0095-1137/09/$08.00⫹0 doi:10.1128/JCM.00853-09
Copyright © 2009, American Society for Microbiology. All Rights Reserved.
Prevalence of and Risk Factors for Colonization by Methicillin-Resistant
Staphylococcus aureus among Adults in Community Settings in Taiwan
䌤
Jann-Tay Wang,
1Chun-Hsing Liao,
2Chi-Tai Fang,
1Wei-Chu Chie,
3Mei-Shu Lai,
3Tsai-Ling Lauderdale,
4Wen-Sen Lee,
5Jeng-Hua Huang,
6and Shan-Chwen Chang
1,7*
Department of Internal Medicine, National Taiwan University Hospital, Taipei 100, Taiwan
1; Department of Internal Medicine,
Far Eastern Memorial Hospital, Taipei County 220, Taiwan
2; Graduate Institute of Preventive Medicine, College of Public Health,
3and Graduate Institute of Clinical Pharmacy, College of Medicine,
7National Taiwan University, Taipei 100, Taiwan;
Division of Clinical Research, National Health Research Institute, Zhunan 350, Taiwan
4; Department of
Internal Medicine, Wan Fang Hospital, Taipei 100, Taiwan
5; and Department of Internal Medicine,
Taipei Cathay General Hospital, Taipei 100, Taiwan
6Received 28 April 2009/Returned for modification 13 June 2009/Accepted 15 July 2009
In order to determine the prevalence of methicillin (meticillin)-resistant
Staphylococcus aureus (MRSA)
colonization among adults in community settings in Taiwan and identify its risk factors, we conducted the
present study. For a 3-month period, we enrolled all adults who attended mandatory health examinations at
three medical centers and signed the informed consent. Nasal swabs were taken for the isolation of
S. aureus.
For each MRSA isolate, we performed multilocus sequence typing, identification of the staphylococcal cassette
chromosome
mec, tests for the presence of the Panton-Valentine leukocidin gene, and tests for drug
suscep-tibilities. Risk factors for MRSA colonization were determined. The results indicated that the MRSA
coloni-zation rate among adults in the community settings in Taiwan was 3.8% (119/3,098). Most MRSA isolates
belonged to sequence type 59 (84.0%). Independent risk factors for MRSA colonization included the presence
of household members less than 7 years old (
P < 0.0001) and the use of antibiotics within the past year (P ⴝ
0.0031). Smoking appeared to be protective against MRSA colonization (
P < 0.0001).
Before the late 1990s, nearly all methicillin
(meticillin)-re-sistant Staphylococcus aureus (MRSA) infections occurred in
patients with specific risk factors who were in health care
facilities (31). However, the emergence of MRSA infections
among previously healthy persons in community settings
(with-out exposure to health care facilities) was noted thereafter (6,
31). Therefore, MRSA infections are now classified as
health care-associated MRSA (HA-MRSA) infections and
community-associated MRSA (CA-MRSA) infections (38).
Strains responsible for CA-MRSA infections differ from
those for HA-MRSA infections in several phenotypic and
ge-netic features (1, 28). CA-MRSA strains carry type IV or V
staphylococcal cassette chromosome mec (SCCmec) elements,
are usually Panton-Valentine leukocidin (PVL) producing, and
are not multidrug resistant; HA-MRSA strains carry type I, II,
or III SCCmec elements, are usually not PVL producing, and
are multidrug resistant (15, 22, 28).
Initially, CA-MRSA infections were mostly reported in
young children (36). However, as CA-MRSA infections
be-came more common, infections were reported among people
of all ages and contributed to the increase of
community-associated S. aureus infections with significance (25, 29, 36).
MRSA colonization is an important risk factor for subsequent
MRSA infection (30), so several studies in the United States
have characterized the MRSA colonization rate in a
commu-nity setting (13, 16). These studies demonstrated that the nasal
colonization rates among healthy children increased from
0.8% in 2001 to 9.2% in 2004 (13). The colonization rate was
0.84% among people participating in the 2001 to 2002 National
Health and Nutrition Examination Survey (NHANES) (16).
In Taiwan, MRSA strains of sequence type 59 (ST59),
de-termined by multilocus sequence typing (MLST) and carrying
type IV or V SCCmec elements, were recently found to be the
major strains of CA-MRSA (5, 7, 27). Other studies
demon-strated that these CA-MRSA strains were responsible for the
rapid increase in the number of CA-MRSA infections among
children and adults in Taiwan (7, 37). The MRSA colonization
rates among Taiwanese children increased from 1.5% from
2001 to 2002 to 7.2% from 2005 to 2006 (18, 19). However, the
MRSA colonization rate among adults in community settings
in Taiwan is unclear. This study was conducted to determine
the prevalence and risk factors for the colonization of MRSA
among adults in community settings in Taiwan.
