Human Serum Albumin for Scintigraphic Detecting of
Protein-losing Enteropathy
Yuh-Feng Wang
1,2, Yi-Chun Chen
3, Mei-Hua Chuang
4, Kuo-Chih Tseng
5,
Jainn-Shiun Chiu
2, Mei-Ing Chung
1 1Graduate Institute of Pharmaceutical Sciences, Kaohsiung Medical University, Kaohsiung, Taiwan 2
Department of Nuclear Medicine, Buddhist Dalin Tzu Chi General Hospital, Chiayi, and College of Medicine, Tzu Chi University, Hualien, Taiwan
3
Division of Nephrology, Department of Internal Medicine, Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan 4
Department of Pharmacy, Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan 5
Division of Gastroenterology, Department of Internal Medicine, Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan
Received 5/22/2008; revised 8/29/2008; accepted 9/1/2008.
For correspondence and reprints contact: Mei-Ing Chung, Ph.D., Graduate Institute of Pharmaceutical Sciences, Kaohsiung Medical University. 100 Shi-Chuan 1st Road, Kaohsiung 807, Taiwan. Tel: (886)7-3121101 ext. 2672, Fax: (886)7-3210683, E-mail: meinch@kmu.edu.tw
Background: Abdominal scintigraphy performed with technetium-99m (99mTc)-labeled human serum albumin (HSA) provides direct evidence of protein-losing enteropathy (PLE). However, because of transfusion-induced infections, the safety of serum products is a major concern. We previously developed an on-site preparation of 99m
Tc-HSA using domestic serum prod-ucts, which showed good labeling efficiency, stability, and bioavailability. The purpose of this pilot study was to initially evaluate those preparations of 99mTc-labeled HSA for clinical application.
Methods: HSA was locally acquired and appropriately screened for infectious diseases. 99mTc-HSA was pre-pared according to our previous protocol. It was intra-venous administration to patients with clinically suspect-ed PLE. Sequential abdominal images were acquirsuspect-ed by a gamma camera. Sites of obvious PLE were local-ized after the radiolabeled HSA was administered. Patients were monitored for adverse events.
Results: Five patients with clinical suspicious of PLE were enrolled in this pilot study. Areas of protein loss were demonstrated in two patients. Another three cases, the origin of diminished serum albumin levels remained undetermined. All patients tolerated with pro-cedure well with no specific adverse effects or major complaints.
Conclusion: Scintigraphy with 99m
Tc-HSA is noninva-sive and can be used to detect, localize, and follow up cases of occult or intermittent PLE. Our pilot study demonstrated that this on-site prepared 99mTc-HSA was appropriate for clinical application. Procedure of on-site radiolabeling from the native serum product was easy to be performed and achieved good labeling efficiency and bioavailability. It might also reduce the incidence of transfusion-induced infections. This technique resulted in good image quality and improved patient safety. Key words: albumin, 99mTc-HSA, infection, protein-los-ing enteropathy
Introduction
Protein-losing enteropathy (PLE) is the loss of essential proteins from the gastrointestinal tract [1]. It causes hypoal-buminemia and potential clinical complications such as chronic limb edema, increased susceptibility to infections, a heightened risk of atherosclerosis due to dyslipidemia, and chronic diarrhea. PLE often impairs patients’ activity and usually lowers their albumin levels.
Several clinical studies have been applied to diagnose PLE. Abdominal scintigraphy performed with technetium-99m (99m
Tc)-labeled human serum albumin (HSA) provides direct evidence of protein loss from the gastrointestinal tract [2]. This method demonstrates the physiologic bowel leak. It also enables localization of involved segments, assessment of the therapeutic results, and detection of recurrences after therapy [3].
Clinically acquired HSA is commercially available as cold kits that contain powdered or crystallized HSA extract-ed from blood. When mixextract-ed with 99m
Tc-pertechnetate, it forms 99m
Tc-HSA. However, the safety of serum products is a serious concern because of transfusion-induced infectious diseases such as human immunodeficiency virus infection, viral hepatitis, and Creutzfeldt-Jakob disease. Therefore, the World Health Organization recommends the use of serum products derived from patients’ native populations.
