Tuberculosis Patients - A Prospective Observational
Study
Jia-Yih Feng1,2, Wei-Juin Su1,3*, Yu-Chi Chiu1, Shiang-Fen Huang1, Yung-Yang Lin4,5, Ruay-Ming Huang6, Ching-Hsiung Lin7, Jhi-Jhu Hwang8, Jen-Jyh Lee9, Ming-Chih Yu10, Kwok-Woon Yu11, Yu-Chin Lee1,3
1 Department of Chest Medicine, Taipei Veterans General Hospital, Taipei, Taiwan, Republic of China, 2 Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan, Republic of China,3 School of Medicine, National Yang-Ming University, Taipei, Taiwan, Republic of China, 4 Institute of Clinical Medicine and Institute of Brain Science, National Yang-Ming University, Taipei, Taiwan, Republic of China,5 Laboratory of Neurophysiology and Department of Neurology, Taipei Veterans General Hospital, Taipei, Taiwan, Republic of China,6 Hua-Lien Hospital, Department of Health, Executive Yuan, Hua-Lien County, Taiwan, Republic of China, 7 Division of Chest Medicine, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan, Republic of China,8 Division of Pulmonary and Critical Care, Department of Internal Medicine, Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan, Republic of China,9 Department of Internal Medicine, Buddhist Tzu Chi General Hospital, Tzu Chi University, Hualien, Taiwan, Republic of China,10 Department of Internal Medicine, Wan Fang Hospital, Taipei, Taiwan, Republic of China, 11 Division of Clinical Microbiology, Department of Pathology and Laboratory Medicine, Taipei Veterans General Hospital, Taipei, Taiwan, Republic of China
Abstract
Background:Despite effective anti-TB treatments, tuberculosis remains a serious threat to public health and is associated with high mortality. Old age and multiple co-morbidities are known risk factors for death. The association of clinical presentations with mortality in pulmonary tuberculosis patients remains an issue of controversy.
Methods:This prospective observational study enrolled newly diagnosed, culture-proven pulmonary tuberculosis patients from five medical centers and one regional hospital, which were referral hospitals of TB patients. Radiographic findings and clinical symptoms were determined at the time of diagnosis. Patients who died for any reason during the course of anti-TB treatment were defined as mortality cases and death that occurred within 30 days of initiating treatment was defined as early mortality. Clinical factors associated with overall mortality and early mortality were investigated.
Results:A total of 992 patients were enrolled and 195 (19.7%) died. Nearly one-third (62/195, 31.8%) of the deaths occurred before or within 30 days of treatment initiation. Older age (RR = 1.04, 95%CI: 1.03–1.05), malignancy (RR = 2.42, 95%CI: 1.77– 3.31), renal insufficiency (RR = 1.77, 95%CI: 1.12–2.80), presence of chronic cough (RR = 0.63, 95%CI: 0.47–0.84), fever (RR = 1.45, 95%CI: 1.09–1.94), and anorexia (RR = 1.49, 95%CI: 1.07–2.06) were independently associated with overall mortality. Kaplan-Meier survival analysis demonstrated significantly higher mortality in patients present with fever (p,0.001), anorexia (p = 0.005), and without chronic cough (p,0.001). Among patients of mortality, those with respiratory symptoms of chronic cough (RR = 0.56, 95%CI: 0.33–0.98) and dyspnea (HR = 0.51, 95%CI: 0.27–0.98) were less likely to experience early mortality. The radiological features were comparable between survivors and non-survivors.
Conclusions:In addition to demographic characteristics, clinical presentations including the presence of fever, anorexia, and the absence of chronic cough, were also independent predictors for on-treatment mortality in pulmonary tuberculosis patients.
Citation: Feng J-Y, Su W-J, Chiu Y-C, Huang S-F, Lin Y-Y, et al. (2011) Initial Presentations Predict Mortality in Pulmonary Tuberculosis Patients - A Prospective Observational Study. PLoS ONE 6(9): e23715. doi:10.1371/journal.pone.0023715
Editor: Adithya Cattamanchi, San Francisco General Hospital, University of California San Francisco, United States of America Received February 18, 2011; Accepted July 23, 2011; Published September 13, 2011
Copyright: ß 2011 Feng et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work has been funded by the Institute for Biotechnology and Medicine Industry, Taiwan, and the Taipei Veterans General Hospital (V97C1-064, V98C1-039, V98A-086, V99C1-181, V99A-023 and V100A-002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist. * E-mail: wjsu@vghtpe.gov.tw
Introduction
Pulmonary tuberculosis (PTB) is an infectious disease with airborne transmission that is associated with high morbidity and mortality worldwide. Despite the advances in anti-tuberculosis (anti-TB) medications and the use of a Direct Observation Therapy/Short Course (DOTS) strategy, the tuberculosis mortal-ity rates remain high in many areas, including Taiwan [1–4].
Globally, it has been estimated that tuberculosis causes 1.7 million deaths each year, or about three deaths each minute [1].
