www.elsevier.com / locate / chroma
A
nalysis of chitin oligosaccharides by capillary electrophoresis with
laser-induced fluorescence
*
Chin-Yu Wang, You-Zung Hsieh
Department of Applied Chemistry, National Chiao Tung University, 1011 Ta-Hsueh Road, Hsinchu 300, Taiwan
Abstract
A method, using capillary electrophoresis (CE) with laser-induced fluorescence (LIF) detection for analyzing chitin oligosaccharides is described. Chitin oligosaccharides were derivatized with 9-aminopyrene-1,4,6-trisulfonate (APTS) via reductive amination at 37 8C for 16 h (optimized conditions). The APTS–chitin oligosaccharides were analyzed using either an acidic citric acid–phosphate buffer or an alkaline borate buffer. The effects of buffer types, buffer pH values, and buffer concentrations on the separation were examined. The analytes were successfully separated by using a pH 4.6 citric acid–phosphate within 19 min. The APTS-derivatized chitin monosaccharide (D-glucosamine) migrated first. The analytes
were also completely separated by using a pH 9.0 borate buffer within 24 min. Moreover, the specificity of enzyme digestion on chitin polysaccharides using the optimized APTS labeling procedure and the CE–LIF method was demonstrated. 2002 Elsevier Science B.V. All rights reserved.
Keywords: Derivatization, electrophoresis; Oligosaccharides; Chitin; Aminopyrenetrisulfonate
1
. Introduction In order to obtain bioactive oligosaccharides,
chemi-cal and biocatalytichemi-cal procedures are applied to Chitin is the N-acetylated product of chitosan and depolymerize the polysaccharides to oligosaccha-is found in the shells of crabs and shrimps, and as rides. However, an enhanced efficiency and spe-cell wall components of most fungi, molds and cificity can be obtained by the use of an enzymatic yeasts [1,2]. These compounds have extensive appli- degradation procedure. Consequently, interest in cations, in the biomedical field, in personal care these bioactive compounds has increased considera-products, biotechnology, and in food products [3]. bly in recent years. Thus, the determination of chitin Oligomers with a degree of polymerization (DP) of oligomers becomes important in the complete about 6 are potentially useful components of medical characterization of chitin polysaccharides and other materials [4]. Chitin–oligosaccharides and related glycoconjugates.
derivatives, which are amino polysaccharides, have Carbohydrates generally do not contain distinctive properties including a variety of biologi- chromophoric or fluorophoric groups and, as a result, cal activities and the fact that they are biodegradable. the determination of these compounds is a challeng-ing topic. Several analytical techniques for the separation of different carbohydrates have been reported [5–14]. However, capillary electrophoresis *Corresponding author. Tel.: 573-1785; fax:
1886-3-(CE) [15–27] has attracted a considerable amount of
572-3764.
E-mail address: yzhsieh@mail.nctu.edu.tw(Y.-Z. Hsieh). attention recently because of its high sensitivity,
0021-9673 / 02 / $ – see front matter 2002 Elsevier Science B.V. All rights reserved. P I I : S 0 0 2 1 - 9 6 7 3 ( 0 2 ) 0 1 2 6 0 - 8
rapid analysis time, and high resolution. CE in 2 . Experimental conjunction with indirect detection is a universal
method and can be used for carbohydrates analysis 2 .1. Apparatus [18,19]. However, the disadvantages of limited
back-ground electrolyte, unsatisfactory sensitivity, and The instrument used in these studies was a Beck-resolution of complex carbohydrates has limited its man P/ACE MDQ capillary electrophoresis system development in this area. Generally, in order to (Beckman Instruments, Fullerton, CA, USA) obtain more sensitive detection and to enlarge the equipped with a laser-induced fluorescence detector differences between adjoining carbohydrate mole- using an 8-mW 488-nm argon-ion laser as the cules, a chromophoric or fluorophoric reagent is excitation source. The fluorescence emission was introduced into carbohydrates by derivatization. The collected with a bandpass filter of 520620 nm. A major derivatization mechanisms are based on reduc- personal computer using the P/ACE MDQ software tive amination with various labeling reagents such as program was used to control the instrument. Data 4-aminobenzonitrile, 6-aminoquinoline, 8-amino- analyses were performed using the P/ACE MDQ naphthtalene-1,3,6-trisulfonic acid (ANTS), and 3-(- software. A 50-mm I.D. fused-silica capillary tube 4-carboxybenzoyl)-2-quinolinecarboxaldehyde (CB- (Polymicro Technologies, Phoenix, AZ, USA) was QCA) [20–27]. Such derivatives can then be de- used at a total length of 60.2 cm (50 cm to the tected by UV and / or fluorescence methods. detector). The capillary column was assembled in a Due to the inherently high sensitivity of laser- cartridge format. The temperature of the capillary induced fluorescence (LIF), it is currently in heavy tube during electrophoresis was maintained at 25 8C use for the detection of labeled carbohydrates after by means of an MDQ thermostating system. The high-performance CE separation. In addition, LIF is electrophoretic separation was performed with an characterized by its good specificity and a large applied voltage of 25 kV at either positive or linear dynamic range. A recent development in the negative polarity. Samples were pressure injected at fluorescent labeling of carbohydrates was the intro- 3.5 kPa.
