Increased Risk of Coronary Heart Disease in Patients with Chronic Osteomyelitis: A Population-Based Study in a Cohort of 23 Million
Lien-Cheng Hsiao1, Chih-Hsin Muo2, Yu-Ching Chen3, Che-Yi Chou4,5, Chun-Hung
Tseng6,7, and Kuan-Cheng Chang1,4
1Division of Cardiology, Department of Internal Medicine, China Medical University
Hospital, Taichung, 40447, TAIWAN; 2Institute of Environmental Health, College of
Public Health, China Medical University, Taichung, 40402, TAIWAN; 3Department
of Biomedical Informatics, Asia University, Taichung, 41354, TAIWAN; 4Graduate
Institute of Clinical Medical Science, China Medical University, Taichung 40402, TAIWAN; 5Department of Nephrology, China Medical University
Hospital, Taichung, 40447, TAIWAN; 6Department of Neurology, China Medical
University Hospital, Taichung, 40447, TAIWAN; 7School of Medicine, China
Medical University, Taichung, 40402, TAIWAN
Running head: Chronic Osteomyelitis and Coronary Artery Disease
Word count: 2620
Address correspondence to:
Kuan-Cheng Chang, M.D., Ph.D.
Division of Cardiology, Department of Medicine, China Medical University Hospital 2, Yuh-Der Road, Taichung 40447, Taiwan
Tel: 886-4-22052121, ext. 2311 Fax: 886-4-23602467 E-mail: kuancheng.chang@gmail.com
Chun-Hung Tseng, MD., Department of Neurology, China Medical University Hospital, and School of Medicine, China Medical University, No.91 Hsueh-Shih Road, Taichung, 40402, Taiwan (Tel: 886-4-2205-3366; Fax: 886-4-2234-4055; e-mail: d8333@mail.cmuh.org.tw)
Abstract
Objectives: Chronic inflammatory disease may trigger vascular atherosclerosis. This study aimed to determine whether chronic osteomyelitis (COM) is linked to an increased risk of coronary heart disease (CHD).
Methods: A national insurance claim dataset of more than 23 million enrollees was used to identify 15,054 patients with newly-diagnosed COM and 60,216 randomly selected age- and gender-matched controls between 2001 and 2009 for comparing the risk and incidence of CHD. The study period was from the entry date to the first date of the following events: the diagnosis of CHD, death, withdrawal from the Taiwan National Health Insurance program or the end of 2010. The analysis of the CHD risk was performed using Cox proportional hazards regression model.
Results: During a follow-up period of 67,927 person-years, the overall incidence rate of CHD in COM cohort was 1.95-times higher than non-COM cohort (16.66 vs. 8.52 per 1000 person-years). After controlling age, gender and four co-morbidities (hypertension, diabetes, hyperlipidemia, and stroke), the risk remained significantly higher in the COM cohort than the control group (adjusted hazard ratio [HR] =1.65, 95% confidence interval [CI] =1.54-1.78, p<0.001). In age-stratified analysis, the younger population had a stronger association between COM and CHD risk than the elderly (from HR =3.42, 95% CI =1.60-7.32 in age <35 to HR =1.39, 95% CI =1.15-1.68 in age ≥80).
Conclusions: This study demonstrates that COM is an independent risk factor for CHD, particularly in the younger population. Further studies are necessary to explore the underlying mechanisms linking COM and CHD.
What is already known about this subject?
1. Although a number of traditional risk factors have been identified in the development of coronary heart disease (CHD), such as hypertension,
hyperlipidemia and type 2 DM, it has been observed that about 10-50% of people developed CHD without these classical risk factors.
2. Infection and inflammation have been proposed as novel risk factors in atherosclerotic disease.
What does this study add?
1. Chronic osteomyelitis (COM), a long-term infection of bone and bone marrow, could lead to a subsequent chronic inflammatory process in the body.
2. To improve CHD prevention and management, it would be important to understand whether COM serves as a novel risk factor in the development of CHD.
How might this impact on clinical practice?
1. Results presented in this study suggest that COM is a risk factor for CHD, independent of age, gender, hypertension, diabetes, hyperlipidemia, and stroke. 2. This implication has paved the way for further exploration in the pathogenesis,
risk prediction and treatment of COM in relation to CHD prevention in the future.