Investigating the clinical factors associated with mortality is of paramount importance for the management of PTB patients. The early identification of high risk patients can enable clinicians to provide more aggressive and intensive treatments. Age and underlying co-morbidities have been frequently reported as independent predictors of mortality in previous studies [4–6]. By
comparison, the extensiveness of radiological presentations and the bacilli loads in sputum have been less frequently mentioned as independent risk factors [7]. Studies that evaluated the impacts of drug susceptibility profiles on mortality also reported controversial results [3,6]. Most of these predictors of mortality were non-modifiable factors.
Interestingly, the predictive values of the initial presentations of PTB on mortality have rarely been evaluated and have shown inconsistent results [3,8–10]. Although the symptoms/signs of PTB are usually non-specific, typical presentations, such as chronic cough, afternoon fever, and unexplained body weight loss, remain important hints to remind clinical physicians to consider a diagnosis of tuberculosis. In addition, the clinical presentations reflect the interactions between pathogens and host immune responses. The major purpose of the present study was to evaluate the associations of initial presentations for predicting the mortality of PTB patients. The mortality profiles of PTB patients and other clinical predictors of mortality, including bacilli genotyping, were also investigated.
Materials and Methods Ethics
This study was approved by the Institutional Review Board of Taipei Veterans General Hospital; Chest Hospital, DOH, Institutional Review Board; Changhua Christian Hospital Institu-tional Review Board; Kaohsiung Medical University Chung-Ho Memorial Hospital Institutional Review Board; Taipei Medical University- Wang Fang Medical Center Institutional Review Board; Buddhist Tzu Chi General Hospital and Tzu Chi University, Hualien, Research Ethics Committee; and Chest Hospital, Department of Health, Executive Yuan, Research Ethics Committee. Written informed consent was obtained from each patient or their authorized representative(s) before enrollment.
Patients and setting
This prospective observational study was conducted at six hospitals in Taiwan: five referral medical centers and 1 regional hospital specializing in pulmonary diseases. Newly diagnosed, culture-proven tuberculosis patients from January 2007 to June 2009 were eligible for enrollment. Patients without pulmonary involvement and those who were younger than 18 years of age were excluded. Demographic profiles (age, gender, co-morbidities) and clinical characteristics (history of previous anti-TB treatments, and smoking habits) were obtained from the patients by enrollment interview. The presenting symptoms/signs were determined according to the subjective statements of the patients and/or their caregivers in critical ill patients. In patients with altered mental status, the presence of dyspnea was determined when the respiratory rate excessed 25/minute. Drug susceptibility profiles were collected from medical records.
Initial presentations were divided into pulmonary symptoms/ signs (chronic cough lasting longer than 3 weeks, hemoptysis, and dyspnea) and constitutional symptoms/signs (body weight loss, fever, malaise, and anorexia). Chest radiographs were reviewed by the doctor in-charge at each hospital. Patients were categorized as having cavitation, lobar/segmental consolidation, or bilateral disease if these were present on chest radiographs at the time of diagnosis.
Treatment of pulmonary tuberculosis and follow-up
All of the patients were treated with standard anti-TB treatments that included isoniazid, rifampicin, ethambutol, and pyrazinamide in the initial phase. The regimen was modified when
drug susceptibility results were available or when significant adverse effects occurred. All of the patients were followed until death or upon completion of anti-TB treatment. The treatment outcome was determined according to the records in the Centers for Disease Control (CDC) registration database, Taiwan. Patients who died for any reason before or during the course of anti-TB treatment were defined as mortality cases and mortality that occurred within 30 days of initiating PTB treatment was defined as early mortality [11] Defaulted cases and patients who were transferred out were excluded from analysis.
Mycobacteriology and genotyping
Sputum AFB smears and cultures were performed in each hospital using standard methods. Sputum smears were examined through Ziehl-Neelsen staining. The isolation of MTB in sputum cultures was performed in liquid medium (BACTEC) and/or Lowenstein-Jensen solid medium. Drug susceptibility testing was performed using the proportion method with Middlebrook 7H11 media. Genomic DNA was extracted from cultures as described previously [12]. All clinical isolates were genotyped using a commercial spoligotyping kit (Isogen Bioscience B.V., Maarssen, Netherlands). The ‘‘Beijing strain’’ was defined as a deletion from spacer 1 to spacer 34 in the direct repeat region and the presence of (at least 3) spacers in 35–43.
Statistical analysis
The cumulative incidence of mortality was estimated by Kaplan-Meier analysis. The statistical significances and risk ratios of overall and early (deaths within 30 days) mortality by clinical characteristics were analyzed using unadjusted and adjusted Poisson regression analysis with a stepwise selection procedure. The proportional-hazards assumption was tested on the basis of Schoenfeld residuals after a fitted Cox regression model. For time varying covariates, i.e. the hazard ratios for dying varied with follow-up time, averaged hazard ratios were reported. A p value of , 0.1 in unadjusted model was required for a variable to be entered into the adjusted model. Variables were included in the final model for p,0.05 and were excluded if p,0.1. Survival time was estimated by the Kaplan-Meier method and a log-rank test was used to evaluate factors associated with death. The patients of defaults and transferred out were censored in the Kaplan-Meier survival analysis. A p value of , 0.05 (two-tailed) was considered statistically significant for all tests. Statistical analysis used SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) and STATA 11.0 (Stata corporation, College Station, Texas, USA).