MATERIALS AND METHODS
Study population. From 1 October 2007 to 31 December 2007, all adults (ages, ⬎18 years) who attended mandatory health examinations (as a part of the workplace health promotion program) at three medical centers located in north-ern Taiwan and signed the informed consent were enrolled in this study. Three well-trained study assistants took a nasal swab from each enrolled person. The swabs were sent to the central laboratory located at National Taiwan University Hospital (a major teaching hospital in Taiwan with a total capacity of 2,200 beds) and were cultured within 6 h. When an enrolled person was found to be a MRSA carrier, his or her household members were invited to participate in the study. After the informed consent was signed, nasal swabs from these household mem-bers were also taken and sent to be cultured. This study has been approved by the institute review boards of the three medical centers.
Bacterial culture and identification of MRSA. Each swab was plated onto a sheep blood agar plate. All plates were incubated at 35°C ambient air for 48 h. Isolates suspected of being S. aureus from sheep blood agar were first checked by
* Corresponding author. Mailing address: Department of Internal
Medicine, National Taiwan University Hospital, No. 7 Chung-Shan
South Road, Taipei 100, Taiwan. Phone: 886-2-23123456, ext. 5401.
Fax: 886-2-23958721. E-mail: changsc@ntu.edu.tw.
䌤
Published ahead of print on 22 July 2009.
catalase and Gram stain if deemed necessary, and all S. aureus isolates were confirmed by coagulase latex agglutination. S. aureus isolates were spotted onto ChromAgar MRSA to check for methicillin resistance. All isolates were pre-served.
Drug susceptibility tests. The MICs of all MRSA isolates were determined for gentamicin, clindamycin, erythromycin, ciprofloxacin, minocycline, rifampin (ri-fampicin), trimethoprim-sulfamethoxazole, and vancomycin using the agar dilu-tion method proposed by the Clinical and Laboratory Standards Institute (CLSI) (10). In brief, a Steers’ replicator was used to apply 104CFU of bacteria onto Mueller-Hinton agar containing serial twofold dilutions of each antimicrobial agent (256 to 0.03 mg/liter). The agar plates were incubated at 35°C for 18 h before reading. The MIC was defined as the lowest concentration of antimicro-bial agents completely inhibiting the growth of bacteria. S. aureus ATCC 25923 was used as the internal control in each run of the test. The breakpoints used to determine susceptibility were as defined by the CLSI (11).
Molecular typing and detection of the PVL gene. Chromosomal DNA was prepared as described previously (17). The presence of the PVL gene lukF-lukS was determined by PCR with the use of a primer as described elsewhere (26). Typing of the SCCmec elements (I to V) and the mecA gene was performed by methods described by Ito et al. (22, 23). MLST was performed as described by Enright et al. (14).
Data collection. A standardized questionnaire was used to collect information on the risk factors for CA-MRSA colonization. The data collected were age; sex; educational degree; marital status; whether the subject was living in a dormitory or not; the number of household members; the presence of any household member who was a health care worker; the presence of any household member who was less than 7 years old; the presence of any household member who was bedridden; the presence of chronic diseases; smoking habits; hospitalizations within the previous year; a history of caring for inpatients within the past year; outpatient clinic visits within the past year; the use of antibiotics within the previous year; tattoos, acupuncture treatments, parenteral drug use, and/or di-alysis treatments within the previous year; a history of skin and soft-tissue infection within the previous year; whether the subject takes a shower every day; a history of visiting public places (e.g., hot-spring baths, swimming pools, sauna baths, gymnasiums, and dancing saloons) within the previous year; and economic status.
Statistics. Continuous variables were given as means⫾ standard deviations and compared using Student’s t test. The categorical variables were compared with a chi-square test or Fisher’s exact test if the expected values were below 5. The prevalence of MRSA colonization was determined. To analyze the risk factors for carrying MRSA, we used polytomous logistic regression to compare people with MRSA to those without S. aureus and people with MRSA to those with methicillin-susceptible S. aureus (MSSA). All parameters were initially tested by univariate analysis; those with a P value of⬍0.05 and those being biologically meaningful were used for the multivariate analysis. However, pa-rameters with colinearity, tested by correlation matrices, were not simultaneously considered in the final model. In the multivariate analysis, stepwise model com-parison was used to determine the best model. Statistical analyses were per-formed using SAS 9.1.3 (SAS Institute, Inc., Cary, NC). All tests were two-tailed, and a P value of⬍0.05 was considered statistically significant.
RESULTS
During the 3-month study period, there were 3,098 people
enrolled. Among them, 686 people were found to carry S.
aureus. A total of 119 of these 686 people carried MRSA and
567 had MSSA. The comparisons of demographics and other
parameters of the enrolled people are shown in Table 1. There
were statistically significant differences between these three
groups in the parameters of sex, educational degree, the
pres-ence of any household member who was a health care worker,
the presence of any household member less than 7 years old,
smoking habits, and the use of antibiotics within the past year.
Based on a post-hoc analysis, we found that people with
MRSA (i) tended to have less education than those with MSSA
or without S. aureus colonization (P
⫽ 0.0875 and 0.0650,
respectively), (ii) were more likely to have household members
who were less than 7 years old than the other two groups (both
P
⬍ 0.0001), (iii) were less likely to be smokers than those
without S. aureus colonization (P
⫽ 0.0077), and (iv) were
more likely to have used antibiotics during the past year than
the two other groups (P
⫽ 0.0012 and 0.0004, respectively).