Coronary heart disease (CHD), also known as coronary artery disease (CAD), refers to a spectrum of illnesses ranging from coronary atherosclerosis, myocardial infarction, and post-infarct heart failure.1,2 Nowadays, CHD remains one of the major
causes of mortality and morbidity in the world.3-5 Fortunately, with the introduction of
life style modification, modern pharmacology and innovative coronary intervention, the number of CHD-associated death has been steadily reduced in the past decades.3,6,7
According to Framingham Heart Study (FHS) and following epidemiological studies, a number of CHD risk factors, including hypertension, hyperlipidemia, diabetes mellitus, obesity and smoking, have been identified to play crucial roles in the pathogenesis of coronary artery atherosclerosis and these so-called conventional risk factors for CHD were helpful to predict the risk of CHD.3,8,9 On the other hand,
lipoprotein (a) [Lp(a)], homocysteinemia, hypercoagulability, imbalanced oxidative stress and infection have been investigated as novel issues (non-traditional risk factors) in atherosclerotic disease, including CHD.3,10-13 Furthermore, it has been
suggested that atherosclerotic change, the main etiology of CHD, is an inflammatory process resulting from mutual involvement of immune mechanisms and metabolic risk factors.14,15
Recently, it has been shown that patients with chronic inflammatory disease have an increased risk of cardiovascular diseases.16 For example, periodontal disease, a
chronic inflammatory disorder caused by a chronic bacterial infection, has been proposed to be associated with the development and progression of CHD.17 Similarly,
chronic osteomyelitis (COM), a long-term infection of bone and bone marrow, could lead to a subsequent chronic inflammatory process in the body.18,19 To improve CHD
prevention and management, it would be important to understand whether COM serves as a novel risk factor in the development of CHD. However, current knowledge regarding the interaction between COM and CHD remains limited. In the present
study, we sought to determine the association of COM with the incidence and risk of CHD using a nationwide insurance dataset of 23 million enrollees in Taiwan.
Methods Data Sources
This retrospective cohort study used inpatient database, a part of National Health Insurance Research Databases (NHIRDs), which was established by the National Health Research Institutes (NHRI). NHRI constructed all medical claims from the beneficiary files in National Health Insurance program which covered 98% of Taiwan population since 1996.20 According to Personal Information Protection Act, the
identification code of patients was scrambled during NHIRDs compiling before sent to researchers. Due to the blindness of researchers to the patient identities, this study escaped from the review of Ethics Committee. The information included birthday, gender, inpatient admission and discharge. A variety of personal health habits, such as physical activity, smoking and alcohol consumption, were not available in the Taiwan National Health Insurance dataset. The diagnosis of disease code was based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) in NHIRDs. National Health Insurance (NHI) prevents coding errors and misdiagnosis by regularly monitoring disease coding and auditing claims submitted for reimbursement by hospitals that contract with NHI to offer NHI participants inpatient services. Hence, the consistent information could allow us to conduct the present research of correlation between COM and CHD.
Study Population
All 15,054 patients with newly diagnosed osteomyelitis (ICD-9-CM code 730.1) and without past history of coronary heart disease (CHD, ICD-9-CM code 410, 411.1, 411.81, 411.89, 412, 413, 414.00-414.05, 414.8 and 414.9) were collected from 2001
to 2009 as osteomyelitis cohort. The date of their diagnosis of osteomyelitis was used as the entry date. The comparison group consisted of randomly selected insured people without the history of osteomyelitis and CHD, matched with the COM group across age strata (every 5 years), gender and the entry-year and entry-month with a sample size 4-fold of the COM group. The co-morbidities included hypertension (ICD-9-CM code 401-405), diabetes mellitus (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272.0-272.4) and stroke (ICD-9-CM code 430-438) and were defined before the entry date. The study period was from the entry date to the first date of the following events: the diagnosis of CHD, death, withdrawal from the National Health Insurance program or the end of 2010.