Results
PTB Patient Mortality
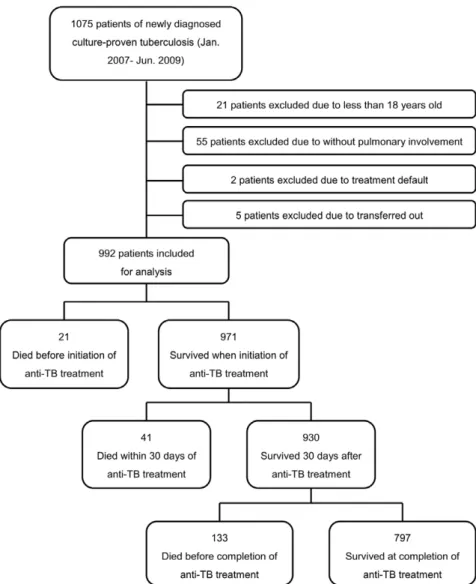
The study profile showing the number of cases and reasons for exclusion is shown in Figure 1. Ultimately, 992 patients with newly diagnosed, culture-proved pulmonary tuberculosis were included for analysis.
Overall, 992 patients were followed up for 646.8 person-year. Of these patients, 62 (6.3%) died within 30 days, 185 (18.6%) died within one year, and 195 (19.7%) died before completing treatment. The 30-day and one-year cumulative mortality was 6.25% (95% CI:4.91%–7.94%) and 22.55% (95% CI: 18.95%– 26.71%), respectively. The survival times for PTB patients in the first 60 days and within one year are shown in Figure 2. The first 40 days had the largest numbers of mortality cases, and these numbers gradually declined thereafter.
All the 21 pre-treatment mortality cases were diagnosed by positive sputum culture results and enrolled before mortality. However, the
anti-TB treatment was not started immediately after diagnosis and the patients died before initiation of treatment. The delay in anti-TB treatment was mostly due to concomitant hepatic and renal dysfunction, inability to take oral medications, or by clinical judgment of the in charge physicians. The intervals from diagnosis to mortality ranged from 13 days to 1 day in these patients.
Clinical Presentations of Survivors and Non-survivors
The demographic characteristics and clinical presentations of the study population are shown in Table 1. Their mean age was 64.6 (619.2) years and they were predominantly males (77.6%). There were 13 (1.3%) HIV-positive patients. Compared with survivors, non-surviving patients were more likely to be male (85.6% vs. 75.7%, p = 0.003), older in age (76.2612.6 vs. 61.5619.5, p,0.001), have some malignancy (31.3% vs. 8.9%, p,0.001), have renal insufficiency (11.8% vs. 3.3%, p,0.001), and were post-gastrectomy (7.2% vs. 2.1%, p,0.001). The proportions of HIV-positive patients were comparable between survivors and non-survivors, and there were no differences in first-line anti-TB drug resistance rates. The proportions of Beijing strain infections were similar between the two groups.
Regarding radiological features, the non-survivors were less likely to have cavity formations (11.3% vs. 20.6%, p = 0.003), and were more likely to have lobar/segmental consolidations (87.2% vs. 79.9%, p = 0.02) and bilateral involvements (49.7% vs. 39.6%, p = 0.01). Regarding clinical symptoms/signs, the non-survivors were less likely to present with chronic cough of . 3 weeks (42.6% vs. 62.5%, p,0.001), and were more likely to present with dyspnea (30.3% vs. 22.5%, p = 0.022), fever (42.6% vs. 27.4%, p,0.001), malaise (21% vs. 13.3%, p = 0.006), and anorexia (25.6% vs. 16.2%, p = 0.002). Multivariate Poisson regression analysis identified age, malignancy, renal insufficiency, chronic cough, fever, and anorexia as independent predictors associated with overall mortality.
Independent risk factors associated with overall mortality from a multivariate Cox proportional hazards model are summarized in Table 2. Older Age (HR = 1.05, 95% CI: 1.04–1.06), malignancy (HR = 2.85, 95% CI: 2.08–3.91), renal insufficiency (HR = 2.27, 95% CI: 1.43–3.59), absence of chronic cough . 3 weeks (HR = 0.54, 95% CI: 0.40–0.72), presence of fever (HR = 1.72, 95% CI: 1.29–2.30), and anorexia (HR = 1.49, 95% CI: 1.07– 2.07) were independent predictors of mortality.
