Fusion of the transposase with a classical nuclear localization
signal to increase the transposition efficiency of Ac transposon
Yuh-Chyang CHARNG
1,*, Hui-Ping LI
1, Hung-Chun CHANG
3, Kuan-Te LI
1, Tzong-Hsiung HSEU
2, and
Jenn TU
31Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China 2Department of Life Science, National Tsing Hua University, Taiwan, Republic of China 3Institute of Botany, Academia Sinica, Taipei, Taiwan, Republic of China
(Received January 29, 2004; Accepted June 29, 2004)
Abstract. A new strategy was applied to improve the transposition efficiency of the maize transposon Activator
(Ac) in heterologous plants. The Ac transposase was fused with a classical nuclear localization signal (NLS) of SV40 to promote the transport of transposase into a nucleus. Base on this, two NLS-TPase constructs were yielded, one containing the full length transposase gene (termed as SV40TPase), the other containing the truncated transposase gene (lacking its first NLS-like signal, termed as SV39TPase). These two NLS-TPase genes were expressed in transgenic tobacco plants under the control of PR-1a promoter. Excision of non-autonomous transposable element (Ds) from luciferase (LUC) reporter gene constructs was employed to analyze the induction of Ac transposase containing NLS. Applying the LUC assay and PCR analysis, these new NLS-TPase sources triggered higher Ds excision efficiencies then the native transposase. Furthermore, the SV40TPase showed more ability then the SV39TPase to trigger the Ds element. The usage of this new inducible transposon for plant functional genomics is discussed.
Keywords: Ac transposase; Inducible transposon; Luciferase reporter gene; Nuclear localization signal.
Abbreviations: HPT, hygromycin phosphotransferase; NLS, Nuclear localization signal; NPT, neomycin
phosphotransferase; LUC, luciferase; SA, salicylic acid; TPase, transposase.
Introduction
The maize transposon Activator (Ac) is an autonomous transposable element with a size of 4565 bp. It codes for a single gene product, the transposase (TPase), which to-gether with the inverted repeats and about 300 bp from each end of the transposon and putative host factors is the only prerequisite for transposition of the Ac element in plants. The Ac element is active in a wide range of plant species, including several members of the Solanaceae, and in rice, carrot and Arabidopsis (Becker et al., 1986; Van Sluys et al., 1987; Knapp et al., 1988; Yoder et al., 1988; Houba-Herin et al., 1990; Izawa et al., 1991), and has proven to be a powerful genetic tool for yielding knockout mu-tants for plant functional genomic studies (for a review, see Haring et al., 1991). Recently, the International Rice Genome Sequencing Project (IRGSP) completed its se-quencing of the entire rice (Oryza sativa) genome. Vari-ous strategies, including transposon tagging, have been used to produce a large population of mutant plants ad-equately assigning function to the abundant sequence in-formation (for review see Jeon and An, 2001). However, using transposon as a tool to create knockout mutants in plants with large genomes, seems to require increased *Corresponding author. Tel: +886-2-23630231 ext. 4118; Fax:
+886-2-23620879; E-mail: bocharng@ccms.ntu.edu.tw
transposition efficiency. To this end, Scofield et al. have fused the Ac TPase with the cauliflower mosaic virus (CaMV) 35S RNA promoter and found, in tobacco, no di-rect proportionality between the amounts of TPase mRNA and Ac/Ds transposition activity (Scofield et al., 1992). Furthermore, transpositions occur only at TPase transcrip-tion level, below a critical threshold (Scofield et al., 1993). One possible explanation is that above this threshold the TPase starts to aggregate and transpositions cease (Heinlein et al., 1994), forcing us to consider another ap-proach to improving transposition efficiency.
In principle, in order to perform the transposition events, the TPase can be transported into the nuclei, a process mediated by specific signals called nuclear localization se-quences (NLSs) (Stochaj and Silver, 1992a). The TPase has three NLSs near its amino-terminal end, NLS (44-62), NLS (159-178), and NLS (174-206), each of which is sufficient to direct GUS to the nucleus (Boehm et al., 1995). However, NLS (44-62) and NLS (159-178) are bipartite NLSs while the structure of NLS (174-206) is not in one of the three major NLS categories. Interestingly, all three sequences were de-termined to be “weak” NLSs or NLS-like signals (Heinlein et al., 1994; Wang et al., 1998 and personal communication). We predict then that fusing a classical-NLS sequence (e. g. a source from SV40) to the TPase protein could pro-mote the transport of the Ac TPase and consequently in-crease transposition efficiency.