Statistical Analysis
All statistic analyses were performed with the use of the SAS software version 9.1 (SAS Institute Inc., Carey, NC) and the significant level was set at 0.05 for a two-tailed p value. The difference of characteristics between two cohorts was analyzed using chi-square test. The incidence (per 1000 person-years) of CHD was calculated and the hazard ratios (HRs) of CHD were estimated in Cox proportional hazards regression. Model 1 was adjusted for age and gender. Model 2 controlled age, gender, hypertension, diabetes, hyperlipidemia and stroke. We also assessed the risks of CHD stratified by gender, age group (< 35, 35-49, 50-64, 65-79 and ≥ 80 years) or co-morbidities including hypertension, diabetes, hyperlipidemia and stroke. Additionally, t here were four separate models (hypertension, diabetic mellitus, hyperlipidemia and stroke) stratified by COM to assess the join effects on the risk of CHD. For example, the hypertension group included hypertension patients with or without any other co-morbidity, and those with only other co-morbidities were excluded from that particular model. Furthermore, the association between the risk for CHD and the severity of COM was examined. The osteomyelitis severity was defined as the
division of total length of hospital stay due to COM during the follow-up duration by the length of follow-up duration. By using the tertile method, the severity of COM was further classified into three levels: mild (the first tertile), moderate (the second tertile) and severe (the third tertile). Kaplan-Meier model was used to describe the disease-free rate curve and log-rank test used to test the difference between case and control cohorts.
Results
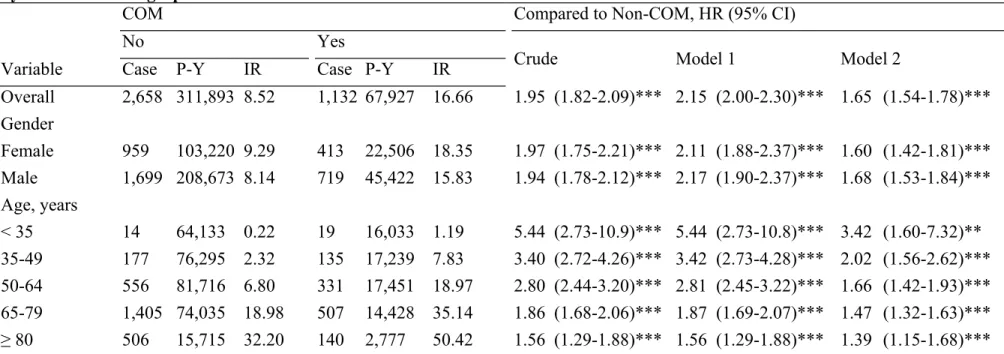
All 15,054 osteomyelitis patients and 60,216 controls were selected in the present study. The mean age was 54.0 years old (standard deviation 19.1) and men was the majority (66.4% vs. 33.6%) in osteomyelitis patients. Compared to controls, osteomyelitis patients had higher percentages of hypertension, diabetes mellitus, hyperlipidemia and stroke, as shown in Table 1. During a follow-up period of 67927 person-years, the overall incidence rate of CHD in osteomyelitis cohort was 1.95-times higher than non-osteomyelitis cohort (16.66 vs. 8.52 per 1000 person-years, Table 2). After controlling age, gender and four co-morbidities, the risk remained significantly higher in the COM cohort than the control group (adjusted hazard ratio [aHR] =1.65, 95% confidence interval [CI] =1.54-1.78, p<0.001, Model 2 in Table 2). Regardless of female or male, the risks of CHD in the COM group were significantly increased when compared with the control group (both p<0.001, Table 2). The incidence of CHD rose from 1.19 to 50.4 and 0.22 to 32.20 per 1000 person-years with increasing age in osteomyelitis and non-osteomyelitis cohort, respectively. It is worth to be highlighted that in age-stratification analysis, the highest risk was in the youngest age group and the hazard ratio declined from 3.42 to 1.39 with advancing age. The Kaplan-Meier analysis reveals that during a follow-up of ten years, the CHD-free survival rate in patients with osteomyelitis was significantly lower than the
control group (log-rank p<0.0001).