Figure 1. Study profile demonstrating the number of cases and reasons for exclusion. doi:10.1371/journal.pone.0023715.g001
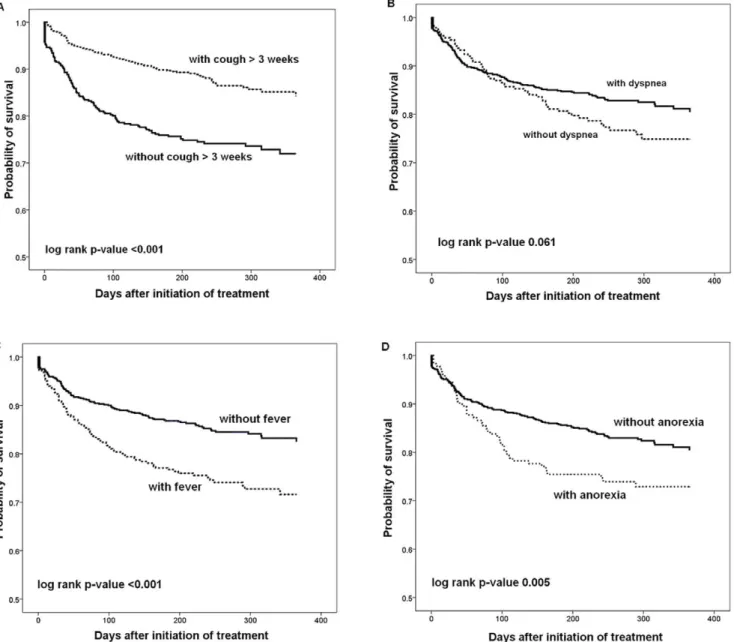
Kaplan-Meier survival curves according to the presence or absence of clinical symptoms/signs are shown in Figure 3. Patients who presented with fever, anorexia, and absence of chronic cough of . 3 weeks had higher mortalities (p,0.001 for fever, p = 0.005 for anorexia, and p,0.001 for chronic cough). An absolute difference in survival between patients with and without chronic cough was evident after the initiation of anti-TB treatment.
The characteristics of patients with early mortality
The demographic characteristics of patients with early (within 30 days) and late (after 30 days) mortality are shown in Table 3. As compared with late mortality, patients with early mortality are less likely to be sputum smear positive (RR: 0.63, 95% CI: 0.41–0.97), to present with chronic cough . 3 weeks (RR: 0.51, 95% CI:
0.32–0.82) and dyspnea (RR: 0.50, 95% CI: 0.28–0.88). Other clinical factors, including age, gender, underlying comorbidities, drug susceptibility profiles, and radiological presentations were comparable between patients with early or late mortality. Multivariate Poisson regression analysis identified that the initial presentations of chronic cough . 3 weeks and dyspnea were independently associated with a lower probability of early mortality within 30 days.
Discussion
Despite effective first line anti-TB drugs, pulmonary tubercu-losis remains a disease with a high mortality rate. The mortality rates for pulmonary tuberculosis reported in previous studies
Figure 2. Survival time of pulmonary tuberculosis patients after initiation of anti-TB treatment. (A) within one year. (B) within 60 days. * Number of cases died before initiation of anti-TB treatment.
ranged from 5% to 30%, depending upon underlying co-morbidities, disease severity, and HIV-infection status of the included patients [2,6,13,14]. In the present study, 19.7% of the patients died before completing the treatment, which was relatively higher as compared with previous reports in non-HIV endemic areas. The higher mortality rate among the patients we enrolled was probably due to older age and more underlying co-morbidities, as the study was performed in referral medical centers. In line with previous reports [3,15], a higher proportion of TB patients died in the early phase of commencing treatment. Nearly one-third of the deaths occurred within 30 days, and more
than half of the deaths occurred within 60 days after initiating anti-TB treatment. Our observations highlight the importance of identifying predictors that are associated with mortality in the start of anti-TB treatment.
The risk factors associated with mortality have been extensively evaluated before. Age, co-morbidities of malignancy, renal disease, and respiratory disorders, HIV infection, and malnutrition are all well-established clinical predictors of mortality in tuberculosis patients [4–6,8,14,16]. For HIV-infected patients, CD4 T cell counts and HIV viral loads have been identified as significant factors of mortality in tuberculosis [2,17]. In addition, low Table 1. Demographic characteristics and clinical presentations of pulmonary tuberculosis patients with or without on-treatment mortality.a
All patients, n = 992 Survival status P value Survivors, n = 797 Non-survivors, n = 195
Mean age (SD) 64.6 (19.2) 61.5 (19.5) 76.2 (12.6) ,0.001
Male gender 770 (77.6%) 603 (75.7%) 167 (85.6%) 0.003
Previous TB history 94 (9.5%) 76 (9.5%) 18 (9.2%) 0.90
Smoking habit 297 (29.9%) 248 (31.1%) 49 (25.1%) 0.10
Initial sputum smear positive 484 (48.8%) 393 (49.3%) 91 (46.7%) 0.51 Concomitant extrapulmonary TB 38 (3.8%) 30 (3.8%) 8 (4.1%) 0.83 Comorbid diseases Diabetes 223 (22.5%) 175 (22.0%) 48 (24.6%) 0.43 COPD 75 (7.6%) 55 (6.9%) 20 (10.3%) 0.11 Malignancy 132 (13.3%) 71 (8.9%) 61 (31.3%) ,0.001 Renal insufficiency 49 (4.9%) 26 (3.3%) 23 (11.8%) ,0.001 Liver cirrhosis 34 (3.4%) 25 (3.1%) 9 (4.6%) 0.31 HIV positiveb 13 (1.3%) 12 (1.5%) 1 (0.5%) 0.28 Post gastrectomy 31 (3.1%) 17 (2.1%) 14 (7.2%) ,0.001
Drug susceptibility test
Isoniazid resistance 121 (12.2%) 98 (12.3%) 23 (11.8%) 0.85
Rifampicin resistance 65 (6.6%) 54 (6.8%) 11 (5.6%) 0.57
Ethambutol resistance 73 (7.4%) 59 (7.4%) 14 (7.2%) 0.92
Streptomycin resistance 111 (11.2%) 93 (11.7%) 18 (9.2%) 0.33
MDR 59 (5.9%) 49 (6.1%) 10 (5.1%) 0.59