duction of 9-aminopyrene-1,4,6-trisulfonate (APTS)
for the CE–LIF of mono- and oligosaccharides [28– 2 .2. Chemicals 32]. Due to its extensive aromaticity and the larger
number of fused rings, APTS–carbohydrate deriva- APTS, Na HPO , boric acid and glacial acetic2 4
tives were observed in a substantially higher molar acid were purchased from Fluka (Buchs, Switzer-absorptivity and quantum efficiency than most of the land). Borax, N-acetylglucosamine and sodium commonly used fluorophore carbohydrate deriva- cyanoborohydride (NaBH CN) were obtained from3
tives. In addition, the presence of negatively charged Sigma (St. Louis, MO, USA). The chitin oligo-functional groups seems to enhance the separation of saccharides, including di-N-acetylchitobiose,
tri-N-mono- and oligosaccharides. acetylchitotriose, tetra-N-acetylchitotetraose,
penta-In this study, a CE–LIF method was developed for N-acetylchitopentaose, and
hexa-N-acetylchitohexa-analyzing chitin–oligosaccharides (1–6 DP) deriva- ose, were purchased from Seikagaku (Tokyo, Japan). tized with APTS via reductive amination. Results Citric acid was obtained from Merck (Darmstadt, from three different labeling methods are compared. Germany). All other chemicals were of analytical The electrophoretic separation was carried out under grade. Water was purified in a Milli-Q water system positive and negative high voltages. The effects of (Millipore, Bedford, MA, USA) and filtered through buffer types, buffer pH values, and buffer concen- a 0.22-mm filter.
trations on the quality of the separation are
dis-cussed. The optimized capillary zone electrophoresis 2 .3. Derivatization of chitin oligosaccharides with (CZE)–LIF methods were employed to analyze the APTS
specificity of hydrolytic enzymes that act on chitin–
stored at 220 8C prior to use. The procedure for the reductive amination of chitin oligosaccharides with APTS followed previously described protocol [28– 32]. Briefly, 10 ml of a 10-mg / ml oligosaccharide solution in a 500-ml Eppendorf tube was mixed with 2 ml of 0.2 M APTS in 15% glacial acetic acid and 10 ml of freshly prepared 1 M aqueous sodium cyanoborohydride. Reaction mixtures were incubated at 37, 55, and 75 8C for 16, 2, or 1 h, respectively. The APTS-derivatized oligosaccharide samples were suitably diluted with 100 mM, pH 10.2 borate buffer and stored under 220 8C without any pretreatment procedure prior to the CE separation. The two enzymatically digested chitin oligosaccharides sam-ples were gifts from Professor Y.-K. Lee in our department. The chitin substrate concentration in the enzymatic digestion incubation was 5% (w / v). 2
.4. Capillary electrophoresis procedures
The procedure used to condition a new capillary involved treatment with 1.0 M NaOH, water, 0.1 M NaOH, and water for 30 min sequentially. For the electrophoretic separation using borate buffer at positive polarity, the capillary was washed between
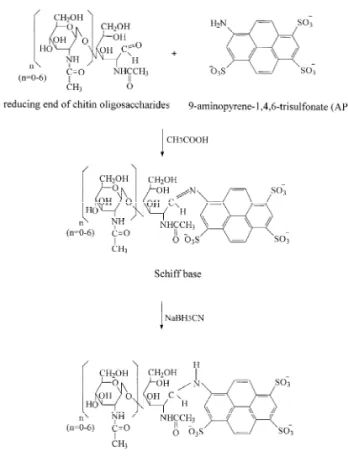
Fig. 1. Reaction scheme for the derivatization of chitin
oligo-runs with 0.1 N NaOH and then water (3 min of high saccharides with APTS followed by reductive amination. pressure rinsing at 20 p.s.i.; 1 p.s.i.56894.76 Pa),
followed by reconditioning with the running buffer,
typically for 3 min. The electrophoretic separation stable secondary amine by NaBH CN. As shown in3
using acidic citric acid–phosphate buffer was under Fig. 1, even in the low pH buffer, the APTS–chitin negative high voltage. The capillary was washed oligosaccharide molecules contain negative charges, between runs only with water for 3 min in this case. which are contributed to the three-sulfonate groups
on APTS.