In our previous study, we formulated a protocol using a domestic serum product to prepare 99m
Tc-HSA on-site [4]. Our results showed sufficient labeling efficiency, ideal prod-uct stability, and a good biodistribution and biological half-life. The purpose of this pilot study was to evaluate the appli-cation of on-site prepared 99m
Tc-HSA in PLE patients.
Materials and Methods Preparation of 99mTc-HSA
On-site 99mTc-HSA was prepared using previously described protocol [4]. Briefly, HSA (CBSF 20% human albumin solution) was obtained from Chinese Blood Services Foundation and appropriately screened for free of infectious diseases such as hepatitis and human immunodefi-ciency virus infection. Labeling was done by mixing 0.1 mg of HSA with 0.5 ml stannous solution (Amerscan stannous agent; Amersham PLC, Buckinghamshire, UK) and 30 mCi
of Tc-pertechnetate. Mixing was performed manually by gently shaking the tubes to avoid bubble formation. Labeling efficiency was determined by means of thin layer chro-matography done with a commercial quality-control kit (Tec-Control; Mayjoy Co., CA, USA) and developed with ace-tone. A labeling efficiency higher than 90% was essential for clinical application.
Patient population
Internal medicine clinicians referred patients with sus-pected PLE. Hypoalbuminemia was confirmed with serum biochemical analysis. Exclusion criteria included: (1) hypoalbuminemia causes other than PLE; (2) malnutrition or hepatic problems, as determined from clinical history, physi-cal examination and blood examination; (3) protein loss from the urinary tract, as found with urinalysis. No specific prepa-ration of the patients was needed before imaging examina-tion.
Image acquisition
Images were acquired with a dual-head gamma camera (DST-XLi; GE Medical Systems, Buc, France) equipped with general purpose, low-energy collimators with large field of view. After intravenous injection of 99mTc-HSA (370 MBq), patients were positioned supine and dynamic images were acquired concurrently. The region of interest (ROI) was the abdominal region. Serial static images were acquired at 5, 10, 30 min, and at 1, 2, 4, 6, and 24 h after the dynamic acquisition. Adverse effects were monitored throughout of the study.
Image interpretation
An independent nuclear medicine physician interpreted the scintigraphic images. The images were visually interpret-ed for possible extravascular accumulation of the tracer to the intestine. Protein loss from the gastrointestinal tract was subsequently verified by counting of the patient’s stool; a positive result was a finding more than the background. Ethics
This study was performed in accordance with the internationally agreed ethical principles for the conduct of
medical research and was approved by the Institutional Review Broad of Buddhist Dalin Tzu Chi General Hospital.
Results
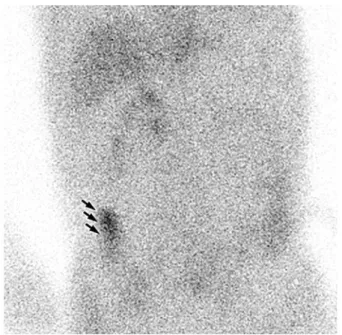
Five patients were enrolled in this pilot study (Table 1). Two patients were proved to have PLE, and three had serum albumin levels of undetermined origin. The initial appear-ance of bowel activity varied in these two patients. It was found at 23 h in one patient (case 2, Figure 1), and during a repeat study after 3 days in the other patient (case 4). The area of initial presentation was thought to be the site of pro-tein loss.
Colonoscopy was performed in 4 patients. 99m
Tc-labeled HSA scans were positive in one patient and negative in three. For the patient with positive scan, pathology reports indicated results consistent with colitis. For the patients with negative scans, their pathology reports noted ulceration or hyperplastic polyps.
All patients tolerated the imaging procedure well. No notable adverse effects or major complaints occurred.
Discussion
Albumin is a water-soluble protein synthesized in the liver. A major component of plasma protein, albumin primar-ily provides colloid osmotic pressure. Therefore, the major symptom of hypoalbuminemia is bilateral lower-limb edema. The clinical picture might be one of anasarca and pleural effusion, pericarditis, or chylous ascites [5]. Other manifesta-tions include fatigue, abdominal pain, weight loss, inability to gain weight, moderate diarrhea, and deficiencies of or
fat-soluble vitamins due to malabsorption [6].