Beijing strain infection 545 (54.9%) 433 (54.3%) 112 (57.4%) 0.43 Radiographic presentations Cavity formation 186 (18.8%) 164 (20.6%) 22 (11.3%) 0.003 Lobar/segmental consolidation 807 (81.4%) 637 (79.9%) 170 (87.2%) 0.020 Bilateral involvement 413 (41.6%) 316 (39.6%) 97 (49.7%) 0.010 Respiratory symptoms Chronic cough 581 (58.6%) 498 (62.5%) 83 (42.6%) ,0.001 Hemoptysis 167 (16.8%) 132 (16.6%) 35 (17.9%) 0.64 Dyspnea 238 (24.0%) 179 (22.5%) 59 (30.3%) 0.022 Constitutional symptoms
Body weight loss 213 (21.5%) 180 (22.6%) 33 (16.9%) 0.08
Fever 301 (30.3%) 218 (27.4%) 83 (42.6%) ,0.001
Malaise 147 (14.8%) 106 (13.3%) 41 (21.0%) 0.006
Anorexia 179 (18.0%) 129 (16.2%) 50 (25.6%) 0.002
a
The data are presented as n (%) unless otherwise stated.
b
HIV testing was not routinely done in each patient. The estimated HIV testing rate ranged around 15–20%.
TB, tuberculosis; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; MDR, multi-drug resistance; RR, risk ratio; CI, confidence interval. doi:10.1371/journal.pone.0023715.t001
socioeconomic status, multidrug resistance, and delayed diagnosis have also been reported to be significant predictors of mortality [18–21]. In the present study, we found that older age and the underlying co-morbidities of malignancy and renal insufficiency were independent demographic characteristics associated with mortality, whereas drug susceptibility profiles and genotypes of MTB isolates were not. Drug resistance, especially multidrug resistance tuberculosis (MDRTB), is widely reported to be associated with mortality [3,9,21]. However, the associations between drug resistance and mortality were insignificant in our report. In Taiwan, MDRTB patients are encouraged to be transferred to referral TB centers and routinely requested to join a Direct Observation Therapy/Short Course (DOTS) program. The treatment regimen is adjusted according to the complete second line anti-TB drugs susceptibility profiles and is under the stringent supervision of CDC. All of these measures help to improve the treatment outcome of MDRTB patients. Our results indicated that the major determinants for mortality among PTB patients in Taiwan, a TB endemic area with high medical accessibility and abundant medical resources, were those factors related to the underlying conditions of the patients, and not bacterial factors.
The mortality rates were comparable between PTB patients with and without HIV-infections in the present study. Taiwan is not a HIV endemic area and HIV testing is not routinely performed in general tuberculosis patients. The estimated HIV prevalence rate of adults in Taiwan was 0.16% in 2008. By comparison, the estimated HIV prevalence rate was 0.64% in overall newly diagnosed TB patients and 1.8% in newly diagnosed TB patients with available HIV testing results [22]. The HIV prevalence rate is likely to be underestimated as the HIV test is not routinely done for our patients. The insignificant correlation between HIV infection and mortality deserves further verification in future studies.
All of the patients we enrolled were culture-proved cases and, therefore, the genotyping data were available for these patients.
The Beijing genotype is distributed worldwide and is the predominant MTB strain in Eastern Europe and Southeast Asia [23,24]. Although previous studies reported higher treatment failures and relapse rates for patients infected with the Beijing strain [25,26], the mortality rates were comparable between patients infected with Beijing and non-Beijing strains in the present study. Based on these results, the impact of the Beijing strain on mortality is probably limited in Taiwan. However, the clinical characteristics, such as drug resistance, associated with the Beijing strain vary between geographic areas and its role in treatment outcomes may also be different [23]. Further studies are required to clarify the impact of the Beijing strain on mortality.
The association between clinical presentations and mortality remains an issue of controversy. Kourbatova et al. reported bilateral lung involvement, cavitary lesions on radiograms, and symptoms lasting . 4 weeks as independent risk factors of mortality for tuberculosis patients [9]. Hoa et al. also found that tuberculosis patients with prolonged coughs had an increased risk of death [10]. However, Venkatarama et al. reported that only symptomatic dyspnea was independently associated with mortality for hospitalized tuberculosis patients [8]. Low at al. identified the absence of cough and cavitary lesions on radiograms as significant predictors of mortality by multivariate analysis [3].
