shown), Fig. 4. Effect of 24p3 protein on the PKA activity. The 0.25 pmole
PKA activity was determined (cf. Methods) in absence (ⵧ) or presence of phosphorylated (䊏) or unphosphorylated 24p3 protein (聺). Amount of added 24p3 protein: A, 2.5 pmole; B, 5.0 pmole; C. 10.0 pmole; D, 12.5 pmole; E, 25.0 pmole. Each value represents the mean ⫾ SEM of triplicates from a representative experiment.
Fig. 5. Immunodetection of phosphorylated 24p3 protein in uterine
tissue. Mouse uterine tissues were collected (cf. Methods). The 24p3 protein was immunoprecipitated from uterine tissue and analyzed by Western-blot analysis with antibody against 24p3 protein or phosphoserine. Lane 1 is purified 24p3 protein as a control. Lane 2 is the immunoprecipitated protein from mouse uterine tissue. Panel A, detected by 24p3 protein induced antibody; panel B, detected by antiphosphoserine antibody; panel C, detected by anti-rabbit IgG antibody as a control.
even if the 24p3 protein increased autophosphorylation of CKII. Elucidation of the interrelationship between 24p3 protein and PKA is more worthy to elucidate. Fur-ther studies are necessary to determine if enhancement of PKA activity will occur in vivo.
In summary, we show for the first time that 24p3 pro-tein is a serine phosphorylated-glycopropro-tein in mouse uteri. The phosphorylation and glycosylation, the post-translational modifications of a protein, which have op-tional, affect the activity of secretion (Kukuruzinska and Leman, 1998; Price et al., 1994). As a secretory protein, whether phosphorylation (in this paper) or glycosylation (Chu et al., 1996) of the 24p3 protein are a necessary re-quirement for the secretion of the 24p3 protein from the tis-sue will require future study. Because the phosphorylation/ dephosphorylation of a lipid-binding protein may regulate the directional orientation of lipid flux in cells (Buelt et al., 1992) as a lipocalin of 24p3 protein (Chu et al., 1997), the study of phosphorylation of the 24p3 protein is important. Phosphorylation may represent a mechanism, which regu-lates a 24p3 protein association with the hydrophobic mol-ecule and regulates biological activity of the 24p3 protein. The outcome may determine how the phosphorylation role is identified in the hydrophobic molecule interaction or protein secretion. Based on the result of the autoradiogram study, the fact that the 24p3 protein facilitated the au-tophosphorylation of PKA and enhanced the PKA activ-ity, indicating an interrelation between the 24p3 protein and kinase in vivo, merits further attention. Further study is needed to elucidate the significance of the 24p3 protein within the reproductive system.
ACKNOWLEDGMENT
This work was partially supported by grant NSC89-2311-B001-064 from National Sciences Council, Taiwan.
REFERENCES
Abe, K., Sakuke, K., Tanaka, M., Uehara, Y., Matsuno, K., Miyazaki, T., and Katoh, N. (1991). Biochem. Biophy. Res. Commun. 176, 1123–1129.
Bairoch, A. and Apweiler, R. (1997). J. Mol. Med. 75, 312–316. Bairoch, A. and Apweiler, R. (1998). Nucleic Acids Res. 26, 38– 42. Bowen, B., Steinberg, J., Laemmli, V. K., and Weitraub, H. (1980)
Nu-cleic Acid Res. 8, 1–20.
Buelt, M. K., Xu, Z., Banaszak, L. J., and Bernlohr, D. A. (1992).
Bio-chemistry 31, 3493–3499.
Casnellie, J. E. (1991). Meth. Enzymol. 200, 115–120.
Chu, S. T., Huang, H. L., Chen, J. M., and Chen, Y. H. (1996).
Biochem. J. 316, 545–550.
Chu, S. T., Lin, H. J., and Chen, Y. H. (1997). J. Peptide Res. 49, 582–585.
Chu, S. T., Lin, H. J., Huang, H. L., and Chen, Y. H. (1998). J. Peptide
Res. 52, 390 –397.
Chu, S. T., Lee, Y. C., Nein, K. M., and Chen, Y. H. (2000). Mol.
Re-prod. Dev. 57, 26 –36.
Davis, T. R., Tabatabai, L., Bruns, K., Hamilton, R. T., and Nilsen-Hamilton, M. (1991). Biochem. Biophys. Acta 1095, 145–152. Flower, D. R., North, A. C. T., and Attwood, T. K. (1991). Biochem.
Biophys. Res. Commun. 180, 69–74.
Glover, C. V., Shelton, E. R., and Brutlag, D. L. (1983). J. Biol. Chem.
258, 3258 –3265.
Gonzalez, A., Klann, E., Sessoms, J. S., and Chen, S. J. (1993). Anal.
Biochem. 215, 184 –189.
Goueli, B. S., Hsiao, K., Tereba, A., and Goueli, S. A. (1995). Anal.
Biochem. 225, 10–17.
Hathaway, G. M. and Traugh, J. A. (1979). J. Biol. Chem. 254, 762 –768.
Hraba-Renevey, S., Turler, H., Kress, M., Salomon, C., and Weil, R. (1989). Oncogene 4, 601– 608.
Kjeldsen, L., Jonsen, A. H., Sengeløy, H., and Borregaard, N. (1993).
J. Biol. Chem. 268, 10425–10430.
Kukuruzinska, M. A. and Lennon, K. (1998). Crit. Rev. Oral Biol.
Med. 9:415–448.
Kurosawa, M. (1994). J. Pharmacol. Toxicol. Meth. 31, 135–139. Liu, Q. and Nilsen-Hamilton, M. (1995). J. Biol. Chem. 270,
22565–22570.
Meheus, L. A. Fransen, L. M., Raymackers, J. G., Blockx, H. A., van Beeumen, J. J., van Bun, S. M., and van de Voorde, A. (1993).
J. Immunol. 151, 1535–1547.
Monaco, H. L. and Zanotti, G. (1992). Biopolymer 32, 457– 465. Price, P. A., Rice, J. S., and Williamson, M. A. (1994). Prot. Sci. 3,
822–830.
Rubin, C. S. and Rosen, O. M. (1975). Annu. Rev. Biochem. 44, 831–887.