452
Strontium Bands in Relation to Age Marks in Otoliths of European
Eel Anguilla anguilla
Wann-Nian Tzeng1,*, Kenneth P. Severin2, Håkan Wickström3 and Chia-Hui Wang1 1Department of Zoology, College of Science, National Taiwan University, Taipei, Taiwan 106, R.O.C.
2Department of Geology and Geophysics, University of Alaska Fairbanks, Alaska 99775-0760, USA 3National Board of Fisheries, Institute of Freshwater Research, S-178 93, Drottningholm, Sweden
(Accepted July 30, 1999)
Wann-Nian Tzeng, Kenneth P. Severin, Håkan Wickström and Chia-Hui Wang (1999) Strontium bands in relation to age marks in otoliths of European eel Anguilla anguilla. Zoological Studies 38(4): 452-457. Higher-concentration strontium (Sr) bands and hyaline zones in otoliths of European eel, Anguilla anguilla (L.), from both brackish waters and freshwater lakes were examined by wavelength dispersive x-ray spectrometry on an elec-tron microprobe and by visible light microscopy, respectively. The positions of higher-concentration (> 0.3 wt %) Sr bands and hyaline zones were identical in otoliths of eels from brackish waters; however, no corresponding higher-concentration Sr bands were discernible in otoliths of eels from fresh water. The higher-concentration Sr bands were deposited when the eels migrated from brackish water to high-saline seawater during winter. The number of hyaline zones in the otoliths corresponds to the age of the eel. Accordingly, higher-concentration Sr bands in otoliths can be used to determine fish age and migratory history in brackish waters.
Key words: European eel, Otolith, Strontium, Annulus.
*To whom correspondence and reprint requests should be addressed. Tel: 886-2-23639570. Fax: 886-2-23636837. E-mail: wnt@ccms.ntu.edu.tw.
E
uropean eel Anguilla anguilla (L.) is the most abundant among 18 species of the genus Anguilla in the world (Tesch 1983). It spawns in the Sargasso Sea, and its larvae (leptocephali) drift with the Gulf Stream and North Atlantic Current to the continental shelf of northern Europe, where they metamorphose into glass eels. They become pigmented elvers in estuaries. Their migration from the Sargasso Sea to the estuaries requires 7 to 8 mo (Lecomte-Finiger 1992). The elvers penetrate rivers and streams, and complete their growth stage in fresh water. Some elvers may remain in marine or brackish waters along the coast until maturation (Tsukamoto et al. 1998). Male eels grow in rivers for 3 to 7 yr with a mean of 5 yr, while female eels grow from 4 to 15 yr with a mean of 7 yr (Vollestad and Jonsson 1986). In late autumn the eels become mature, metamor-phose from yellow eels to silver eels, and migrate back to the Sargasso Sea where they spawn and presumably die (Bertin 1956, Tesch 1983).The otolith of the eel is optically composed of
alternating hyaline and opaque zones which are de-posited with seasonal changes in fish growth (Sinha and Jones 1967, Hogman 1968, Casselman 1982). Hyaline zones are bright and opaque zones are dark when the otolith is viewed with transmitted light, while opaque zones are bright and hyaline zones are dark when viewed with reflected light (Williams and Bedford 1974). Hyaline zones are deposited during slow growth in winter, and are composed primarily of organic materials with lower amounts of calcium carbonate, while opaque zones are deposited during fast growth in summer, and are composed primarily of inorganic calcium carbonate (Penttila and Dery 1988). The hyaline zones are generally deposited annually, and are usually designated as the annuli.
Strontium (Sr)/Calcium (Ca) ratio in otoliths has been used to study the migratory environmental his-tory of European and Japanese eels (Otake et al. 1994, Tzeng and Tsai 1994, Tzeng 1995, Arai et al. 1997, Tzeng et al. 1997) and other fishes (Radtke et al. 1988, Kalish 1990, Secor 1992, Limburg 1995).
The ratio is positively correlated with ambient salinity (Tzeng 1996), but negatively correlated with tem-perature and fish growth rate (Sadovy and Severin 1992 1994, Townsend et al. 1992, Tzeng 1994). Salinity, temperature, and growth rate often change seasonally, suggesting that a temporal relationship may exist between annulus formation and Sr deposition. If this is the case, otolith microchemistry could be used to determine the age and growth his-tory of fish. However, no studies have validated the relationship between annulus formation and otolith microchemistry (Casselman 1982, Proctor et al. 1990, Seyama et al. 1991, Secor 1992, Tzeng et al. 1997).