To date, the nuclear targeting signals of more than 70 mammalian and yeast proteins have been characterized (Dingwall and Laskey, 1991; Garcia-Bustos et al., 1991). Studies indicate that the nuclear transport machinery is highly conserved between animals, yeast and plants (Nelson and Silver, 1989; van der Krol and Chua, 1991; Lassner et al., 1991; Stochaj and Silver, 1992b; Hicks and Raikhel, 1993; Wagner and Hall, 1993). Three different cat-egories of NLSs were identified. The SV40 large-T antigen NLS (ppKKKRKv) is the prototype NLS category, charterized by a short uninterrupted stretch of basic amino ac-ids (Kalderon et al., 1984; Lanford and Butel, 1984). The second most common category is the bipartite NLSs, con-sisting of two clusters of basic residues separated by a spacer peptide. The paradigm for the bipartite class is the Xenopus laevis nucleoplasmin NLS (KRpaatkkagqa KKKKI) (Dingwall and Laskey, 1991). The third category is represented by the yeast MAT alpha-2 NLS (KipiK) and contains only one or two basic residues contiguous to hy-drophobic amino acids (Hall et al., 1984).
Accordingly, we decided to use the SV40 large-T anti-gen NLS (ppKKKRKv) sequence to fuse with the TPase gene. Based on the inducible transposon system, the pro-totype NLS of SV40 containing TPase was used as the source to trigger the transposition of the non-autonomous Ds element. We found that the NLS-containing TPase sources triggered higher Ds transposition efficiencies than did the native TPase source. Furthermore, it has been re-ported that the N-terminally truncated TPase derivative is inefficiently transported into the nucleus and aggregates predominantly in the cytoplasm (Heinlein et al., 1994). We constructed a similarly truncated TPase lacking the first NLS-like sequence of the native TPase. This construct was then fused with the prototype NLS of SV40. We found that this truncated TPase derivative triggered slightly lower Ds transposition efficiency than did the NLS-containing full length TPase. The role of the NLSs for the transport of the TPase and the subsequent transposition was discussed.
Materials and Methods
DNA Manipulation
Recombinant DNA technology was performed accord-ing to Sambrook et al. (1989). The materials and methods required for the construction of plasmids pBC SK+ and pBinHygTs have been previously reported (Charng et al., 1995).
In order to generate modified NLSs constructs, which consist of the NLS of the SV40, two DNA fragments were yielded by polymerase chain reactions. To this end, the following synthetic oligonucleotide primers were used: primer SV39 (harboring 18 nucleotides coding for NLS and the remaining nucleotide identical to the Ac sequence from position 1404 to 1416, 5'-GGATCCATGAAGAAGAAGCG-CAAAGCTATTGTTCATG-3'); primer SV40 (harboring 18 nucleotides coding for NLS and the remaining nucleotide identical to the Ac sequence from position 990 to 1104,
5'-GGATCCATGAAGAAGAAGCGCAAGACGCC TCCGGT-TGG-3'), and primer CSV (complementary to the Ac se-quence from position 1845 to 1823, 5'-AGTACTCATGTTC TACAATATTG-3'). Each polymerase chain reaction con-tained approximately 0.1µg template DNA (plasmid pBinHygTs), 0.25 µg of each primer, 0.2 mM dNTPs, 1.5 m MgCl2, 1 unit of Taq DNA polymerase, and 10X buffer. The samples were subjected to 40 cycles of amplification with each cycle consisting of 1 min at 94°C, 30 s at 50°C, and 30 at 72°C. After reactions an agarose gel electrophosis was performed to recover the DNA fragments. Each DNA fragment was then ligated with a 3.4 kb SmaI fragment of plasmid pBC SK+. Then a Nsi I excised 1.7 kb TPase frag-ment from the pPCV720ORF was ligated to the Nsi I treat-ed pBCSV40 and pBCSV39 vector, yielding the plasmids pLc40TP and pLc39TP. These two plasmids were digest-ed with Bam HI, yielding the 2.5 kb fragment. This frag-ment was then ligated to the Bam HI treated pBinHyg binary vector (Charng et al., 1995), resulting in two plas-mids designated pBH40Ts and pBH39Ts.
Plant Transformation
All transformations were performed with tobacco plants containing the Ds::reporter gene construct, and the transgenic tobacco plants were regenerated as described by Charng et al. (1997).
In Vivo and In Vitro Assays for Luciferase Gene Activities
Luciferase enzyme activity was determined as described by Howell et al. (1989) using a Lumat LB 9501 luminometer from Berthold, München, Germany.
For in vivo assaying, the plant material was sprayed with 0.15 mg/l of luciferin aqueous solution, placed in a dark room and then measured by the luminometer immediately. The luminometer consisted of an intensified CCD camera (Hamamatsu, Japan), with a Nikon 35 mm lens, connected to a personal computer. The live plant material image and the luminescent image were taken separately, and the latter revealed callus with luciferase activity. Genomic DNA Isolation
Genomic DNA was isolated from transformed plants with the use of a kit (BIO101, Vista, AC). Half gram calli were collected, frozen with liquid nitrogen in a mortar, and ground with a pestle. The nuclei were collected and lysed by protease treatment according to the manufacturer’s instructions. Genomic DNA was precipitated by adding ethanol and dissolved in 100 µl TE (10 mM Tris-HCl, 1 mM EDTA pH 8.0).