Figs. 2 and 3 illustrate the effect of buffer pH on
3
. Results and discussion the electrophoretic separation of the APTS–chitin
oligosaccharides. The ionic strength of each buffer The introduction of a charged fluorophore into a was adjusted so as to be approximately equal. In carbohydrate molecule increases detection sensitivity acidic citric acid–phosphate buffer solutions, the and also supplies an additional charge to a neutral electrophoretic mobility of the APTS–chitin oligo-carbohydrate molecule, thereby permitting its elec- saccharides toward the outlet (anode) is provided by trophoretic separation by CE. Fig. 1 shows the negatively charged sulfonate groups under the nega-reaction scheme for the derivatization of chitin tive applied voltage. These electrophoretic mobilities oligosaccharides with APTS by reductive amination. are significantly higher than the electroosmotic flow The reaction mechanism involves the reaction of the (EOF) toward the cathode. Consequently, the migra-primary amino group of APTS with the reducing end tion sequence of analytes to the anode is based on of the chitin oligosaccharides to form Schiff base. their apparent electrophoretic mobility. The APTS– The intermediate is subsequently reduced to a more chitin monosaccharide with the highest
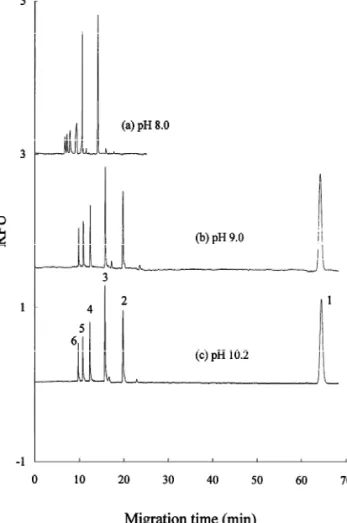
charge-to-Fig. 2. Separation of APTS-derivatized chitin oligosaccharides
Fig. 3. Separation of APTS-derivatized chitin oligosaccharides using (a) pH 2.6, (b) pH 3.6, and (c) pH 4.6 citric acid–phosphate
using (a) pH 8.0 phosphate buffer, (b) pH 9.0 borate buffer, and buffers. Conditions: light source: 488 nm argon-ion laser, 8.0 mW;
(c) pH 10.2 borate buffer. Conditions: applied voltage: 25 kV; emission bandfilter: 520620 nm; buffers: citric acid–phosphate;
outlet: cathode. Other conditions are the same as those in Fig. 2. sample injection: 5 s; applied voltage: 225 kV; outlet: anode;
capillary temperature: 25 8C.