The objectives of this study were to clarify the temporal relationship between hyaline zone forma-tion and the higher-concentraforma-tion Sr band deposiforma-tion in otoliths of the European eel, and to understand the mechanism of Sr band deposition.
MATERIALS AND METHODS
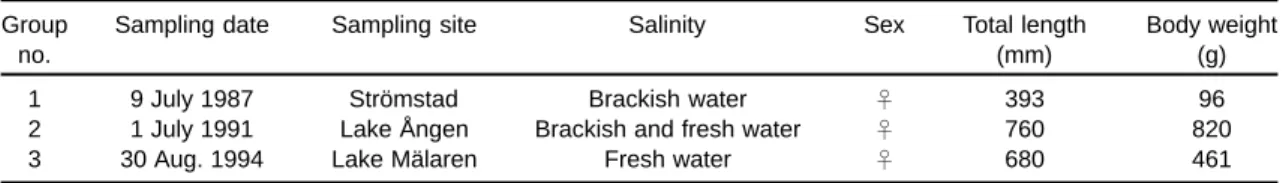
Otoliths of 12 European eels, Anguilla anguilla, collected from brackish waters and freshwater lakes of Sweden in a previous study on otolith microchem-istry (Tzeng et al. 1997) were used as materials in this study. They were divided into 3 different life his-tory groups according to their otolith microchemistry. Relationships between annuli and microchemistry of sagittal otoliths of the 12 eels were examined, and 1 eel from each group was selected for description (Table 1). The eels of Group 1 were collected from brackish waters off Strömstad on the west coast of Sweden in 1987. The eels of Group 2 were stocked in Lake Ången on the east coast of Sweden at the yellow eel stage after being caught in brackish wa-ters on the west coast of Sweden in 1979, and were recaptured in 1991. The eels of Group 3 were col-lected from Lake Mälaren on the east coast of Swe-den in August 1994.
Procedures of preparing the otoliths, including embedding, sectioning, polishing, and coating, for microprobe quantitative analysis of Sr and Ca and x-ray mapping of Sr concentration are described in
Tzeng et al. (1997). After microprobe analysis, otoliths were repolished to remove carbon coating, etched with EDTA to enhance the hyaline zones (annuli), and photographed with both transmitted and reflected light microscopes. The positions of annuli were compared with those of the higher-concentra-tion Sr bands in the x-ray map.
RESULTS
Brighter areas in the Sr maps indicate higher Sr concentrations (Fig. 1a, c, e). Sr content increased from the primordium and reached the highest (> 0.8 wt %) at metamorphosis when the leptocephalus metamorphosed to glass eel. Beyond the elver stage, Sr contents dramatically decreased, and the patterns of distribution of Sr concentrations differed among otoliths from the 3 groups.
Sr concentrations in Group 1 otoliths beyond the elver mark averaged approximately 0.3 wt %. The otoliths revealed 5 distinct higher-concentration Sr bands (> 0.3 wt %) and 5 dark hyaline zones (Fig. 1a, b). The positions of the 5 high-Sr bands and the 5 hyaline zones in the otoliths were identical. The hy-aline zones were dark while opaque zones were bright, irrespective of being viewed with transmitted or reflected light, although it was noted that hyaline zones of otoliths were bright in transmitted light (Penttila and Dery 1988). The 1st opaque zone was wider than others, indicating that the eel grew faster in the 1st year. Sr levels of the 2nd band were low, and the corresponding hyaline zone was relatively faint. On the other hand, Sr levels of the 4th band were higher, and the corresponding hyaline zone was clearer, indicating that the slow growth period was longer.