Analysis of Ds Excision Events by Polymerase Chain Reaction
For the analysis of Ds transposition from the Ds::LUC construct in transgenic tobacco plants, three synthetic oli-gonucleotide primers were used: ACP (complementary to t h e A c s e q u e n c e f r o m n u c l e o t i d e 4 8 0 4 6 2 , 5 '
-CTGGGAGACAGGGAGAGTC-3'), primer LUC (complementary to the luciferase coding sequence from position 577 to 556 as numbered by De Wet et al. (1987), 5'-CGGGAGGTAGATGAGATGTGAC-3'), and primer 35S (identical to the CaMV 35SRNA promoter sequence, 5'-TCCTTCGCAAGACCCTTCCT-3'). Each reaction mixture contained ca. 0.1 µg template DNA, 0.25 µg of each primer, 0.2 mM deoxynucleoside triphosphate, 1 U of Taq DNA polymerase, 1.5 mM MgCl2 and 10x buffer. The amplifica-tion protocol comprised 40 cycles of 1 min at 94°C, 30 s at 50°C, and 30 s at 72°C.
Results
Construction of the PR-1a::SVTPase Chimeric Gene and its Introduction into Tobacco
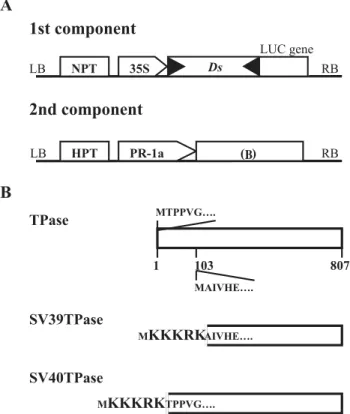
In order to improve the usage of the inducible transposon system, we had previously transformed a two-component system into Nicotiana tabacum cvs. Samsun nn; i.e. the first component for detection of Ds excision efficiency and the second component for providing the TPase source (Figure 1). In this study, various second components were constructed based on the previous two-component system (Charng et al., 1997).
For the first component, a Ds element was inserted be-tween the CaMV 35S promoter and the coding region of the luciferase (LUC) reporter gene from firefly (Ds::LUC; Figure 1A). This leads to the inactivation of the LUC gene, which is restored upon removal of the Ds element from the chimeric gene by transposon excision. This gene construct has been inserted in the binary vector Bin19 (Bevan, 1984) and used to transform tobacco plants. Transformants were selected by maintaining the leaf disks and the regenerat-ing plantlets on kanamycin-containregenerat-ing medium.
For the second component, as a source for the NLS con-taining TPase, plasmid pBinHygTs was used (Charng et al., 1995). This plasmid contains the PR-1a promoter fu-sion with a full length cDNA clone of the Ac TPase tran-script and was used to provide the native TPase source in this report (as a control). Based on this construct, we then generated two additional second components (Figure 1B). First, the full length Ac TPase gene was fused to the typical NLS of SV40 (referred as PR-1a::SV40TPase). The second construct, designated PR-1a::SV39TPase, was a deleted derivative of the PR-1a::SV40TPase. It lacks the first NLS and retains only the second NLS of the putative TPase, but harbors the prototype NLS of SV40.
The kanamycin-resistant progeny of self-pollinated transformants containing the first component (Ds::LUC construct) were transformed with the second component, and the transformants were selected on hygromycin B- and kanamycin-containing medium. All transformed tobacco plants contained the same Ds::LUC construct and a sec-ond component, according to the TPase source. We termed the transformed tobacco plants N- (containing the native TPase), 39- (containing the SV39TPase construct), or 40-(containing the SV40TPase construct). The Ds excision
events were monitored by analyzing the transformants for reporter gene activities.