Fig. 3. The EOF toward the outlet (cathode) is significantly faster than the electrophoretic mobilities mass ratio migrated first. The shortest separation of the APTS–chitin oligosaccharides, even when
2
time was achieved using the pH 3.6 buffer. The complexed with B(OH)4 toward the anode. There-results indicate that the degree of dissociation of the fore, all analytes migrated toward the cathode. The three sulfonate groups of APTS is repressed in the migration sequence of APTS–chitin oligosaccharides pH 2.6 buffer. The APTS–chitin monosaccharide was reversed compared with the data shown in Fig. peak is split into two peaks when the pH 3.6 buffer 2. Although, the fastest separation was achieved is used, indicating that the derivative was unstable in using a pH 8.0 phosphate buffer, its separation this buffer. Consequently, of the acidic buffers efficiency was not adequate. This is probably due to examined, a citric acid–phosphate buffer at pH 4.6 the absence of complexing interactions with
2
was selected for the analysis of the APTS–chitin B(OH) . All the APTS–chitin oligosaccharides were4
oligosaccharides. effectively separated in both pH 9.0 and 10.2 buffers
The larger APTS–chitin oligosaccharides migrated with similar electrophoretic velocities. However, the faster than the smaller oligosaccharides in alkaline entire separation time was extended to 66 min with phosphate or borate buffer solutions as shown in these high ionic strength buffers. Since the
sepa-ration was adequate using the pH 9.0 borate buffer, it was selected for further improvement with respect to reducing the separation time.
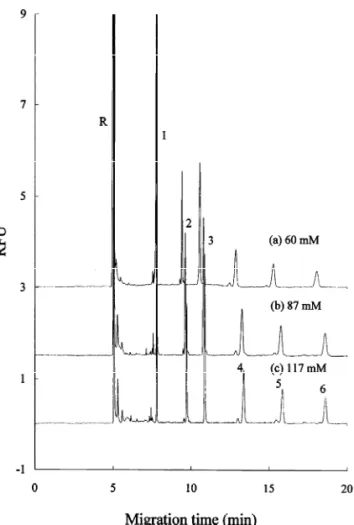
Figs. 4 and 5 display the effects of the buffer concentrations on the CE separation of APTS–chitin oligosaccharides. The effect of buffer concentration on the apparent electrophoretic mobilities of these derivatives was not obvious in acidic citric acid– phosphate (pH 4.6) buffer as shown in Fig. 4. The analytes were completely separated within 19 min. It appears that the EOF was approximately the same in these buffers. Nevertheless, the separation time was significantly affected in the pH 9.0 borate buffer as shown in Fig. 5. The apparent electrophoretic mo-bilities of the APTS–chitin oligosaccharides were decreased with increasing concentration of borate
Fig. 5. Effect of borate buffer concentration on the CE separation of APTS-derivatized chitin oligosaccharides. Conditions: buffer: pH 9.0 boric acid–borax buffer with different concentrations (a) 80 mM, (b) 120 mM, and (c) 160 mM. Other conditions are the same as those in Fig. 3.
buffer. This is likely due to a decrease in EOF and / or an increase in the extent of anionic borate complexation. All the analyte peaks were adequately resolved using borate buffers. While considering buffer capacity and separation time, 87 mM citric acid–phosphate buffer and 120 mM borate buffer were selected for the subsequent analysis of en-zymatically degraded of chitin polysaccharides.
The efficiency of the APTS derivatization reaction with chitin was evaluated for different reaction temperatures and reaction times using the optimized separation conditions. Table 1 summarizes the peak
Fig. 4. Effect of citric acid–phosphate buffer concentration on the area ratio of APTS–chitin oligosaccharides based on CE separation of APTS-derivatized chitin oligosaccharides.
Con-different APTS–carbohydrate derivatization methods
ditions: buffer: pH 4.6 citric acid–phosphate buffer with different
[28–30]. The experimental results indicate that the
concentrations (a) 60 mM, (b) 87 mM, and (c) 117 mM. Other
Table 1
The peak area ratio of APTS-derivatized chitin oligosaccharides based on three derivatization methods Chitin Peak area ratio
oligosaccharides 37 8C (16 h) 55 8C (2 h) 75 8C (1 h) A B A B A B Monomer 2.71 (0.11) 1.98 (3.27) 1.98 (0.67) 1.00 (1.12) 1.00 (0.71) 1.00 (1.31) Dimer 2.64 (1.26) 1.96 (2.50) 1.96 (0.32) 0.98 (0.24) 1.00 (0.89) 1.00 (1.21) Trimer 2.79 (0.10) 1.78 (1.89) 1.78 (0.50) 1.21 (0.11) 1.00 (0.09) 1.00 (2.28) Tetramer 2.92 (0.53) 1.74 (2.76) 1.74 (0.51) 1.22 (0.65) 1.00 (0.81) 1.00 (2.64) Pentamer 2.91 (0.66) 1.84 (2.40) 1.85 (0.20) 1.17 (0.70) 1.00 (0.25) 1.00 (1.74) Hexamer 2.63 (0.39) 1.87 (2.62) 1.87 (2.01) 1.14 (0.92) 1.00 (0.64) 1.00 (1.23) Peak area ratio5peak area (37, 55, or 75 8C) / peak area (75 8C) and RSD (%) of triplet derivatizations. A5CE using acidic pH 4.6, 87 mM citric acid–phosphate buffer; B5CE using alkaline pH 9.0, 120 mM boric acid–borax buffer.