Group 2 otoliths had 2 different levels of Sr centration beyond the elver mark (Fig. 1c). Sr con-centration in the inner layer averaged approximately 0.3 wt %, which was significantly greater than that in the outer layer (< 0.05 wt %). There are 9 high-Sr bands and 9 hyaline zones in the inner layer, and their positions were identical between Sr bands and hyaline zones (Fig. 1c, d). In the outer layer there are
Table 1. Life history of 3 European eels used in this study
Group Sampling date Sampling site Salinity Sex Total length Body weight
no. (mm) (g)
1 9 July 1987 Strömstad Brackish water ð 393 96
2 1 July 1991 Lake Ången Brackish and fresh water ð 760 820
11 more-or-less distinct hyaline zones, but there are no corresponding high-Sr bands. These indicate that the eel lived in brackish water for 9 yr and in fresh water for 11 yr. In the boundary between the high and low levels of Sr concentration, the growth of the otoliths seems to be retarded, and thus Sr bands and hyaline zones are narrow and complicated. This may lead to an underestimation of the numbers of Sr bands and/or hyaline zones.
Sr concentration in Group 3 otoliths averaged less than 0.05 wt %. Other than the area around the nucleus, there were no areas of high Sr levels even though there were 14 distinct hyaline zones visible with light microscopy (Fig. 1e, f).
DISCUSSION
Regardless of later life history, the nucleus of any eel is deposited during a marine life phase. The nuclei of the otoliths of all 3 eels are hyaline and also have high Sr contents, as do those of other eels (Otake et al. 1994, Tzeng and Tsai 1994, Arai et al. 1997, Wang and Tzeng 1998). In addition, all the higher-concentration Sr bands correspond to narrow hyaline zones. This is similar to what Seyama (1991) found in the otolith of a red emperor (Lutjanus
sebae). However, the mechanism of the formation
of higher-concentration Sr bands in otoliths is not clear. Incorporation of Sr into otoliths is a
cated biogeochemical process influenced by abiotic factors such as temperature, salinity, and water chemistry, as well as by biotic factors such as genetics, developmental stage, growth rate, food, and physiological condition of the fish (Dodd 1967, Yamada et al. 1979, Kalish 1989, Gallahar and Kingsford 1992, Radtke and Shafer 1992, Sadovy and Severin 1992, Otake et al. 1994, Tzeng and Tsai 1994, Tzeng et al. 1997).
Hyaline zones are formed during the slow-growth phase of the otolith. Generally, otolith slow-growth is proportional to somatic growth, and seasonal growth of eels is lower in low-temperature periods (Bruun 1963, Sinha and Jones 1967, Campana and Neilson 1985, Tzeng et al. 1994). Thus, higher-con-centration Sr bands corresponding to hyaline zones in otoliths of brackish water eels are deposited dur-ing low temperature periods when fish growth is slow. Sr concentrations are higher in seawater than in brackish water (Tzeng and Tsai 1994). Sr/Ca ra-tios in otoliths of eels are negatively correlated to ambient temperature, and highly positively
corre-lated to salinity (Tzeng 1994 1996). Temperatures in the coastal waters of Sweden decrease sharply dur-ing winter. At this time eels generally migrate from coastal waters to deeper water for overwintering (Tesch 1983). Salinities are higher in deeper water than in coastal waters in the studied area (Tomczak and Godfrey 1994). These facts may indicate that higher-concentration Sr bands corresponding to hya-line zones in otoliths of brackish water eels are de-posited at low temperatures during winter when eels migrate from brackish water to high-saline deep wa-ter (Fig. 1a-d). However, Sr contents are lower and no regular higher-concentration Sr bands corre-sponding to the hyaline zones are observed in otoliths of freshwater eel (Fig. 1c-f). This indicates that fresh water does not provide enough strontium for incorporation during the formation of hyaline zones, and the effect of temperature and growth rate on the formation of the higher-concentration Sr bands is not obvious.
Hyaline zones apparently are formed once a year, which was validated in a previous study (Tzeng et al. 1994). Higher-concentration Sr bands are de-posited synchronously with the hyaline zones in otoliths of eels from brackish waters. This suggests that higher-concentration Sr bands in otoliths can be used to estimate the age of brackish water eels.
In conclusion, the higher-concentration Sr bands in otoliths are deposited when eels migrate from brackish water to high-saline sea water. The deposition of higher-concentration Sr bands is syn-chronous with the hyaline zones that are formed once a year. Thus, the higher-concentration Sr bands in otoliths can be used to determine the age of eels in brackish water and their migratory history. Acknowledgments: This study was financially sup-ported by the National Science Council, Republic of China (NSC 86-2311-B002-042, a research project of Prof. W.N. Tzeng). The authors are grateful to the anonymous reviewers for helpful comments.