Spontaneous Transposition of Ds Element in Shoot Derived from Primary Transformed Calli
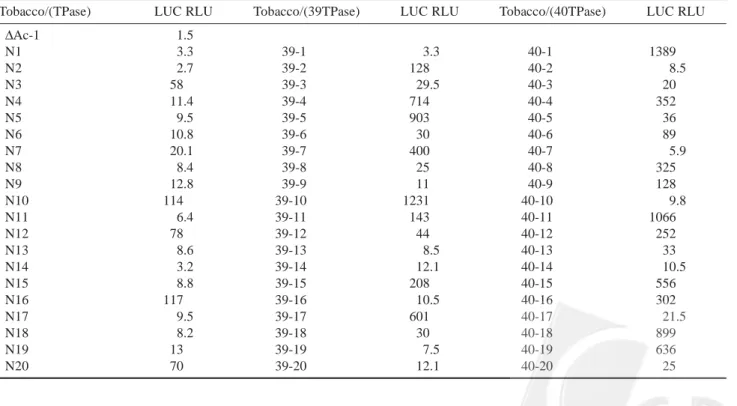
To determine whether Ds undergoes spontaneous transposition, we assayed luciferase activity in the primary regenerated shoots of tobacco transformants. Twenty in-dependent transformed lines for each construct were ex-tracted for luciferase activity assay. For the transformed lines harboring the native TPase source (PR-1a::TPase), 5 out of 20 independent transformed tobacco shoots (N3, N10, N12, N16 and N20) exhibited luciferase activity. For the transformed plants containing PR-1a::SV39TPase
Figure 1. Schematic representation of two-component system
used to access the activity of the Ac transposase under the in-fluence of NLS of SV40. A, The two-component system. The
Ds::LUC gene construct (first component) was used to analyze Ac transposase activity in transgenic tobacco plants via excision
of non-autonomous Ds element. For the selection of transformed p l an t s , t h e f i r s t c o m p o n e n t c o n t a i n s t h e n e o m y c i n phosphotransferase (NPT) gene, and the second component con-tains the hygromycin phosphotransferase (HTP) gene. “35S” in-dicates the promoter region for expression of the LUC reporter gene. RB and LB indicate the left and right border sequences of the T-DNA. B, The NLS derived TPase sequences used to sup-port the transposase source under the control of the PR-1a pro-moter in transformed tobacco plants. Shown are the transposase local amino acids 1-6 and 103-108 of the putative transposase (TPase), inserted by the prototype of NLS (highlighted) to yield SV40TPase and SV39TPase, respectively.
construct, 8 out of 20 independent transformed shoots (39-2, 39-4, 39-5, 39-7, 39-10, 39-11, 39-15 and 39-17) exhibited luciferase activity, and of the transformed shoots contain-ing PR-1a::SV40TPase construct, 11 out of 20 independent transformed shoots (1, 4, 6, 8, 9, 11, 40-12, 40-15, 40-16, 40-18 and 40-19) exhibited luciferase ac-tivity (Table 1). These results indicate that Ds can be triggered spontaneously, and the transposition efficiencies were 25%, 40% and 55% for PR-1a::TPase, PR-1a:: SV39TPase and PR-1a::SV40TPase construct, respectively. To verify that the observed reporter gene activities were due to excision of the Ds element, we analyzed genomic DNA from plants by multiplex PCR with primers LUC (complementary to the luciferase coding sequence), ACP (complementary to the Ac sequence), and 35S (identical to a region of the 35S promoter) (Figure 2A). With primers 35S and ACP, a 580 bp PCR product was obtained with DNA from LUC– tissue of either transformed tobacco plants
N1, 39-1 or 40-2 (Figure 2B; lane 1, 3 and 5). In the pres-ence of all three primers, no 670 bp product was gener-ated from DNA of those transformants with 35S and LUC. The distance between these two primers is about 4 kb in the intact Ds::reporter gene construct. Together, these re-sults indicate that Ds element had not undergone transpo-sition in LUC– tissue. In contrast, genomic DNA from LUC+
tissue of either transformed tobacco plants N16, 39-10 or 40-1 yielded PCR products of 580 and 670 bp (Figure 2B), indicating that the LUC+ tissue contained both cells in
which Ds had undergone transposition and cells in which it had not.
Table 1. Spontaneous transposition of the Ds element in primary transformed tobacco plants. Luciferase activity was measured in
shoots of transformed tobacco plants harboring the native transposase (TPase), SV39TPase (39TPase) or SV40TPase (40TPase) construct. Activity is expressed in relative light units (emission was measured for 2 s) per microgram of total protein; values of <50 RLU mg-1 produced no typical luciferase-luciferin activities corresponding to the absence of luciferase activity, referred to as LUC-.
RLU, relative light units.