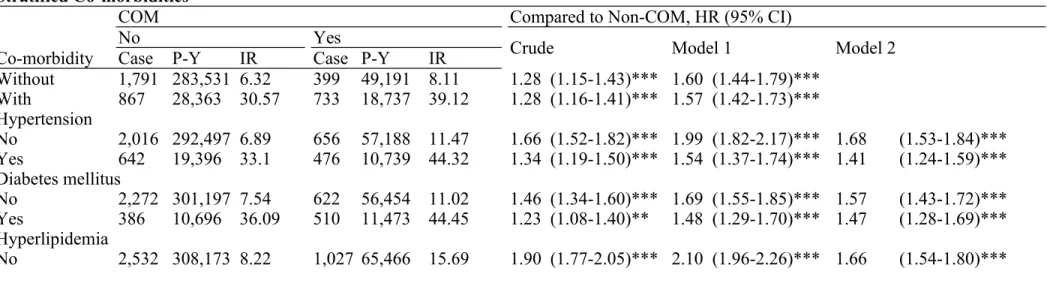
Table 3 shows the incidence and hazard ratios for CHD, stratified by co-morbidity. The incidence of CHD in study subjects with any co-morbidity was 5.16-fold higher than those without any co-morbidity (33.97 vs. 6.58 per 1000 person-years, data not shown). Among subjects with co-morbidity, the risk in patients with osteomyelitis was significantly elevated in comparison with controls (aHR =1.57, 95% CI=1.42-1.73, p<0.001, Model 1 in Table 3). In co-morbidity stratification analysis, COM cohort was at a significantly higher risk of developing CHD in the presence of hypertension (aHR =1.41, 95% CI =1.24-1.59, p<0.001), diabetes mellitus (aHR =1.47, 95% CI =1.28-1.69, p<0.001) or stroke (aHR =1.54, 95% CI =1.28-1.87, p<0.001), respectively (Model 2 in Table 3). Further, in the absence of any co-morbidity such as hypertension, diabetes, hyperlipidemia and stroke, the risk of CHD development was still statistically higher in the COM cohort (adjusted for age and gender, aHR =1.60, 95% CI =1.44-1.79, p<0.001, Model 1 in Table 3).
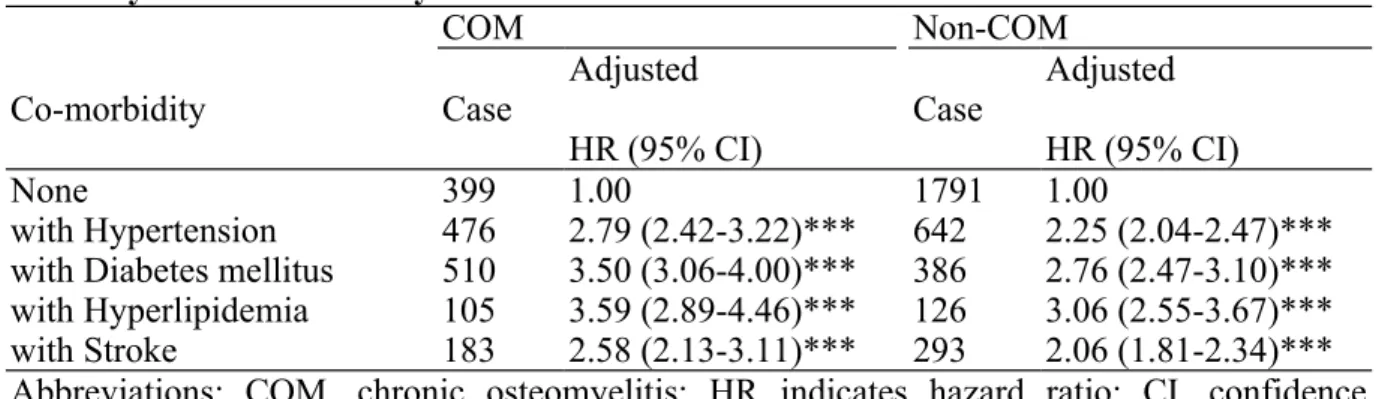
The join effects of COM combined with any of the four co-morbidities on CHD risk are presented in Table 4. In osteomyelitis population, patients with hyperlipidemia had the highest risk of CHD (aHR =3.59, 95% CI =2.89-4.46) compared to those without any co-morbidity, and followed by those with diabetes mellitus (aHR =3.50, 95% CI =2.89-4.46), hypertension (aHR =2.79, 95% CI =2.42-3.22) and stroke (aHR =2.58, 95% CI =2.13-3.11). The control group followed the same trend of effects. The association between the risk of CHD and severity of osteomyelitis was shown in Table 5. Compared to the control group, the risk was increased with severity (mild: aHR =0.95, 95% CI =0.85-1.06; moderate: aHR =1.88, 95% CI =1.68-2.11; severe: aHR =5.36, 95% CI =4.78-6.01, trend test p <0.0001).