Among the 119 MRSA isolates from the 119 people
(hence-forth the “index people”), 100 were classified as ST59, 11 as
ST508, 5 as ST89, 2 as ST239, and 1 as ST6. Of the 100 isolates
of ST59, 65 carried the type IV SCCmec element (ST59-IV)
and 35 carried the type V SCCmec element (ST59-V). Of the
65 ST59-IV MRSA isolates, only 10 (15.4%) were positive for
the PVL gene. All 35 of the ST59-V isolates were positive for
the PVL gene. All isolates of ST6 and ST508 carried the type
IV SCCmec element, all isolates of ST89 carried the type II
SCCmec element, and both isolates of ST239 carried the type
III SCCmec element (Table 2). All isolates, except two of
ST508, that belonged to ST6, ST89, ST239, and ST508 were
negative for the PVL gene. The overall prevalence of MRSA
was 3.8% (119/3,098; 95% confidence interval, 3.1% to 4.5%).
However, when those isolates carrying type IV and V SCCmec
elements were taken into consideration as CA-MRSA strains,
the prevalence of CA-MRSA carriage among healthy adults in
Taiwan was found to be 3.6% (112/3,098; 95% confidence
interval, 2.9% to 4.3%).
We also screened household members of 70 of the 119 index
people. In total, there were 242 household members screened.
Among these 242 people, 64 people (47 adults and 17 children)
from 39 families carried MRSA. Of these 64 MRSA isolates,
47 were classified as ST59, 11 as ST508, 2 as ST30, 2 as ST89,
1 as ST182, and 1 as ST342. Of the 47 isolates of ST59, 31
carried the type IV SCCmec element and the other 16 carried
the type V SCCmec element. Of the 31 ST59-IV MRSA
iso-lates from household members, 11 (35.5%) were positive for
the PVL gene. All 16 ST59-V isolates from household
mem-bers were positive for the PVL gene. All isolates of ST30,
ST182, ST342, and ST508 carried the type IV SCCmec
ele-ment, and both isolates of ST89 carried the type II SCCmec
element (Table 2). Of the 11 ST508-IV MRSA isolates from
household members, one (9.1%) was positive for the PVL
gene. All isolates of ST30 and ST182 were positive for the PVL
gene. None of the ST89 and ST342 isolates was positive for the
PVL gene.
A comparison of genotypes of the MRSA isolates from
household members and their associated index people
indi-cated that there were 16 (41.0%) families in which the MRSA
isolates from all household members and index people
be-longed to the same genotypes (same results from MLST
typ-ing, same SCCmec element, and identical presence/absence of
the PVL gene). There were six (15.4%) families in which
MRSA isolates from some (but not all) household members
were of the same genotypes as those of the index people. There
were five (12.8%) families in which MRSA isolates from
household members were of the same MLST type and the
same types of SCCmec elements as those of the index people
but different in the presence/absence of the PVL gene. There
were four (10.3%) families in which MRSA isolates from
household members were of the same MLST type as those of
the index people but different in the types of SCCmec elements
(despite the presence/absence of the PVL gene). And there
were eight (20.5%) families in which MRSA isolates from
household members differed from those of the index people in
MLST type.
TABLE 1. Characteristics of people with MRSA, MSSA, and no S. aureus colonization (n
⫽ 3,098)
aParameter
No. (%) of people colonized with:
P value MRSA (n⫽ 119) (nMSSA⫽ 567) (nNo_C⫽ 2,412) Age (mean⫾ SD) 38.1⫾ 12.7 39.5⫾ 11.9 39.9⫾ 11.6 0.2513 Sexb 0.0186 Male 50 (42.0) 275 (48.8) 1014 (42.3) Female 69 (58.0) 288 (51.2) 1381 (57.7) Educationb
Under elementary school 3 (2.6) 3 (0.5) 22 (0.9) 0.0278
Elementary school 8 (6.8) 19 (3.4) 68 (2.9)
Junior high school 4 (3.4) 11 (2.0) 78 (3.3)
Senior high school 18 (15.4) 74 (13.3) 376 (15.9)