Tobacco/(TPase) LUC RLU Tobacco/(39TPase) LUC RLU Tobacco/(40TPase) LUC RLU
∆Ac-1 1.5 N1 3.3 39-1 3.3 40-1 1389 N2 2.7 39-2 128 40-2 8.5 N3 58 39-3 29.5 40-3 20 N4 11.4 39-4 714 40-4 352 N5 9.5 39-5 903 40-5 36 N6 10.8 39-6 30 40-6 89 N7 20.1 39-7 400 40-7 5.9 N8 8.4 39-8 25 40-8 325 N9 12.8 39-9 11 40-9 128 N10 114 39-10 1231 40-10 9.8 N11 6.4 39-11 143 40-11 1066 N12 78 39-12 44 40-12 252 N13 8.6 39-13 8.5 40-13 33 N14 3.2 39-14 12.1 40-14 10.5 N15 8.8 39-15 208 40-15 556 N16 117 39-16 10.5 40-16 302 N17 9.5 39-17 601 40-17 21.5 N18 8.2 39-18 30 40-18 899 N19 13 39-19 7.5 40-19 636 N20 70 39-20 12.1 40-20 25
A NLS of SV40 Fusion with TPase Triggers High-est Ds Excision Efficiency after Induction
The fact that Ds transposition occurred spontaneously in transformed tobacco harboring SV39/40::TPase indicates the NLS-containing TPase is active in tobacco plants. To determine whether the NLS-TPase fusion construct could improve the inducible transposon system, we studied the induction of Ds transposition using salicylic acid (SA) as the inducer in transgenic tobacco calli and plants. All pri-mary transformed LUC– tobacco plants (Table 1) were
al-lowed to self-pollinate, and the progeny were collected for induction experiments. The T1 tobacco seeds of each transformed line were incubated on MS medium contain-ing kanamycin and hygromycin to ensure the presence of the two components. Five independent transformants for each NLS-TPase construct and at least 50 induced leaf discs for each transformed line were assayed. To induce the ex-pression of the Ac TPase, tobacco plants harboring the two components were treated with SA according to the previous report (Charng et al., 1997). The leaf discs from each transformed line were divided into several portions and were induced by SA or incubated directly on callus regeneration selection medium. For induction, the leaf discs were incubated on callus regeneration medium con-taining 1 or 5 mM SA for 24 h and then transferred to cal-lus regeneration selection medium without SA. Reporter gene activities were analyzed 2 weeks after induction. The Ds excision efficiency was analyzed by recording the pres-ence of leaf discs yielding LUC reporter gene activities, either in vivo or in vitro.
Figure 2. PCR analysis of Ds excision from the Ds::LUC
chi-meric gene. A, Structure of the Ds::LUC chichi-meric gene and the location of primers (shown as solid triangles) used for PCR analysis. The sizes of expected PCR products (580 and 670 bp before and after excision of Ds, respectively) are indicated. B, Ethidium bromide-stained agarose gel on which PCR products were separated. PCR was performed with genomic DNA from the indicated transformants. Lane M, 100 bp DNA ladder.
Figure 3. In vivo assay of LUC enzyme activity as revealed
by Ds excision events in transgenic tobacco leaf discs and in re-sponse to NLS-TPase fusion. Induced Ds excision patterns are revealed as light images produced by leaf discs after 14 days cultivation on medium. Reflected-light reference images (a to d) show leaf discs sprayed with luciferase substrate solution. The luminescent images (e to h) detected in dark. (a) and (e): to-bacco plants containing only the first element; (b) and (f): TPase as the second element to trigger the Ds element; (c) and (g): 39TPase as the second element to trigger the Ds element; (d) and (h): 40TPase as the second element to trigger the Ds element.
For in vivo assay of luciferase activities, Ds excision events are revealed as light images produced by the tissue. We observed that reporter gene activities were always re-stricted to the calli (Figure 3). This observation indicated that the Ds excision events were triggered mainly during the development of calli, mostly regenerated from the edge of excised leaf discs (Charng et al., 1997). Furthermore, when the light intensities of LUC+ leaf discs for each
con-struct were used as a record, a similar Ds excision effi-ciency was observed for each NLS-TPase construct (see below).
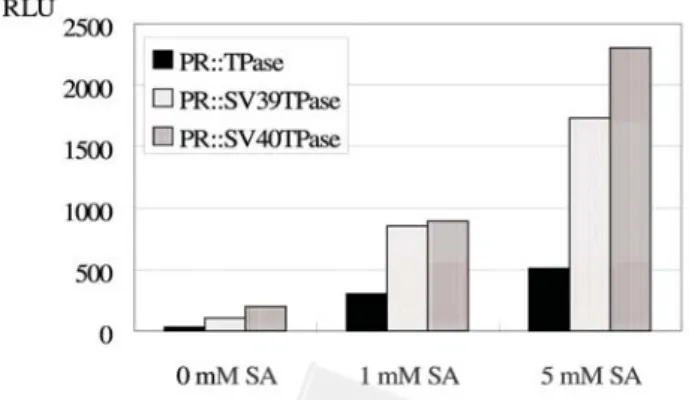
For in vitro assay, the regeneration calli for each trans-formed line were collected and extracted for luciferase ac-tivity assay. Figure 4 shows the mean luciferase acac-tivity yielded by each NLS-TPase transformed line with various SA-treatments. With in vitro assay, light is emitted as a peak because the luciferin-luciferase reaction is rapidly feedback-inhibited by a reaction product. All un-induced leaf discs yielded no typical luciferase activity kinetics but background values (RLU<50). These findings suggest that
Figure 4. Schematic representation of the LUC activities of
SA induced transgenic tobacco containing various NLS-TPase fusions.