It has been observed that approximately 10-50% of people developed CHD without classical risk factors.3,8,21-23 As a result, a number of other possible risk factors
have been proposed to explain the discrepancy including infection, inflammation, excessive oxidative stress and homocysteinemia.3,17 It has been postulated that these
novel risk factors cause atherosclerosis likely through triggering of endothelial injury (endothelial dysfunction), followed by intimal thickening, plaque formation and eventual disruption of vulnerable plaque.3,24,25 To the best of our knowledge, this is the
first report of the association between COM and CHD using a nationwide population-based dataset. In this study, we discovered a significantly higher risk (1.65-fold) of developing CHD among COM patients during a long-term follow-up period. According to our findings, COM patients of ≥35 years of age had a 4% increased risk for CHD with one year increase in age.
With the introduction of stratification analyses, the incidence and risk of CHD significantly increased with age in both COM and non-COM groups and among patients with any of four co-morbidities. These findings are generally in line with previous studies, attesting the reliability of our dataset. More importantly, our data also demonstrate that COM was associated with a higher risk of developing CHD, either with or without any of other traditional risk factors including hypertension, diabetes mellitus, hyperlipidemia and stroke. The combination of COM and these conventional risk factors further enhance the risk of CHD, especially in the group of COM and hyperlipidemia, which carries the highest risk for CHD development (aHR =3.59, 95% CI =2.89-4.46).
An interestingly inverse relationship between CHD risk and the advancement of age was observed in COM cases (from aHR =3.42, 95% CI =1.60-7.32 in age <35 to aHR =1.39, 95% CI =1.15-1.68 in age ≥80). Although, the statistics of strong association between COM and CHD risk in the lowest age group might be affected by
a small number of people enrolled, a consistent inverse relationship between CHD risk and increasing age still remains in other age groups. We believe that the relatively stronger association between COM and CHD in younger population may be attributable to less traditional risk factors in the these patients compared to the elderly, which in turn contributes to a greater association of COM with the CHD risk in younger patients. This finding carries an important implication for clinical practice in real world. Indeed, further studies are necessary to explore the underlying mechanisms linking COM and CHD.
For a long time, it has been thought that CHD mainly results from coronary atherosclerosis due to cholesterol deposition in coronary arteries.14 With time,
nevertheless, the concept of the pathophysiology of CHD has been linked to a chronic dynamic inflammatory process, associated with the interaction between immune response and lipid deposition.3 The role of infection in the trigger and progression of
atherosclerosis has attracted considerable attention in recent years since chronic infection may lead to a chronic inflammatory reaction in the body.17 A number of
infectious pathogens, including herpes simplex virus, cytomegalovirus, human immunodeficiency virus, chlamydia pneumonia and helicobacter pylori, have been found to be linked to atherosclerosis.3,26-28 With accumulating evidence, it has been
proposed that patients with chronic inflammatory disorders are at increased risk of CHD.24 COM patients, with a difficulty in eradication of bacterial pathogen hiding in
bone and surrounding soft tissue, may be embedded in a long-term inflammatory state that might increase the risk of CHD.29
This study has several advantages in terms of study design and statistics. First, it should be highlighted that this research was conducted on the basis of a nationwide population-based dataset consisting of more than 98% of the entire population in Taiwan. Insurance claims for expenditure of in-hospital management have been
strictly monitored and audited by NIH to avoid healthcare frauds. The stringent surveillance program confirms the reliability of the diagnosis.20 Second, the large
sample size, including 15,054 osteomyelitis patients and 60,216 age- and gender-matched controls, improves the validity of data and provides adequate power to find a reliable statistical significance. The demographic profile reveals gender difference and unique age distribution, which is consistent with the previous studies, all attesting the reliability of this dataset.30 Collectively, these strengths allow us to discover a linkage
between presence of COM and increased risk of CHD, as shown in this study. Study Limitations
However, there are still some limitations in the current study. First, we could not exclude the possibility of other coexistent vascular risk factors, such as altered immunity, reduced physical activities and medications for COM, which might also predispose patients to increased CHD risk. Thus, the CHD risks derived from the present study may carry superimposed factors on COM. Second, the personal health habits, such as smoking and alcohol consumption, were not available in the Taiwan National Health Insurance dataset for evaluating their association with the increased risk of CHD in COM patients. However, given that COM increased CHD risk in both genders and there is a very low smoking rate (<3%) among females in Taiwan, this indicates that smoking is unlikely to be a confounding variable for the significant increase in CHD risk in patients with COM. Finally, we have noted that there are relatively high prevalence rates of co-morbidities among patients with COM when compared with controls. However, our results show that even among those without CHD-related co-morbidities, presence of COM was still highly associated with CHD. The time- and severity-dependent effects of COM on CHD risk also demonstrate a consistent finding that COM was associated with a higher risk on the development of CHD.