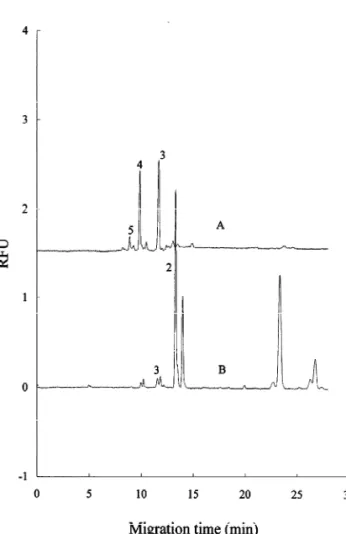
of 37 8C for 16 h. The reaction times were shortened B was used. These analytes were confirmed by the at elevated temperature of 55 or 75 8C. However, the use of spiked standards in the samples. The APTS-reaction efficiencies were decreased. The RSDs for derivatized chitin oligosaccharides were adequately reaction efficiency at 37 8C were less than 3.27%. separated by the CZE–LIF method. Fig. 7 displays Table 2 lists the average migration times, RSDs electropherograms of APTS-derivatized chitin oligo-for the migration time and detection limits of six saccharides from an enzymatic digestion using a APTS–chitin oligosaccharides under the optimized 120-mM pH 9.0 borate buffer. These APTS-deriva-separation conditions. The RSDs for the migration tized chitin oligosaccharides were also adequately time were less than 0.96%. The detection limits of separated. Peak identification was further verified by these analytes were in the range of 5.1–16 amol for a comparison of the results from two different the acidic citric acid–phosphate buffer and from 6.3 separations.
to 17 amol for the alkaline borate buffer. In summary, a CE–LIF method was developed for Fig. 6 shows an electropherogram of APTS-de- the analysis of chitin–oligosaccharides, after deri-rivatized chitin oligosaccharides from an enzymatic vatization with APTS. The optimized derivatization digestion using 87 mM pH 4.6 citric acid–phosphate conditions involved heating the reaction mixture at buffer. Two different enzymes were employed to 37 8C for 16 h. The APTS-derivatized chitin–oligo-degrade the chitin polysaccharides to oligosaccha- saccharides were adequately separated in both pH rides. According to this figure, the major chitin 4.6 citric acid–phosphate buffer and pH 9.0 borate oligomer products of enzyme A digestion were dimer buffer. The APTS–chitin monosaccharide with the and traces of trimer oligosaccharides. Chitin trimer, highest charge-to-mass ratio migrated first in the tetramer and pentamer were produced when enzyme acidic buffer and the migration order of analytes was
Table 2
Average migration times, reproducibilities, and the detection limits of six APTS-derivatized chitin oligosaccharides
Chitin Migration time (min) RSD (%) Detection limit (amol)
oligosaccharide A B A B A B Monomer 7.74 23.10 0.82 0.86 8.4 17 Dimer 9.65 13.19 0.39 0.54 12 14 Trimer 10.81 11.49 0.39 0.51 5.1 6.3 Tetramer 13.25 9.76 0.73 0.44 13 8.2 Pentamer 15.74 8.84 0.84 0.36 14 9.2 Hexamer 18.57 8.19 0.96 0.35 16 9.4
Fig. 7. Electropherogram of APTS-derivatized chitin oligosac-Fig. 6. Electropherogram of APTS-derivatized chitin oligosac- charides from two different enzymatic digestions. Conditions: charides from two different enzymatic digestions. Conditions: buffers: 120 mM boric acid–borax buffer, pH 9.0. Other con-buffers: 87 mM citric acid–phosphate buffer, pH 4.6. Other ditions are the same as those in Fig. 3.
conditions are the same as those in Fig. 2.