University 59 (50.4) 324 (58.2) 1380 (58.4) Graduate or beyond 25 (21.4) 126 (22.6) 437 (18.5) Status of marriageb Married 87 (75.7) 359 (65.5) 1568 (66.9) 0.2589 Divorced 1 (0.9) 11 (2.0) 56 (2.4) Unmarried 27 (23.5) 178 (32.5) 721 (30.7) Working as a HCWb 0.1894 Yes 13 (13.3) 40 (8.1) 212 (10.3) No 85 (86.7) 453 (91.9) 1844 (89.7) Living in a dormitoryb 0.7715 Yes 1 (1.2) 10 (2.0) 37 (2.2) No 85 (98.8) 406 (98.0) 1674 (97.8)
No. of household members (mean⫾ SD) 3.0⫾ 1.6 3.7⫾ 1.5 3.7⫾ 1.6 0.0761
Presence of household members who are HCWsb 0.0085
Yes 10 (8.7) 54 (9.7) 144 (6.1)
No 105 (91.3) 504 (90.3) 2209 (93.9)
Presence of household members less than 7 yr oldb ⬍0.0001
Yes 52 (44.8) 121 (21.7) 610 (25.7)
No 64 (55.2) 437 (78.3) 1759 (74.3)
Presence of household members who are bedriddenb 0.7468
Yes 4 (3.4) 18 (3.2) 64 (2.7) No 113 (96.6) 541 (96.8) 2299 (97.3) Chronically illb 0.4702 Yes 42 (36.5) 180 (32.7) 741 (31.5) No 73 (63.5) 370 (67.3) 1614 (68.5) Smoking habitsb ⬍0.0001 Yes 13 (11.0) 76 (13.5) 505 (21.2) No 105 (89.0) 485 (86.5) 1876 (78.8) Hospitalizationb 0.3568 Yes 9 (7.6) 25 (4.5) 128 (5.4) No 109 (92.4) 534 (95.5) 2256 (94.6)
Caring for an inpatientb 0.6462
Yes 22 (19.3) 90 (16.2) 416 (17.4)
No 92 (80.7) 465 (83.8) 1952 (82.6)
Visiting outpatient clinicsb 0.5940
Yes 78 (67.8) 364 (64.8) 1587 (66.9)
No 37 (32.2) 198 (35.2) 784 (33.1)
Using antibioticsb 0.0016
Yes 35 (30.1) 95 (17.1) 409 (17.2)
No 81 (69.9) 461 (82.9) 1964 (82.8)
Tattoo or acupuncture or using parenteral drug or dialysisb 0.7917
Yes 2 (1.7) 16 (2.8) 64 (2.7)
No 114 (98.3) 546 (97.2) 2310 (97.3)
Skin or soft-tissue injuryb 0.8420
Yes 55 (47.8) 282 (50.8) 1186 (50.4)
No 60 (52.2) 273 (49.2) 1165 (49.6)
Showering every dayb 0.3498
Yes 112 (96.6) 539 (96.3) 2315 (97.4)
No 4 (3.4) 21 (3.7) 62 (2.6)
Visiting public amusement placesb 0.1761
Yes 67 (57.3) 322 (57.5) 1454 (61.4) No 50 (42.7) 238 (42.5) 913 (38.6) Family income (NTD)b Less than 20,000 1 (2.4) 1 (0.6) 17 (2.5) 0.4533 20,000-50,000 3 (7.1) 28 (16.3) 108 (15.8) 50,000-100,000 20 (47.6) 64 (37.2) 244 (35.7) 100,000-200,000 8 (19.0) 38 (22.1) 123 (18.0) 200,000-300,000 3 (7.1) 5 (2.9) 31 (4.5) Over 300,000 7 (16.7) 36 (20.9) 160 (23.4)
aNo_C, no S. aureus colonization; SD, standard deviation; M, male; F, female; HCWs, health care workers; NTD, new Taiwan dollar.
bThere are missing data for some parameters, including the sex category (21 people), education (63), working as a HCW (95), living in a dormitory (885),
presence of household members who are HCWs (72), presence of household members under 7 years old (55), presence of household members who are bedridden (59), chronically ill (78), smoking habits (38), hospitalization (37), caring for inpatients (61), visiting outpatient clinics (50), using antibiotics (53), tattoo or acupuncture or using parenteral drug or dialysis (48), skin or soft-tissue injury (77), showering every day (47), visiting public amusement places (54), and family income (2,201).
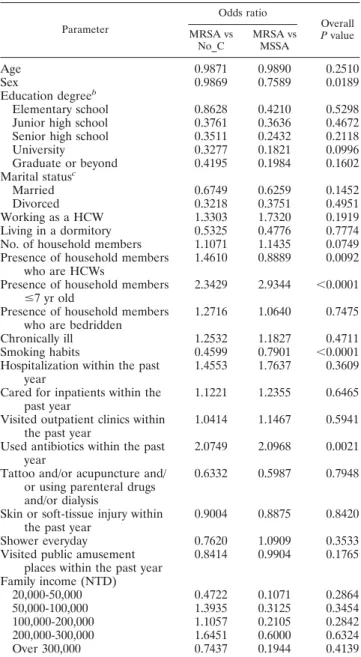
We used polytomous logistic regression to identify risk
fac-tors for MRSA colonization by comparing people with MRSA
to those with MSSA and people with MRSA to those without
carriage of S. aureus. Univariate analysis indicated that the
female gender, the presence of health care workers in the
household, the presence of household members less than 7
years old, being a nonsmoker, and the use of antibiotics during
the past year were risk factors for MRSA colonization (Table
3). Using a multivariate analysis, the presence of household
members less than 7 years old, being a nonsmoker, and the use
of antibiotics during the past year were independent risk
fac-tors for MRSA colonization compared to those without
car-riage of S. aureus. However, the presence of household members
less than 7 years old and the use of antibiotics during the past year
were the only two independent risk factors for MRSA
coloniza-tion compared to those for carriage of MSSA (Table 4).