Conclusion
This is the first study showing that COM is associated with the development of CHD, especially in the younger age group. It is believed that this implication has paved the way for further exploration in the pathogenesis, risk prediction and treatment of COM in relation to CHD prevention in the future.
Acknowledgements
The authors thank the National Health Research Institute in Taiwan for making insurance claims data available for medical analyses.
Funding Sources: This study was supported in part by the National Science Council, Taiwan (NSC 100-2314-B-039-042, NSC 101-2314-B-039-039, and NSC 102-2314-B-039-019), Taiwan Department of Health Clinical Trial and Research Center for Excellence (DOH102-TD-B-111-004), Taiwan Department of Health Cancer Research Center for Excellence (DOH102-TD-C-111-005), and China Medical University Hospital (DMR-101-006, DMR-102-007, and DMR-103-003). All of the aforementioned funding sources had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflict of interest: The authors have declared that no competing interests exist.
Contributor statement: K.-C.C., L.-C.H., and C.-H.T. designed research; C.-H.M., Y.-C.C., C.-Y.C., and L.-C.H. analyzed the data; L.-C.H. and K.-C.C. wrote the paper.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
References
1 Taylor DA, Robertson MJ. The basics of cell therapy to treat cardiovascular disease: one cell does not fit all. Rev Esp Cardiol 2009;62:1032-44.
2 Hsiao LC, Carr C, Chang KC, et al. Stem cell-based therapy for ischemic heart disease. Cell Transplant 2013;22:663-75.
3 Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll
Cardiol 1998;31:1217-25.
4 Fihn SD, Gardin JM, Abrams J, et al.
2012ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354-471.
5 Hsiao LC, Perbellini F, Gomes RS, et al. Murine cardiosphere-derived cells are impaired by age but not by cardiac dystrophic dysfunction. Stem Cells Dev2014 Jan 31. [Epub ahead of print].
6 McGovern PG, Pankow JS, Shahar E, et al. Recent trends in acute coronary heart disease--mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med 1996;334:884-90.
7 Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012;366:54-63.
8 Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290:898-904.
9 Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
10 D'Angelo A, Selhub J. Homocysteine and thrombotic disease. Blood 1997;90:1-11.
11 Dahlen GH. Lipoprotein(a), atherosclerosis and thrombosis. Prog Lipid Res 1991;30:189-94.
12 Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012;308:379-86.
13 Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788-95.
14 Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135-43.
15 Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl
J Med 2005;352:1685-95.
16 Charakida M, O'Neil F, Masi S, et al. Inflammatory disorders and atherosclerosis: new therapeutic approaches. Curr Pharm Des 2011;17:4111-20. 17 Shrihari TG. Potential correlation between periodontitis and coronary heart
disease--an overview. Gen Dent 2012;60:20-4.
18 Ikpeme IA, Ngim NE, Ikpeme AA. Diagnosis and treatment of pyogenic bone infections. Afr Health Sci 2010;10:82-8.
19 Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012;54:393-407.