R
eferences reversed in the alkaline buffer. The detection limits
[1] S. Hirano, S. Tokura, in: Proceedings of the 2nd
Internation-of APTS-derivatized chitin–oligosaccharides (1–6
al Conference on Chitin and Chitosan, Sapporo, Japan, 1982.
DP) ranged from 5.1 to 17 amol.
Chitin–polysac-˚
[2] G. Skjak-Bræk, T. Anthonsen, P. Sandford, Chitin and
charides, depolymerized by chitinase digestion, were
Chitosan, Elsevier, London, 1988.
adequately analyzed using the optimized APTS [3] D. Somashekarn, R. Joseph, Chitosanases—properties and labeling procedure and the CZE–LIF method. En- applications: A review, Bioresour. Technol. 55 (1996) 35.
[4] K. Suzuki, T. Mikami, Y. Okawa, A. Tokoro, S. Suzuki, M.
zyme specificity in the digestion of
chitin–polysac-Suzuki, Carbohydr. Res. 151 (1986) 403.
charides was also investigated.
[5] M. Lafosse, B. Herbreteau, L. Morin-Allory, J. Chromatogr. A 720 (1996) 61.
[6] S. Churms, J. Chromatogr. 500 (1990) 555. [7] S. Honda, Anal. Biochem. 140 (1984) 1. [8] Y.C. Lee, Anal. Biochem. 189 (1990) 151. A
cknowledgements
[9] K. Kakehi, S. Suzuki, S. Honda, Y.C. Lee, Anal. Biochem. 199 (1991) 256.
This research was supported by Grant NSC 90- [10] A. Karcher, H.A. Melouk, Z.E. Rassi, Anal. Biochem. 267 2113-M-009-028 from the National Science Council (1999) 92.
[12] H. Suzuki, O. Muller, A. Guttman, B.L. Karger, Anal. Chem. [22] P. Camilleri, G.B. Harland, G. Okafo, Anal. Biochem. 230
69 (1997) 4554. (1995) 115.
[13] D.J. Harvey, Mass Spectrom. Rev. 18 (1999) 349. [23] P. Jackson, Biochem. J. 270 (1990) 705.
[14] T.R.I. Catadi, C. Campa, G.E. De Benedetto, Fresenius J. [24] C. Chiesa, C. Horvath, J. Chromatogr. 645 (1993) 337. Anal. Chem. 368 (2000) 739. [25] J. Liu, V. Dolnik, Y.-Z. Hsieh, M. Novotny, Anal. Chem. 64 [15] P.D. Grossman, J.C. Colburn (Eds.), Capillary Electropho- (1992) 1328.
resis. Theory and Practice, Academic Press, San Diego, CA, [26] C. Chiesa, R.A. O’Neill, Electrophoresis 15 (1994) 1132. 1992. [27] M. Stefansson, M. Novotny, Anal. Chem. 66 (1994) 1134.
´ ´
[16] F. Foret, L. Krivankova, P. Bocek, Capillary Zone Electro- [28] R.A. Evangelista, M.S. Liu, F.-T.A. Chen, Anal. Chem. 67 phoresis, VCH, Weinheim, 1993. (1995) 2239.
[17] H.-Y. Huang, K.-L. Kuo, Y.-Z. Hsieh, J. Chromatogr. A 771 [29] F.-T.A. Chen, R.A. Evangelista, Anal. Biochem. 230 (1995)
(1997) 267. 273.
[18] H. Schwaiger, P.J. Oefner, C. Huber, E. Grill, G.K. Bonn, [30] A. Guttman, R.A. Evangelista, F.-T.A. Chen, N. Cooke, Electrophoresis 15 (1994) 941. Anal. Biochem. 233 (1996) 234.
[19] J.B.L. Damm, G.T. Overklift, J. Chromatogr. A 678 (1994) [31] A. Guttman, J. Chromatogr. A 763 (1997) 271.
151. [32] M.G. O’Shea, M.S. Samuel, C.M. Konik, M.K. Morell,
[20] A. Rydlund, O. Dahlman, Carbohydr. Res. 300 (1997) 95. Carbohydr. Res. 307 (1998) 1. [21] H. Birrell, J. Charlwood, L. Lynch, S. North, P. Camilleri,