no spontaneous transposition event occurred. When the leaf discs of these transformed lines were induced with 1 mM SA, the transformed lines harboring the native PR-1a:: TPase yielded typical peaks of luciferase light emission, but the LUC activities were low. For the same SA-treatment, transformed plants containing PR-1a::SV40TPase or PR-1a::SV39TPase construct yielded about twofold as much as the transformed plants containing the native PR-1a::TPase construct. On the other hand, when the leaf discs of these transformed lines were induced with 5 mM SA, the transformed lines harboring the native PR-1a:: TPase yielded a threefold increase in LUC activities over 1 mM treated tobacco. Furthermore, the 5 mM SA-treated transformed tobacco containing PR-1a::SV40TPase and PR-1a::SV39TPase construct yielded about fourfold and threefold, respectively, what the tobacco containing na-tive PR-1a::TPase construct with the same treatment yielded. Taken together, these results show that the trans-position efficiency of Ds was induced by SA in a dose-dependent manner. Furthermore, the NLS-containing TPase can trigger higher Ds transposition more efficiency than the native TPase.
DNA Blot Analysis of Somatic Excisions Arising from the NLS TPase Fusions
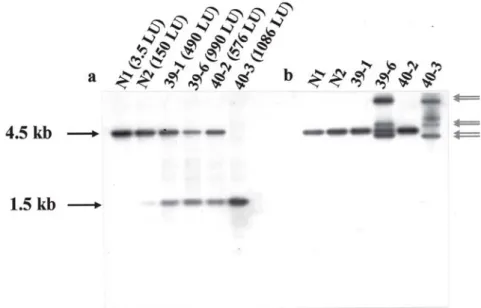
To determine whether the somatic excisions triggered by Tpase-derived constructs were bona fide transposition events, DNA was extracted from SA-induced transgenic tobacco plants with two components, which yielded vari-ous Ds excision efficiencies after 5 mM SA treatment as described above. As probe, the 1.2 kb Bam HI/Eco RV frag-ment comprising the LUC reporter gene was used. Bands of 4.5 kb (if Ds is not excised) and 1.5 kb (if Ds is trans-posed from its donor site) can be expected. As shown in
Figure 5a, plants with the highest luciferase activity (40-3) showed only the 1.5 kb band but no detectable 4.5 kb band. This indicates an early excision event during regeneration in this plant. In tobacco plants, which contained both the 4.5 kb band and the 1.5 kb fragments, transposition of Ds was incomplete or occurred at a later stage of development. In these plants the luciferase activities are proportional to the relative intensity of the 1.5 kb band (Figure 5a). In plant N1, only a 4.5 kb band was observed. This plant showed no enzyme activity and, as such, sug-gested an inactive transposon. The same filter was also probed with a 2.9 kb Ds fragment. Bands corresponding to the non-transposed donor site can hybridize to both the LUC probe and the Ds probe, yielding a band of 4.5 kb. Additional bands of various sizes (all larger than 2.9 kb), which hybridized to Ds probe but not to LUC probe, indi-cated the reinsertion of Ds. As shown in Figure 5b, plant 39-6 yielded three and plant 40-3 yielded four Ds-specific bands.
Discussion
Transposable elements have proven to be a powerful genetic tool for functional genomics studies. Several strat-egies have been applied to maximize the transposition ef-ficiency the Ac/Ds system in heterologous plants: (1) fusion of a constitutive or inducible promoter to control the expression level of TPase (Scofield et al., 1992; Charng et al., 1995), and (2) application of a demethylating agent, 5AzaC, to regulate the transposition mechanism (Scortecci et al., 1997; Charng et al., 2000). Interestingly, by fusing the TPase with the CaMV 35S promoter, Scofield et al. (1992) found that, in tobacco, the accumulation of high levels of the Ac TPase may inhibit subsequent transposon
Figure 5. Southern blot analysis of Ds transposition events. DNA gel blot hybridization of endonuclease Eco RV digested genomic
DNAs isolated from different transgenic tobacco lines with the luciferase gene probe (a) or with the Ds probe (b). Expected LUC-specific bands of the 4.5 kb fragment (inactive luciferase) and the 1.5 kb (active luciferase after Ds transposition) are indicated as black arrows. Reintegrated Ds elements are indicated as hatched arrows. On the top, the name of each sample and the luciferase activities are given.
excision. This compels us to develop another strategy to improve transposition efficiency. The native Ac TPase has three NLSs near its amino-terminal end, NLS (44-62), NLS (159-178) and NLS (174-206). However, all three were de-termined to be “weak” NLSs or NLS-like signals (Wang, 1998) though each is sufficient to direct GUS to the nucleus (Boehm et al., 1995). Here, we fused a classical nuclear lo-calization signal (NLS) of SV40 with the TPase gene and tried to increase the nuclear import efficiency of TPase and consequentially raise its transposition frequency. Indeed, after SA induction, the fusion TPase harboring NLS trig-gered a transposition efficiency that was fourfold the na-tive TPase triggered in tobacco.