20 Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 2003;22:77-88. 21 Enas EA, Yusuf S, Mehta JL. Prevalence of coronary artery disease in Asian
Indians. Am J Cardiol 1992;70:945-9.
22 Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 1999;100:e20-8.
23 Canto JG, Kiefe CI, Rogers WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA 2011;306:2120-7.
24 Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999;340:115-26.
25 Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart 2006;92:441-4.
26 Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med 1989;225:293-6.
27 Kuo CC, Grayston JT, Campbell LA, et al. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15-34 years old). Proc Natl Acad Sci U S A 1995;92:6911-4.
28 Nieto FJ, Adam E, Sorlie P, et al. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation 1996;94:922-7.
29 Eid AJ, Berbari EF. Osteomyelitis: review of pathophysiology, diagnostic modalities and therapeutic options. J Med Liban 2012;60:51-60.
30 Zuluaga AF, Galvis W, Saldarriaga JG, et al. Etiologic diagnosis of chronic osteomyelitis: a prospective study. Arch Intern Med 2006;166:95-100.
Table 1. Comparison of Demographics Between Chronic Osteomyelitis and Non- Osteomyelitis Groups
N=60,216 N=15,054 Variable n % n % Gender Women 20,228 33.6 5,057 33.6 Men 39,988 66.4 9,997 66.4 Age, years < 35 10,944 18.1 2,736 18.1 35-49 13,668 22.7 3,417 22.7 50-64 15,556 25.8 3,889 25.8 65-79 15,576 25.9 3,894 25.9 ≥ 80 4,472 7.43 1,118 7.43 Co-morbidity Hypertension 5,125 8.51 3,374 22.4 Diabetes mellitus 2,830 4.70 3,354 22.3 Hyperlipidemia 924 1.53 734 4.88 Stroke 2,365 3.93 1,367 9.08
Table 2. Incidence and Hazard Ratio for Coronary Heart Disease Between Chronic Osteomyelitis and Non-Osteomyelitis Groups by Stratified Demographics
COM Compared to Non-COM, HR (95% CI)
No Yes
Crude Model 1 Model 2
Variable Case P-Y IR Case P-Y IR
Overall 2,658 311,893 8.52 1,132 67,927 16.66 1.95 (1.82-2.09)*** 2.15 (2.00-2.30)*** 1.65 (1.54-1.78)*** Gender Female 959 103,220 9.29 413 22,506 18.35 1.97 (1.75-2.21)*** 2.11 (1.88-2.37)*** 1.60 (1.42-1.81)*** Male 1,699 208,673 8.14 719 45,422 15.83 1.94 (1.78-2.12)*** 2.17 (1.90-2.37)*** 1.68 (1.53-1.84)*** Age, years < 35 14 64,133 0.22 19 16,033 1.19 5.44 (2.73-10.9)*** 5.44 (2.73-10.8)*** 3.42 (1.60-7.32)** 35-49 177 76,295 2.32 135 17,239 7.83 3.40 (2.72-4.26)*** 3.42 (2.73-4.28)*** 2.02 (1.56-2.62)*** 50-64 556 81,716 6.80 331 17,451 18.97 2.80 (2.44-3.20)*** 2.81 (2.45-3.22)*** 1.66 (1.42-1.93)*** 65-79 1,405 74,035 18.98 507 14,428 35.14 1.86 (1.68-2.06)*** 1.87 (1.69-2.07)*** 1.47 (1.32-1.63)*** ≥ 80 506 15,715 32.20 140 2,777 50.42 1.56 (1.29-1.88)*** 1.56 (1.29-1.88)*** 1.39 (1.15-1.68)*** Abbreviations: COM, chronic osteomyelitis; P-Y, person-years; IR, incidence rate; HR, hazard ratio; CI, confidence interval.
Incidence rate, per 1,000 person-years. Crude, crude hazard ratio without adjustment Model 1, mutually adjusted for age and gender.