Table 5 shows the drug susceptibilities of all 183 MRSA
isolates (from the index people and their families) stratified by
MLST types. The overall susceptibilities were 25.1% for
clin-damycin, 16.9% for erythromycin, 99.5% for
trimethoprim-sulfamethoxazole, 78.1% for gentamicin, 99.5% for
minocy-cline, 98.9% for ciprofloxacin, 100% for rifampin, and 100%
for vancomycin.
DISCUSSION
Several reports from the United States indicated that
com-munity-associated S. aureus infections have increased rapidly
in recent years and that MRSA (not MSSA) accounts for most
of this increase (24, 29). Studies from Taiwan have
demon-strated similar findings among children and adults (7, 37).
Therefore, it is increasingly important to characterize the
MRSA colonization pool among people in communities. The
prevalence of MRSA colonization among children in
commu-nities has been extensively studied in Taiwan and the United
States (5, 13, 18, 19, 21, 32–34), but there are only a few studies
of MRSA colonization among adults in communities (16, 39).
Our study showed that the MRSA colonization rate among
adults in community settings in Taiwan who attended
manda-tory health examinations as a part of workplace health
promo-tion was 3.8% (95% confidence interval, 3.1% to 4.5%).
A previous population-based study showed that the MRSA
colonization rate among people attending the 2001 to 2002
NHANES was 0.84% (16). A study in The Netherlands from
1999 to 2000 indicated that the MRSA colonization rate
among the general Dutch population was 0.03% (39). The
MRSA colonization rate in this study was about 5- to 10-fold
higher than reported in these prior studies. There may be
several reasons for this difference. First, the colonization by
MRSA among adults in communities may be more prevalent in
Taiwan than in the United States and The Netherlands.
Sec-ond, our study was conducted 5 to 7 years after those studies,
so the difference may be due to an overall increase of MRSA
TABLE 2. MLST types and SCCmec elements in the 183 MRSA
isolates (119 index people and 64 household members)
MLST type
No. of isolates for indicated type of SCCmec element
Index people Household members
II III IV V Subtotal II III IV V Subtotal
ST6
0
0
1
0
1
0
ST30
0
0
0
2
0
2
ST59
0
0
65
35
100
0
0
31
16
47
ST89
5
0
0
0
5
2
0
0
0
2
ST182
0
0
0
1
0
1
ST239
0
2
0
0
2
0
ST342
0
0
0
1
0
1
ST508
0
0
11
0
11
0
0
11
0
11
Total
5
2
77
35
119
2
0
46
16
64
TABLE 3. Risk factors for people colonized with MRSA compared
to those colonized with MSSA and those not colonized with
S. aureus by univariate analysis using polytomous
logistic regression
a Parameter Odds ratio Overall P value MRSA vs No_C MRSA vs MSSAAge
0.9871
0.9890
0.2510
Sex
0.9869
0.7589
0.0189
Education degree
bElementary school
0.8628
0.4210
0.5298
Junior high school
0.3761
0.3636
0.4672
Senior high school
0.3511
0.2432
0.2118
University
0.3277
0.1821
0.0996
Graduate or beyond
0.4195
0.1984
0.1602
Marital status
cMarried
0.6749
0.6259
0.1452
Divorced
0.3218
0.3751
0.4951
Working as a HCW
1.3303
1.7320
0.1919
Living in a dormitory
0.5325
0.4776
0.7774
No. of household members
1.1071
1.1435
0.0749
Presence of household members
who are HCWs
1.4610
0.8889
0.0092
Presence of household members
ⱕ7 yr old
2.3429
2.9344
⬍0.0001
Presence of household members
who are bedridden
1.2716
1.0640
0.7475
Chronically ill
1.2532
1.1827
0.4711
Smoking habits
0.4599
0.7901
⬍0.0001
Hospitalization within the past
year
1.4553
1.7637
0.3609
Cared for inpatients within the
past year
1.1221
1.2355
0.6465
Visited outpatient clinics within
the past year
1.0414
1.1467
0.5941
Used antibiotics within the past
year
2.0749
2.0968
0.0021
Tattoo and/or acupuncture and/
or using parenteral drugs
and/or dialysis
0.6332
0.5987
0.7948
Skin or soft-tissue injury within
the past year
0.9004
0.8875
0.8420
Shower everyday
0.7620
1.0909
0.3533
Visited public amusement
places within the past year
0.8414
0.9904
0.1765
Family income (NTD)
20,000-50,000
0.4722
0.1071
0.2864
50,000-100,000
1.3935
0.3125
0.3454
100,000-200,000
1.1057
0.2105
0.2842
200,000-300,000
1.6451
0.6000
0.6324
Over 300,000
0.7437
0.1944
0.4139
aNo_C, no S. aureus colonization; HCWs, health care workers; NTD, new
Taiwan dollar.
bUsing the under-elementary-school category result as the baseline. cUsing the unmarried category result as the baseline.
during this time. Several previous studies have demonstrated
that the MRSA colonization rate of people in communities has
increased over time (5, 13). However, we also understand that
only adults who attended mandatory health examinations as a
part of a workplace health promotion program were enrolled
in our study and thus may not be representative of the adult
populations in communities. Since these attendees are
presum-ably healthier than average, our results may be biased by the
healthy worker effect (2).