In our analysis, the presence of fever, anorexia, and the absence of chronic cough of . 3 weeks were clinical presentations that were independently associated with mortality. The associations of radiological features for predicting mortality were limited. More importantly, we found that the absence of respiratory symptoms, including chronic cough and dyspnea, was the sole independent predictor for early mortality within 30 days. The differences in patient settings, demographic characteristics, and HIV-infection status may have contributed to the inconsistent findings between our results and those of previous reports.
Our findings also provide important information for physi-cians in clinical practice. The presence of constitutional Table 2. Cox proportional hazards models for overall mortality prediction by demographic characteristics and clinical
presentations.
Unadjusted HR (95% CI)a Adjusted HR (95% CI)b
HR (95% CI) p value HR (95% CI) p value
Age 1.06 (1.05–1.07) ,0.001 1.05 (1.04–1.06) ,0.001 Male gender 1.78 (1.19–2.66) 0.005 Malignancyc 3.59 (2.65–4.87) ,0.001 2.85 (2.08–3.91) ,0.001 Renal insufficiency 3.33 (2.16–5.16) ,0.001 2.27 (1.43–3.59) ,0.001 Post gastrectomy 3.03 (1.76–5.23) ,0.001 Cavitary lesion 0.47 (0.30–0.73) 0.001 Lobar/segmental consolidation 1.77 (1.16–2.71) 0.008 Bilateral involvement 1.44 (1.08–1.90) 0.012 Chronic cough 0.46 (0.34–0.61) ,0.001 0.54 (0.40–0.72) ,0.001 Dyspnea 1.40 (1.03–1.90) 0.031 Fever 1.82 (1.37–2.42) ,0.001 1.72 (1.29–2.30) ,0.001 Malaise 1.54 (1.09–2.18) 0.014 Anorexia 1.60 (1.16–2.21) 0.004 1.49 (1.07–2.07) 0.018 a
Unadjusted hazard ratios and 95% confidence intervals for variables that might predict overall mortality (p,0.1).
b
Results of stepwise selection for a fitted cox regression model (Goodness-of-fit, Chi square = 214.226, p,0.001). Variables were included in the final model for a p,0.05 and were excluded if p,0.1.
c
The hazard of dying for patients with malignancy or dyspnea attenuated after 6 months and averaged hazard ratios were reported. HR, hazard ratio; CI, confidence interval.
symptoms, such as fever and anorexia, may represent higher disease severity and associated with increased risk of death. Meanwhile, respiratory symptoms/signs are important clues that can remind clinical physicians of the possibility of pulmonary diseases. The absence of typical respiratory symp-toms may lessen clinical suspicion and result in delayed diagnosis of pulmonary tuberculosis. A delayed diagnosis can lead to increased risks of mortality [27]. However, information regarding the durations of presenting symptoms/signs was not collected in the study design. Therefore, the association between delayed diagnosis and mortality cannot be readily analyzed, and this made it difficult for us to clarify the interaction between presenting symptoms/signs and mortality. However, delays in diagnosis remain the most probable cause for a worse outcome in patients without respiratory symptoms.
Possible predictors of early mortality as compared with late mortality among PTB patients have rarely been analyzed before. Interestingly, we found that only the absence of respiratory
symptoms, but neither age nor co-morbidities, was an independent predictor of mortality within 30 days. The findings again emphasize the importance of early diagnosis and early treatment in the improving outcome of PTB patients. The proportions of previous TB history were comparable between patient with early and late mortality in the present study. However, the impact of TB reaction remains an issue that deserves discussion as critical patients probably are more likely to have TB reactivation and this leads to early mortality. This study was carried out in a TB endemic area and unrecognized previous TB infection is highly possible with uncertain incidence. The proportion of previous TB infection is obviously underestimated. However, a history of previous TB infection does not necessarily indicate the presence of reactivation, as reinfection could be a major cause of recurrent TB after previous cure [28]. Without genotyping profiles of previous infection, it is difficult to differentiate TB reactivation from TB reinfection in these patients. Further studies are needed to elucidate this issue.
Figure 3. Kaplan-Meier survival curves of pulmonary tuberculosis patients stratified by the presence or absence of clinical symptoms/signs. (A) Chronic cough . three weeks. (B) Dyspnea. (C) Fever. (D) Anorexia. Significances were tested using the log-rank test. doi:10.1371/journal.pone.0023715.g003
Several independent risk factors for death were identified in the present study. Unfortunately, all of these were un-modifiable factors. What actions can we take when managing these high risk patients? Irrespective of the cause of mortality, treating the underlying comorbidities of patients as best as possible is definitely important. It would be helpful to encourage the patients to join a DOTS program to enhance compliance of anti-TB treatment. However, the crucial step probably lies in early anti-TB treatment; and early treatment mostly depends on early diagnosis. Maintain-ing a high degree of clinical suspicion in order to diagnosis PTB early is of pivotal importance to improve the treatment outcome of PTB patient.