A curious aspect of the Ac TPase has been reported. A truncated TPase lacking 102 amino acids from the amino-terminus is still functional in transgenic tobacco and Arabidopsis (Li and Starlinger, 1990; Grevelding et al., 1992). In this work, in additional to the full length NLS-TPase construct, we have constructed a similarly trun-cated NLS-fused TPase (SV39TPase). Our results inditrun-cated that when these two kinds of NLS-fused TPase genes were expressed under the control of the PR-1a promoter, the to-bacco plants harboring SV40TPase construct always yielded higher transposition efficiency then the plants har-boring SV39TPase did. Previously, Heinlein et al. (1994) suggested that a combination of several NLSs of the Ac TPase is required for efficient nuclear transport. On the other hand, these authors also suggested that Ac TPase that forms large aggregates in nuclear and the N-terminally truncated TPase derivative is inefficiently transported into the nucleus and aggregates predominantly in the cyto-plasm (Heinlein et al., 1994). The fusion of NLS of SV40 may interfere with the formation of aggregates and leave more free and active TPase to perform the Ds excision. Alternatively, owing a higher number of NLSs, nuclear up-take of SV40TPase may proceed more quickly and lead to higher transposition frequency.
The Ac/Ds transposon system has been widely used to create knockout mutants in many heterologous plants. In order to maximize the Ac transposition efficiencies in many species, we have developed several inducible transposon systems (Charng et al., 1995; 1997; 2000). These systems have demonstrated functionality in tobacco, tomato and rice plants. Here, a new strategy, of fusing the TPase with a putative NLS, was introduced for expanding the usage of the inducible transposon systems. All these efforts will allow us to develop more efficient transposon systems for future plant functional genomic studies.
Acknowledgments. This project was supported by the
Na-tional Science Council (Grant No. NSC 90-2313-B-002-275) of Taiwan.
Literature Cited
Becker, B., J. Shell, H. Lörz, and N. Fedoroff. 1986. Transposi-tion of the maiz3 controlling element “Activator” in tobacco. Proc. Natl. Acad. Sci. USA 83: 4844-4848.
Bevan, M. 1984. Agrobacterium vectors for plant transformation. Nucl. Acids Res. 12: 8711-8721.
Boehm, U., M. Heinlein, U. Behrens, and R. Kunze. 1995. One of three nuclear localization signals of maize Activator (Ac) transposase overlaps the DNA-binding domain. Plant J. 7: 441-451.
Charng, Y.C., C. Ma, J. Tu, and T.T. Kuo. 1997. A 200-bp con-structed inducible PR-1a promoter fusion to the Ac transposase gene drives higher transposition of a Ds ele-ment than the native PR-1a promoter fusion drives. Plant Sci. 130: 73-86.
Charng, Y.C., U.M. Pfitzner, and A.J.P. Pfitzner. 1995. Fusion of the inducible promoter of the PR-1a gene to the
Activa-tor transposase gene can trans-activate excision of a
non-autonomous transposable element by external and by internal stimuli. Plant Sci. 106: 141-155.
Charng Y.C., A.J.P. Pfitzner, U.M. Pfitzner, K.F. Charng-Chang, C.-M. Chen, J. Tu, and T.T. Kuo. 2000. Construction of an inducible transposon, INAc, to develop a gene tagging sys-tem in higher plants. Mol. Breed. 6: 353-367.
De Wet, J.R., K.V. Wood, M. DeLuca, D.R. Helinski, and S. Subramani. 1987. Firefly luciferase gene: structure and ex-pression in mammalian cells. Mol. Cell Biol. 7: 725-737. Dingwall, C. and R.A. Laskey. 1991. Nuclear targeting
se-quences—a consensus? Trends Biochem. Sci. 16: 478-481. Garcia-Bustos, J., J. Heitman, and M.N. Hall. 1991. Nuclear pro-tein localization. Biochim. Biophys. Acta. 1071: 83-101. Grevelding, C., D. Becker, R. Kunze, A. von Menges, V. Fantes,
J. Schell, and R. Masterson. 1992. High rates of Ac/Ds ger-minal transposition in Arabidopsis suitable for gene isola-tion by inserisola-tional mutagenesis. Proc. Natl. Acad. Sci. USA
89: 6085-6089.
Hall, M.N., L. Hereford, and I. Herskowitz. 1984. Targeting of
E. coli beta-galactosidase to the nucleus in yeast. Cell 36:
1057-1065.