Our molecular analysis indicated that most of the MRSA
isolates (112/119) from the index people carried the type IV or
type V SCCmec element, as is typical for CA-MRSA strains
(15, 22, 23, 28). Therefore, the colonization rate of CA-MRSA
strains was 3.6% in this study. In this study, ST59 isolates were
the most common MLST type of isolates. Previous studies
from Taiwan have found that ST59 MRSA isolates were the
most common MLST type of MRSA causing CA-MRSA
in-fections in different geographic areas all over Taiwan (8).
Stud-ies concerning the MRSA colonization in Taiwanese children
also found ST59 is the predominant type among MRSA
iso-lates from child carriers in communities all over Taiwan (7, 18).
Our study adds additional information about the MRSA
car-rier rate and bacterial typing in adults in community settings in
Taiwan. However, ST59 MRSA isolates were rarely found in
other Asian countries according to the findings from a recent
large-scale study (9).
Our molecular analysis that compared MRSA isolates from
the index people and their associated households identified
numerous instances where the genotypes were different. This
strongly suggests that, in addition to household transmission
(20), the spread of MRSA in community settings occurred via
some other routes, such as sport contact, the use of saunas,
exposure to a colonized animal, and so on (3, 4, 12, 40).
We used polytomous logistic regression to identify risk
fac-tors for MRSA colonization by comparing people with MRSA
colonization to those with MSSA colonization and people with
MRSA colonization to those without carriage of S. aureus at
the same time. This allowed us to avoid problems associated
with multiple intergroup comparisons. Studies that reported
the determinants of MRSA colonization in community settings
remained limited (16). Our multivariate analysis indicated that
the presence of household members less than 7 years old, being
a nonsmoker, and the use of antibiotics within the past year
were the independent risk factors for MRSA colonization
com-pared to those without S. aureus colonization. The presence of
household members less than 7 years old and the use of
anti-biotics within the past year were the only two independent risk
factors for MRSA colonization compared to those for MSSA
colonization.
A previous study showed that the MRSA colonization rate
of children in community settings in Taiwan was 7.2% from
2005 to 2006 (18), much higher than the adult colonization rate
(3.8%) in the present study. In addition, among our 17
pedi-atric household members who had MRSA, 12 carried MRSA
of the same genotype as the associated index person. The
hypothesis that transmission from children to their parents
through close household contact might play an important role
in MRSA colonization among adults is worthy of further study.
We also found that the use of antibiotics was associated with
the presence of MRSA. This was expected, because antibiotics
provide selective pressure and thus facilitated the colonization
of drug-resistant pathogens such as MRSA.
TABLE 4. Risk factors for MRSA colonization compared to MSSA colonization and no S. aureus colonization by multivariate analysis using
polytomous logistic regression
Risk factor
MRSA vs No_Ca MRSA vs MSSA
P value of overall model Odds ratio (95% confidence interval) P value of coefficient Odds ratio (95% confidence interval) P value of coefficient
Presence of household members
aged under 7
2.2387 (1.5255–3.2853)
⬍0.0001
2.9110 (1.9048–4.4488)
⬍0.0001
⬍0.0001
Smoking habits
0.4419 (0.2383–0.8195)
0.0096
0.9582 (0.4946–1.8563)
0.8994
⬍0.0001
Using antibiotics within the past year
2.0530 (1.3544–3.1118)
0.0007
2.0322 (1.2826–3.2198)
0.0025
0.0031
aNo_C, no S. aureus colonization.
TABLE 5. Drug susceptibilities of the 183 MRSA isolates with stratification by MLST type
MLST type (no. of isolates)
% Susceptibility for indicated druga
CM ERM TXT GM MIN CIP RIF VAN
ST6 (1)
100
100
100
100
100
100
100
100
ST30 (2)
50
50
100
100
100
100
100
100
ST59 (147)
14.3
11.6
99.3
74.1
99.3
100
100
100
ST89 (7)
0
0
100
100
100
85.7
100
100
ST182 (1)
100
0
100
100
100
100
100
100
ST239 (2)
50
0
100
50
100
50
100
100
ST342 (1)
100
0
100
100
100
100
100
100
ST508 (22)
90.9
54.5
100
95.4
100
100
100
100
Overall (183)
25.1
16.9
99.5
78.1
99.5
98.9
100
100
aCM, clindamycin; ERM, erythromycin; TXT, trimethoprim-sulfamethoxazole; GM, gentamicin; MIN, minocycline; CIP, ciprofloxacin; RIF, rifampin; VAN,
Surprisingly, in a comparison of people with MRSA and
those without S. aureus colonization, we found that smoking
was a protective factor against MRSA colonization. However,
a comparison of people with MRSA and those with MSSA
found that smoking was not such a factor. In reanalyzing our
data, we found that smoking was also an independent
protec-tive factor against MSSA and S. aureus (pooling MRSA and
MSSA together) colonization compared to those without S.
aureus colonization (odds ratio, 0.4612 and 0.4570,
respec-tively; 95% confidence interval, 0.3480 to 0.6111 and 0.3520 to
0.5940, respectively; P
⬍ 0.0001 and 0.0001, respectively).