Our study had several limitations. Five of the six participating hospitals were regional referral medical centers. More patients with more co-morbidities and higher disease severities may have been included in the present study and, as a result, the overall mortality rate may have been overestimated. However, this also shows that our findings can be reliably applied to elderly PTB patients with multiple co-morbidities. Information regarding the durations of presenting symptoms prior to PTB diagnosis was not collected in the study design. This limitation made it more difficult to interpret the association between symptoms/signs and mortality. We did not divide deaths as being due to either TB or other non-TB causes in the study design because it is not always easy to identify the impact of tuberculosis on mortality. All-cause mortality is more objective and more applicable to clinical practice. As the study area not an
HIV endemic area, HIV testing was not routinely conducted in the present study. This also limits the ability of our findings to be applied in non-HIV-endemic areas.
In conclusion, our study found that nearly one-third of the mortality cases among PTB patients occurred within 30 days of initiating anti-TB treatment. Irrespective of the cause of mortality, older age and the underlying co-morbidities of malignancy and renal insufficiency were demographic characteristics that associ-ated with death. Regarding clinical presentations, the presence of fever, anorexia, and the absence of chronic cough were independent predictors of death. As compared with late mortality, the absence of respiratory symptoms, including chronic cough and dyspnea, were significant factors associated with early mortality within 30 days. In contrast, the associations of radiological features with early and overall mortalities were limited.
Acknowledgments
We gratefully acknowledge Dr. Y-C. Chen (Institute of Medical Biometry and Informatics, University of Heidelberg) for his help in statistical analysis. Author Contributions
Conceived and designed the experiments: J-YF W-JS CC S-FH YL Y-CL. Analyzed the data: J-YF W-JS. Contributed reagents/materials/ analysis tools: J-YF W-JS R-MH C-HL J-JH J-JL M-CY K-WY. Wrote the paper: J-YF.
Table 3. Poisson regression analysis for early mortality (within 30 days) prediction by demographic characteristics and clinical presentations.a,b
All mortality
patients, n = 195 Mortality status Unadjusted RR (95% CI)c Adjusted RR (95% CI)d Early mortality,
n = 62
Late mortality,
n = 133 RR (95% CI) P value RR (95% CI) P value Mean Age (SD) 76.2 (12.6) 77.2 (15.3) 75.7 (11.2) 1.01 (0.99–1.03) 0.53
Initial sputum smear positive 91 (46.7%) 22 (35.5%) 69 (51.9%) 0.63 (0.41–0.97) 0.033 Previous TB history 18 (9.2%) 3 (4.8%) 15 (11.3%) 0.50 (0.17–1.43) 0.16 Malignancy 61 (31.3%) 17 (27.4%) 44 (33.1%) 0.83 (0.52–1.33) 0.43 Renal insufficiency 23 (11.8%) 11 (17.7%) 12 (9%) 1.61 (0.99–2.62) 0.08 Post gastrectomy 14 (7.2%) 4 (6.5%) 10 (7.5%) 0.89 (0.38–2.10) 0.79 Isoniazid resistance 23 (11.8%) 4 (6.5%) 19 (14.3%) 0.52 (0.21–1.29) 0.11 Rifampicin resistance 11 (5.6%) 1 (1.6%) 10 (7.5%) 0.27 (0.04–1.80) 0.10 MDR 10 (5.1%) 1 (1.6%) 9 (6.8%) 0.30 (0.05–1.97) 0.13 Beijing strain infection 112 (57.4%) 35 (56.5%) 77 (57.9%) 0.96 (0.63–1.45) 0.85 Cavity formation 22 (11.3%) 7 (11.3%) 15 (11.3%) 1.00 (0.52–1.92) 1.00 Lobar/segmental consolidation 170 (87.2%) 54 (87.1%) 116 (87.2%) 0.99 (0.54–1.83) 0.98 Chronic cough 83 (42.6%) 17 (27.4%) 66 (49.6%) 0.51 (0.32–0.82) 0.003 0.56 (0.33–0.98) 0.041 Hemoptysis 35 (17.9%) 16 (25.8%) 19 (14.3%) 1.59 (1.03–2.46) 0.05 Dyspnea 59 (30.3%) 11 (17.7%) 48 (36.1%) 0.50 (0.28–0.88) 0.009 0.51 (0.27–0.98) 0.043 Fever 83 (42.6%) 27 (43.5%) 56 (42.1%) 1.04 (0.69–1.57) 0.85 Malaise 41 (21.0%) 16 (25.8%) 25 (18.8%) 1.31 (0.83–2.05) 0.26 Anorexia 50 (25.6%) 11 (17.7%) 39 (29.3%) 0.63 (0.35–1.10) 0.09 a
The data are presented as n (%) unless otherwise stated.
b
Only mortality cases are included for analysis.
c
Unadjusted risk ratios and 95% confidence intervals for clinically relevant variables.
d
Results of stepwise selection for a fitted Poisson Regression model (Goodness-of-fit, Chi square = 9.55, p = 0.008). Variables were included in the final model for p,0.05 and were excluded if p,0.1.
TB, tuberculosis; MDR, multi-drug resistance; RR, risk ratio; CI, confidence interval. doi:10.1371/journal.pone.0023715.t003
References
1. World Health Organization Global tuberculosis control-surveillance, planning, financing. (2008) Geneva. WHO/HTM/TB/ 2008.393.
2. Mugusi FM, Mehta S, Villamor E, Urassa W, Saathoff E, et al. (2009) Factors associated with mortality in HIV-infected and uninfected patients with pulmonary tuberculosis. BMC Public Health 12: 409.