Haring, M.A., C.M. Rommens, H.J. Nijkamp, and J. Hille. 1991. The use of transgenic plants to understand transposition mechanisms and to develop transposon tagging strategies. Plant Mol. Biol. 16: 449-461.
Heinlein, M., T. Brattig, and R. Kunze. 1994. In vivo aggrega-tion of maize Activator (Ac) transposase in nuclei of maize endosperm and Petunia protoplasts. Plant J. 5: 705-714. Hicks, G.R. and N.V. Raikhel. 1993. Specific binding of nuclear
localization sequences to plant nuclei. Plant Cell 5: 983-994.
Houba-Herin, N., D. Becker, A. Post, Y. Larondelle, and P. Starlinger. 1990. Excision of a Ds-like maize transposable element (Ac delta) in a transient assay in Petunia is enhanced by a truncated coding region of the transposable element
Ac. Mol. Gen. Genet. 224: 17-23.
Howell, S.H., D.W. Ow, and M. Schnerider. 1989. Use of the firefly luciferase gene as a reporter of gene expression in plants, In S.B. Gelvin, R.A. Schilperoort and D.P.S. Verma (eds.), Plant Molecular Biology Manual, Vol. 2, Kluwer Aca-demic Publishers, Dordrecht, pp. 1-11.
Izawa, T., C. Miyazaki, M. Yamamoto, R. Terada, and S. Iida. 1991. Introduction and transposition of the maize transpos-able element Ac in rice (Orysa sativa L.). Mol. Gen. Genet.
227: 391-396.
Jeon, J. and G. An. 2001. Gene tagging in rice: a high through-put system for functional genomics. Plant Sci. 161:
211-219.
Kalderon, D., W.D. Richardson, A.F. Markham, and A.E. Smith. 1984. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 311: 33-38.
Knapp, S., G. Coupland, H. Uhring, P. Starlinger, and F. Salamini. 1988. Transposition of the maize transposable el-ement Ac in Solanum tuberosum. Mol. Gen. Genet. 213: 285-290.
Lassner, M.W., A. Jones, S. Daubert, and L. Comai. 1991. Tar-geting of T7 RNA polymerase to tobacco nuclei mediated by an SV40 nuclear location signal. Plant Mol. Biol. 17: 229-234.
Lanford, R.E. and J.S. Butel. 1984. Construction and character-ization of an SV40 mutant defective in nuclear transport of T antigen. Cell 37: 801-813.
Li, M.G. and P. Starlinger. 1990. Mutational analysis of the N terminus of the protein of maize transposable element Ac. Proc. Natl. Acad. Sci. USA 87: 6044-6048.
Nelson, M. and P. Silver. 1989. Context affects nuclear protein localization in Saccharomyces cerevisiae. Mol. Cell. Biol.
9: 384-389.
Sambrook, J., E.F. Fritsch, and T. Maniatis (eds.). 1989. Mo-lecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Scofield, S.R., J.J. English, and J.D. Jones. 1993. High level pression of the Activator transposase gene inhibits the ex-cision of Dissociation in tobacco cotyledons. Cell 75: 507-517.
Scofield, S.R., K. Harrison, S.J. Nurrish, and J.D.G. Jones. 1992. Promoter fusions to the Activator transposase gene cause distinct patterns of Dissociation excision in tobacco cotyledons. Plant Cell 4: 573-582.
Scortecci, K.C., Y. Dessaux, A. Petit, and M.A. Van Sluys. 1997. Somatic excision of the Ac transposable element in transgenic
Arabidopsis thaliana after 5-azacytidine treatment. Plant
Cell Physiol. 38: 336-343 .
Stochaj, U. and P. Silver. 1992a. Nucleocytoplasmic traffic of proteins. Eur. J. Cell Biol. 59: 1-11.
Stochaj, U. and P. Silver. 1992b. A conserved phosphoprotein that specifically binds nuclear localization sequences is in-volved in nuclear import. J. Cell Biol. 117: 473-482. Van der Krol, A.R. and N.H. Chua. 1991. The basic domain of
plant B-ZIP proteins facilitates import of a reporter pro-tein into plant nuclei. Plant Cell. 3: 667-675.
Van Sluys, M.A., J. Tempé, and N. Fedoroff. 1987. Studies on the introduction and mobility of the maize Activator ele-ment in Arabidopsis thaliana and Daucus carota. EMBO J. 6: 3881-3889.
Wagner, P. and M.N. Hall. 1993. Nuclear protein transport is functionally conserved between yeast and higher eukaryotes. FEBS Lett. 321: 261-266.
Wang, H.R. 1998. Study on the Nuclear Import of Proteasomes. Thesis, Ludwig-Maximilains-University, Munich, Germany. Yoder, J.I., J. Palys, K. Alpert, and M. Lassner. 1988. Ac trans-position in transgenic tomato plants. Mol. Gen. Genet. 213: 291-296.