Therefore, it seems that smoking is a protective factor against
S. aureus, not only specifically against MRSA, colonization. To
our best knowledge, only a review article described the similar
findings based on the results from a Ph.D. thesis (35). Our
study therefore provides the important evidence that smoking
might be a protective factor against the nasal colonization of S.
aureus. It might be that smoking creates a microenvironment in
the nose that protects against the growth of S. aureus. Clearly,
the effect of smoking on S. aureus colonization requires further
study.
The results of our drug susceptibility tests showed that more
than 95% of the isolates were susceptible to
trimethoprim-sulfamethoxazole, minocycline, and ciprofloxacin; that all
iso-lates were susceptible to rifampin and vancomycin; and that
most isolates were resistant to clindamycin and erythromycin.
These results differ from those reported from the United
States, where the rate of susceptibility to clindamycin of
MRSA isolates causing CA-MRSA infection was as high as
95% (29). This may be due to the predominance of different
strains in these different geographic regions.
In conclusion, the present study showed that the rate is
3.8%. Most (94.1%) of these MRSA isolates in the present
study had typical characteristics of CA-MRSA. Our study also
identifies that the presence of household members less than 7
years old as well as the use of antibiotics within the past year
were the independent risk factors for MRSA colonization, and
smoking appeared to be a protective factor against MRSA
colonization. These findings could be helpful for controlling
the spread of MRSA in community settings.
ACKNOWLEDGMENT
This study has been supported by the Center for Disease Control,
Taiwan (E9638).
REFERENCES
1. Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827.
2. Baillargeon, J. 2001. Characteristics of the healthy worker effect. Occup. Med. (London) 16:359–366.
3. Benjamin, H. J., V. Nikore, and J. Takagishi. 2007. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin. J. Sport Med. 17:393–397. 4. Boucher, H. W., and G. R. Corey. 2008. Epidemiology of methicillin-resistant
Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S344–S349.
5. Boyle-Vavra, S., B. Ereshefsky, C.-C. Wang, and R. S. Daum. 2005. Success-ful multiresistant community-associated methicillin-resistant Staphylococcus
aureus lineage from Taipei, Taiwan, that carries either the novel
staphylo-coccal chromosome cassette mec (SCCmec) type VTor SCCmec type IV. J. Clin. Microbiol. 43:4719–4730.
6. Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Min-nesota and North Dakota, 1997–1999. MMWR Morb. Mortal. Wkly. Rep. 48:707–710.
7. Chen, C. J., L. H. Su, C. H. Chiu, L. Y. Lin, K. S. Wong, Y. Y. Chen, and Y. C. Huang. 2007. Clinical features and molecular characteristics of invasive community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Diagn. Microbiol. Infect. Dis. 59:287–293.
8. Chen, F. J., T. L. Lauderdale, I. W. Huang, H. J. Lo, J. F. Lai, H. Y. Wang, Y. R. Shiau, P. C. Chen, T. Ito, and K. Hiramatsu. 2005. Methicillin-resistant
Staphylococcus aureus in Taiwan. Emerg. Infect. Dis. 11:1761–1763.
9. Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tien-sasitorn, M. Jamklang, T. Chavalit, J. H. Song, and K. Hiramatsu. 2006. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 50:1001–1012.
10. Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA. 11. Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA. 12. Coronado, F., J. A. Nicholas, B. J. Wallace, D. J. Kohlerschmidt, K. Musser,
D. J. Schoonmaker-Bopp, S. M. Zimmerman, A. R. Boller, D. B. Jernigan, and M. A. Kacica. 2007. Community-associated methicillin-resistant
Staph-ylococcus aureus skin infections in a religious community. Epidemiol. Infect.
135:492–501.
13. Creech, C. B., D. S. Kernodle, A. Alsentzer, C. Wilson, and K. M. Edwards. 2005. Increasing rates of nasal carriage of methicillin-resistant
Staphylococ-cus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617–621.
14. Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible Staphylococcus aureus. J. Clin. Microbiol. 38:1008– 1015.
15. Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Pie´mont, N. Brousse, D. Floret, and J. Etienne. 2002. Asso-ciation between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotizing pneumonia in young im-munocompetent patients. Lancet 359:753–759.
16. Graham, P. L., III, S. X. Lin, and E. L. Larson. 2006. A U.S. population-based survey of Staphylococcus aureus colonization. Ann. Intern. Med. 144: 318–325.
17. Hiramatsu, K., H. Kihara, and T. Yokota. 1992. Analysis of borderline-resistant strains of methicillin-borderline-resistant Staphylococcus aureus using polymer-ase chain reaction. Microbiol. Immunol. 36:445–453.