REFERENCES
Arai T, T Otake, K Tsukamoto. 1997. Drastic changes in otolith microstructure and microchemistry accompanying the on-set of metamorphosis in Japanese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 161: 17-22.
Bertin L. 1956. Eels - a biological study. London: Cleaver-Hume Press.
Bruun AF. 1963. The breeding of the North Atlantic freshwater eel. Adv. Mar. Biol. 1: 137-169.
Campana SE, JD Neilson. 1985. Microstructure of fish otolith. Can. J. Fish. Aquat. Sci. 42: 1014-1032.
Casselman JM. 1982. Age and growth assessment of fish from Fig. 1. Higher-concentration Sr bands in Sr x-ray maps (a, c, and
e) and hyaline zones in visible-light photographs (b, d, and f) of otoliths of 3 European eels (a and b, Group 1; c and d, Group 2; e and f ,Group 3; E, elver mark; P, primordium; circles, annuli). Groups 1, 2 and 3 refer to table 1. The Sr maps a, c, and e are modified from Tzeng et al. 1997.
their calcified structure-techniques and tools. In ED Prince, LM Palos, eds. Proceeding of the International Workshop on Age Determination of Oceanic Pelagic Fishes: Tunas, Billfishes, and Shark, NOAA Technical Report NMFS, USA 8: 1-17.
Dodd RJ. 1967. Magnesium and strontium in calcareous skeletons: a review. J. Palaeontol. 41: 1313-1329. Gallahar NK, MJ Kingsford. 1992. Patterns of increment width
and strontium ratios in otoliths of juvenile rock blackfish, Girella elevata (M.). J. Fish Biol. 41: 749-763.
Hogman WJ. 1968. Annulus formation on scale of four species of coregonids reared under artificial conditions. J. Fish. Res. Board Can. 25: 2111-2122.
Kalish JM. 1989. Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. J. Exp. Mar. Biol. Ecol. 41: 749-763.
Kalish JM. 1990. Use of otolith microchemistry to distinguish the progeny of sympatric anadromous and non-anadromous salmonids. US Fish. Bull. 88: 657-666.
Lecomte-Finiger R. 1992. Growth history and age at recruitment of European glass eels (Anguilla anguilla) as revealed by otolith microstructure. Mar. Biol. 144: 205-210.
Limburg KE. 1995. Otolith strontium traces environmental his-tory of subyearling American shad Alosa sapidissima. Mar. Ecol. Prog. Ser. 119(1-3): 25-35.
Otake T, T Ishii, M Nakahara, R Nakamura. 1994. Drastic changes in otolith strontium/calcium ratios in leptocephali and glass eels of Japanese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 113: 189-193.
Penttila J, LM Dery. 1988. Age determination methods for north-west Atlantic species. NOAA Technical Report NMFS 72, US Department of Commerce, 135 pp.
Proctor CH, JS Gune, RE Thresher, IR Harrowfield. 1990. Elec-tron probe X-ray microanalysis of calcified tissues: an alter-native method of determining age of fish. In DA Hancock, ed. The measurement of age and growth in fish and shellfish. Australian Society for Fish Biology Workshop 22-23 August 1990, Proceedings No. 12 of Bureau of Rural Resources, Canberra, Australia. pp 109-111.
Radtke RL, RA Kinzie III, SD Folsom. 1988. Age at recruitment of Hawaiian freshwater gobies. Environ. Biol. Fish. 23: 205-213.
Radtke RL, DJ Shafer. 1992. Environment sensitivity of fish otolith microchemistry. Aust. J. Mar. Freshwater Res. 43: 935-951.
Sadovy Y, KP Severin. 1992. Trace elements in biogenic aragonite: correlation of body growth rate and strontium lev-els in the otoliths of the white grunt, Haemulon plumieri (Pisces: Haemulidae). Bull. Mar. Sci. 50: 237-257. Sadovy Y, KP Severin. 1994. Elemental patterns in red hind
(Epinephelus guttatus) otoliths from Bermuda and Puerto Rico reflect growth rate, not temperature. Can. J. Fish. Aquat. Sci. 51: 133-141.
Secor DH. 1992. Application of otolith microchemistry analysis to investigate anadromy in Chesapeake Bay striped bass Morone saxatilis. US Fish. Bull. 90: 798-806.