3. Low S, Ang LW, Cutter J, James L, Chee CB, et al. (2009) Mortality among tuberculosis patients on treatment in Singapore. Int J Tuberc Lung Dis 13: 328–334.
4. Chiang CY, Lee JJ, Yu MC, Enarson DA, Lin TP, et al. (2009) Tuberculosis outcomes in Taipei: factors associated with treatment interruption for 2 months and death. Int J Tuberc Lung Dis 13: 105–111.
5. Sterling TR, Zhao Z, Khan A, Chaisson RE, Schluger N, et al. (2006) Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis 10: 542–549.
6. Kim HJ, Lee CH, Shin S, Lee JH, Kim YW, et al. (2010) The impact of nutritional deficit on mortality of in-patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 14: 79–85.
7. Silva DR, Menegotto DM, Schulz LF, Gazzana MB, Dalcin Pde T (2010) Factors associated with mortality in hospitalized patients with newly diagnosed tuberculosis. Lung 188: 33–41.
8. Rao VK, Iademarco EP, Fraser VJ, Kollef MH (1998) The impact of comorbidity on mortality following in-hospital diagnosis of tuberculosis. Chest 114: 1244–1252.
9. Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, et al. (2006) Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis 10: 1224–1230. 10. Hoa NP, Thorson AE (2006) Excess mortality and tuberculosis among
individuals with prolonged cough: a population-based study from Vietnam. Int J Tuberc Lung Dis 10: 851–856.
11. Beadsworth MB, van Oosterhout JJ, Diver MJ, Faragher EB, Shenkin A, et al. (2008) Hypoadrenalism is not associated with early mortality during tuberculosis treatment in Malawi. Int J Tuberc Lung Dis 12: 314–318.
12. Su WJ, Huang CY, Huang CY, Perng RP (2001) Utility of PCR assays for rapid diagnosis of BCG infection in children. Int J Tuberc Lung Dis 5: 380–384. 13. Na´jera-Ortiz JC, Sa´nchez-Pe´rez HJ, Ochoa-Dı´az H, Arana-Ceden˜o M,
Lezama MS, et al. (2008) Demographic, health services and socio-economic factors associated with pulmonary tuberculosis mortality in Los Altos Region of Chiapas, Mexico. Int J Epidemiol 37: 786–795.
14. Harries AD, Hargreaves NJ, Kemp J, Jindani A, Enarson DA, et al. (2001) Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet 357: 1519–1523.
15. Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM (2001) High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis 5: 1000–1005.
16. Tocque K, Convrey RP, Bellis MA, Beeching NJ, Davies PD (2005) Elevated mortality following diagnosis with a treatable disease: tuberculosis. Int J Tuberc Lung Dis 9: 797–802.
17. Garin B, Glaziou P, Kassa-Kelembho E, Yassibanda S, Mbelesso P, et al. (1997) High mortality rates among patients with tuberculosis in Bangui, Central African Republic. Lancet 350: 1298.
18. Kliiman K, Altraja A (2010) Predictors and mortality associated with treatment default in pulmonary tuberculosis. Int J Tuberc Lung Dis 14: 454–463. 19. Pablos-Me´ndez A, Sterling TR, Frieden TR (1996) The relationship between
delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA 276: 1223–1228.
20. Liu YC, Lin HH, Chen YS, Su IJ, Huang TS, et al. (2010) Reduced health provider delay and tuberculosis mortality due to an improved hospital programme. Int J Tuberc Lung Dis 14: 72–78.
21. Kawai V, Soto G, Gilman RH, Bautista CT, Caviedes L, et al. (2006) Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in Peru. Am J Trop Med Hyg 75: 1027–1033. 22. Yang CH, Feng CF, Tang CC, Chan PC, CDC Taiwan (2009) Epidemiology of
Human Immunodeficiency Virus Testing Among Tuberculosis Patients in Taiwan. Poster presentation at 2009 49th ICAAC, Abstract H-234. 23. European Concerted Action on New Generation Genetic Markers and
Techniques for the Epidemiology and Control of Tuberculosis (2006) Beijing/ W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis 12: 736–743.
24. Feng JY, Su WJ, Liu LY, Tsai CC, Chang SC (2009) Radiological presentation of pulmonary tuberculosis infected by the W-Beijing Mycobacterium tubercu-losis strain. Int J Tuberc Lung Dis 13: 1387–1392.
25. Feng JY, Su WJ, Tsai CC, Chang SC (2008) Clinical impact of Mycobacterium tuberculosis W-Beijing genotype strain infection on aged patients in Taiwan. J Clin Microbiol 46: 3127–3129.
26. Lan NTN, Lien HTK, Tung LB, Borgdorff MW, Kremer K, et al. (2003) Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg Infect Dis 9: 1633–1635.
27. Zahar JR, Azoulay E, Klement E, De Lassence A, Lucet JC, et al. (2001) Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensive Care Med 27: 513–520.
28. van Rie A, Warren R, Richardson M, Victor TC, Gie RP, et al. (1999) Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med 341: 1174–1179.