18. Huang, Y. C., K. P. Hwang, P. Y. Chen, C. J. Chen, and T. Y. Lin. 2007. Prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among Taiwanese children in 2005 and 2006. J. Clin. Microbiol. 45:3992– 3995.
19. Huang, Y. C., L. H. Su, C. J. Chen, and T. Y. Lin. 2005. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without iden-tifiable risk factors in northern Taiwan. Pediatr. Infect. Dis. J. 24:276–278. 20. Huijsdens, X. W., M. G. van Santen-Verheuvel, E. Spalburg, M. E. Heck, G. N. Pluister, B. A. Eijkelkamp, A. J. de Neeling, and W. J. Wannet. 2006. Multiple cases of familial transmission of community-acquired methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 44:2994–2996. 21. Hussain, F. M., S. Boyle-Vavra, C. D. Bethel, and R. S. Daum. 2001.
Com-munity-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr. Infect. Dis. J. 20:763–767.
22. Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of the three types of staph-ylococcal cassette chromosome mec integrated in the chromosome in me-thicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45: 1323–1336.
23. Ito, T., X. X. Ma, F. Takeuchi, K. Okuma, H. Yuzawa, and K. Hiramatsu. 2004. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Che-mother. 48:2637–2651.
24. Kaplan, S. L., K. G. Hulten, B. E. Gonzalez, W. A. Hammerman, L. Lam-berth, and J. Versalovic. 2005. Three-year surveillance of community-ac-quired Staphylococcus aureus infections in children. Clin. Infect. Dis. 40: 1785–1791.
25. Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, S. K. Fridkin, and Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771.
26. Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leu-kocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132.
Siu. 2005. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J. Clin. Microbiol. 43:132–139. 28. Ma, X. X., T. Ito, C. Tiensasitorn, M. Jamklang, P. Chongtrakool, S.
Boyle-Vavra, R. S. Daum, and K. Hiramatsu. 2002. Novel type of staph-ylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Che-mother. 46:1147–1152.
29. Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDou-gal, R. B. Carey, D. A. Talan, and EMERGEncy ID Net Study Group. 2006. Methicillin-resistant Staphylococcus aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674.
30. Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, H. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosoco-mial transmission of multidrug-resistant strains of Staphylococcus aureus and
Enterococcus. Infect. Control Hosp. Epidemiol. 24:362–386.
31. Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O’Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infections. JAMA 290: 2976–2984.
32. Nakamura, M. M., K. L. Rohling, M. Shashaty, H. Lu, Y. W. Tang, and K. M. Edwards. 2002. Prevalence of methicillin-resistant Staphylococcus
aureus nasal carriage in the community pediatric population. Pediatr. Infect.
Dis. J. 21:917–922.
33. Shopsin, B., B. Mathema, J. Martinez, E. Ha, M. L. Campo, A. Fierman, K. Krasinski, J. Kornblum, P. Alcabes, M. Waddington, M. Riehman, and B. N. Kreiswirth. 2000. Prevalence of methicillin-resistant and methicillin-suscep-tible Staphylococcus aureus in the community. J. Infect. Dis. 182:359–362. 34. Suggs, A. H., M. C. Maranan, S. Boyle-Vavra, and R. S. Daum. 1999.
Methicillin-resistant and borderline methicillin-resistant asymptomatic
Staphylococcus aureus colonization in children without identified risk factors.
Pediatr. Infect. Dis. J. 18:410–414.
35. van Belkum, A., D. C. Melles, J. Nouwen, W. B. van Leeuwen, W. van Wamel, M. C. Vos, H. F. Wertheim, and H. A. Verbrugh. 2009. Co-evolutionary aspects of human colonization and infection by Staphylococcus aureus. Infect. Genet. Evol. 9:32–47.
36. Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffer-nan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978–984.
37. Wang, J. L., S. Y. Chen, J. T. Wang, G. H. M. Wu, W. C. Chiang, P. R. Hsueh, Y. C. Chen, and S. C. Chang. 2008. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired me-thicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin. Infect. Dis. 46:799–806.
38. Weber, J. T. 2005. Community-associated methicillin-resistant
Staphylococ-cus aureus. Clin. Infect. Dis. 41:S269–S272.
39. Wertheim, H. F., M. C. Vos, H. A. Boelens, A. Voss, C. M. Vandenbro-ucke-Grauls, M. H. Meester, J. A. Kluytmans, P. H. van Keulen, and H. A. Verbrugh. 2004. Low prevalence of methicillin-resistant
Staphylo-coccus aureus (MRSA) at hospital admission in the Netherlands: the
value of search and destroy and restrictive antibiotic use. J. Hosp. Infect. 56:321–325.
40. Wulf, M. W., M. Sorum, A. van Nes, R. Skov, W. J. Melchers, C. H. Klaassen, and A. Voss. 2008. Prevalence of methicillin-resistant
Staphy-lococcus aureus among veterinarians: an international study. Clin.