Seyama H, JS Edmonds, MJ Moran, Y Shibata, M Soma, M Morita. 1991. Periodicity in fish otolith strontium, sodium,
and potassium corresponds with visual banding. Experientia (Basel) 47(11-12): 1193-1196.
Sinha VPR, JW Jones. 1967. On the age and growth of the freshwater eel (Anguilla anguilla). J. Zool. Soc. London 153: 99-117.
Tesch FW. 1983. Der Aal: Biologie und Fischerei. 2nd ed. Hamburg: Parey.
Tomczak M, JS Godfrey. 1994. Regional oceanography: an introduction. London: Pergamon, 422 pp.
Townsend DW, RL Radtke, S Corwin, DA Libby. 1992. Stron-tium: calcium ratios in juvenile Atlantic herring Clupea harengus L. otolith as a function of water temperature. J. Exp. Mar. Biol. Ecol. 160: 131-140.
Tsukamoto K, I Nakai, WV Tesch. 1998. Do all freshwater eels migrate? Nature 396: 635.
Tzeng WN. 1994. Temperature effects on the incorporation of strontium in otolith of Japanese eel Anguilla japonica. J. Fish Biol. 45: 1055-1066.
Tzeng WN. 1995. Migratory history recorded in otoliths of the Japanese eel, Anguilla japonica, elvers as revealed from SEM and WDS analyses. Zool. Stud. 34 (Supplement 1): 234-236.
Tzeng WN. 1996. Effects of salinity and ontogenetic movement on strontium: calcium ratio in otolith of the Japanese eel, Anguilla japonica Temminck and Schlegel. J. Exp. Mar. Biol. Ecol. 199: 111-122.
Tzeng WN, KP Severin, H Wickström. 1997. Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Mar. Ecol. Prog. Ser. 149: 73-81.
Tzeng WN, YC Tsai. 1994. Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its migration from the ocean to the rivers of Taiwan. J. Fish Biol. 45: 671-683.
Tzeng WN, CH Wang, H Wickström. 1998. Can European eel, Anguilla anguilla (L.), complete their life cycle in marine environment? In Abstract of International Conference on Fisheries and Food Security Beyond the Year 2000. The Fifth Asian Fisheries Forum, 11-14 Nov. 1998, Chiang Mai, Thailand. Asian Fisheries Society, Chulalongkorn University, Thailand, p. 252.
Tzeng WN, HF Wu, H Wickström. 1994. Scanning electron mi-croscope analysis of annulus microstructure in otolith of European eel, Anguilla anguilla. J. Fish Biol. 45: 479-492. Vollestad LA, B Jonsson. 1986. Life-history characteristics of the European eel Anguilla anguilla in the Imsa River, Norway. Trans. Amer. Fish. Soc. 115: 864-871.
Wang CH, WN Tzeng. 1988. Interpretation of geographic varia-tion in size of American eel Anguilla rostrata elvers on the Atlantic coast of North America using their life history and otolith aging. Mar. Ecol. Prog. Ser. 168: 35-43.
Williams T, BC Bedford. 1974. The use of otoliths for age determination. In TB Bagenal, ed. The aging of fish. Oxford, UK: Unwin Brothers, pp 114-123.
Yamada SB, TJ Mulligan, SJ Fairchild. 1979. Strontium marking of hatchery-reared coho salmon (Oncorhychus kisutch, Walbaum). J. Fish Biol. 14: 267-275.
ÚwÁÕÛW§ªÖôP~ü§öY
¿U~1 Kenneth P. Severin2 Håkan Wickström3 ýÎf1
QÎGlL´öÎúÇãLè)ÀRÚwÁÕÛºª(×ÖôP~ü§öY+²Go{ÓÛÐHô ºÚwÁºÕÛ)³Üú㺪ÖôX{)ªÖôºìmP~üºìm@2+ýO)ÓÛòyÌ)hS ³ªÖôX{+ÀúªÖôiàOÁ½óVuqÐHôjåìª3׺üôÉÒΨº+Ñ~üºÆØ iH¾,Á½º~Ö+]¹)ѪÖôºÆØiHÀúÁ½bÐHôɺ~ÖÎäjåiú+ öäü/ÚwÁ)ÕÛ)Ö)~ü+ 1êßO9jÇÊ«Çt 2üêüÔµ[FairbanksjÇaèÎayìÇt 3çåêß®~¡HôãsÒ