* p<0.05, ** p<0.01, *** p<0.001
Table 3. Incidence and Hazard Ratio for Coronary Heart Disease Between Chronic Osteomyelitis and Non-Osteomyelitis Groups by Stratified Co-morbidities
COM Compared to Non-COM, HR (95% CI)
No Yes
Crude Model 1 Model 2
Co-morbidity Case P-Y IR Case P-Y IR
Without 1,791 283,531 6.32 399 49,191 8.11 1.28 (1.15-1.43)*** 1.60 (1.44-1.79)*** With 867 28,363 30.57 733 18,737 39.12 1.28 (1.16-1.41)*** 1.57 (1.42-1.73)*** Hypertension No 2,016 292,497 6.89 656 57,188 11.47 1.66 (1.52-1.82)*** 1.99 (1.82-2.17)*** 1.68 (1.53-1.84)*** Yes 642 19,396 33.1 476 10,739 44.32 1.34 (1.19-1.50)*** 1.54 (1.37-1.74)*** 1.41 (1.24-1.59)*** Diabetes mellitus No 2,272 301,197 7.54 622 56,454 11.02 1.46 (1.34-1.60)*** 1.69 (1.55-1.85)*** 1.57 (1.43-1.72)*** Yes 386 10,696 36.09 510 11,473 44.45 1.23 (1.08-1.40)** 1.48 (1.29-1.70)*** 1.47 (1.28-1.69)*** Hyperlipidemia No 2,532 308,173 8.22 1,027 65,466 15.69 1.90 (1.77-2.05)*** 2.10 (1.96-2.26)*** 1.66 (1.54-1.80)***
Yes 126 3,721 33.87 105 2,462 42.65 1.25 (0.97-1.62) 1.55 (1.19-2.03)** 1.29 (0.98-1.70) Stroke
No 2,365 303,165 7.80 949 64,154 14.79 1.89 (1.76-2.04)*** 2.13 (1.98-2.30)*** 1.64 (1.52-1.78)*** Yes 293 8,728 33.57 183 3,773 48.50 1.45 (1.20-1.74)*** 1.68 (1.39-2.02)*** 1.54 (1.28-1.87)*** Abbreviations: COM, chronic osteomyelitis; P-Y indicates person-years; IR, incidence rate; HR, hazard ratio; CI, confidence interval. Incidence rate, per 1,000 person-years.
Crude, crude hazard ratio without adjustment. Model 1, mutually adjusted for age and gender.
Model 2, mutually adjusted for age, gender, hypertension, diabetes, hyperlipidemia and stroke in Cox proportional hazards regression. * p<0.05, ** p<0.01, *** p<0.001.
Table 4. Joint Effects of Associated Co-morbidities on Chronic Osteomyelitis and Non-Osteomyelitis for Coronary Heart Disease
COM Non-COM
Co-morbidity Case Adjusted
HR (95% CI) Case
Adjusted HR (95% CI)
None 399 1.00 1791 1.00
with Hypertension 476 2.79 (2.42-3.22)*** 642 2.25 (2.04-2.47)*** with Diabetes mellitus 510 3.50 (3.06-4.00)*** 386 2.76 (2.47-3.10)*** with Hyperlipidemia 105 3.59 (2.89-4.46)*** 126 3.06 (2.55-3.67)*** with Stroke 183 2.58 (2.13-3.11)*** 293 2.06 (1.81-2.34)*** Abbreviations: COM, chronic osteomyelitis; HR indicates hazard ratio; CI, confidence interval.
Adjusted for age and gender. *** p<0.001.
p>0.05 in all interaction.
Table 5. Incidence and Hazard Ratio for Coronary Heart Disease Stratified by the Severity of Chronic Osteomyelitis
COM severity Event P-Y IR Adjusted HR (95% CI)
Compared group 2,658 311,893 8.52 1.00
Mild (T1) 376 44,309 8.49 0.95 (0.85-1.06)
Moderate (T2) 363 18,088 20.07 1.88 (1.68-2.11)***
Severe (T3) 393 5,530 71.06 5.36 (4.78-6.01)***
p for trend <0.0001
hazard ratio; CI, confidence interval; T1, first tertile; T2, second tertile; T3, third tertile. Incidence rate, per 1,000 person-years.
COM severity = (total length of hospital stay due to chronic osteomyelitis during the follow-up duration) ÷ (length of follow-up duration).
Adjusted HR: adjusted for age, gender, hypertension, diabetes, hyperlipidemia and stroke in Cox proportional hazards regression.