The cause of diminished serum albumin levels may an inability to synthesize albumin (a predominant feature of chronic liver disease), increased catabolism (as in inflamma-tory processes), or an unknown loss from the urinary or gas-trointestinal tract. Hepatic conditions or underlying inflam-mation can be evaluated effectively by scrutinizing the patient’s clinical history, physical findings, and diagnostic results. Protein loss from the urinary tract is easily detected
Table 1. Participants enrolled for the 99m
Tc-HSA scintigraphy Patient
Gender Age Clinical diagnosis Serum albumin Urinary Image Pathology
no. (year) (g/dl) protein result
1 M 78 Terminal ileitis 2.4 - - Transverse colon: inflammatory exudate, consistent with ulcer 2 M 65 Hereditary 2.7 1+ + Transverse colon: chronic
polyneuropathy nonspecific colitis
3 F 49 Chronic enterocolitis 1.4 - - Rectum: consistent with hyperplastic polyp.
4 F 40 Dermatositis 2.8 - + N/A
5 F 43 Chronic diarrhea 2.0 - - Multiple ulcerations
Figure 1. 99m
Tc-HSA scintigraphic image of a 65-year-old suspected PLE male patient, 23 h after intravenous injection of radiotracer, demonstrates 99m
Tc-HSA accumulation to the right lower quadrant of abdomen (arrows). This is a direct demonstration of protein-losing from the ascending colon.
with routine urinalysis. However, losses from the gastroin-testinal tract are not so conveniently identified.
Indirect studies available for clinical confirmation of gastrointestinal protein loss include measurements of fecal alpha-1 antitrypsin clearance [7,8], chromium-51 albumin levels [9], and iodine-131 polyvinyl pyrrolidone concentra-tions. Exudative enteropathy can be confirmed on the basis of elevated 24-h clearance of alpha-1 antitrypsin in the stool. However, these studies do not help in localizing involved segments. Scintigraphy with radiolabeled albumin provides a direct and physiologic demonstration of bowel leaks [10]. For this study, 99m
Tc-HSA appeared to be stable and appropri-ate for clinical application in current practice. Abdominal scintigraphy after an intravenous administration of 99m
Tc-HSA permitted us to visualization of the site of intestinal albumin loss.
Intravenously injected 99m
Tc-HSA remained in the blood pool for a prolonged duration and was distributed throughout the body in the same way the patient’s own serum albumin was. It was a suitable tracer for transiently imaging the vascular compartment and for evaluating inter-mittent protein lost. However, commercial labeling kits for HSA are derived from human serum collected in the country of manufacture. In recent decades, various transfusion-trans-mitted infectious diseases have raised concerns about the safety of serum products. The World Health Organization recommends the use of native rather than foreign serum products.
In this pilot study, all patients compiled well with the procedure. No evidence of major adverse effect, such as allergy or infection, was found. The source of protein loss was successfully demonstrated in two patients, and the find-ing lead to appropriate clinical management [11]. However, the source was not identified in three patients. Possible caus-es of the negative rcaus-esult of the 99m
Tc-HSA study are: (1) the amount of protein loss might have been too small to be detected; (2) the duration of loss might have been indetermi-nate or beyond the study period; and (3) the patients’ hypoal-buminemia might have been associated with other causes (e.g., occult or unknown inflammation).
To our knowledge, minimal detectable rates of albumin leakage from the gastrointestinal tract have not been
report-ed. However, scintigraphy performed with Tc-HSA to detect PLE is fairly similar to scanning with 99m
Tc-labeled red blood cells to investigate gastrointestinal bleeding. In previ-ous reports, minimal detectable bleeding rates were 0.1-0.2 ml/min [12]. If protein leaks at a rate less than 0.1 ml/min, it might be neglected as background radioactivity.
Gastrointestinal protein loss is not continuous and tends to be intermittent. Therefore, serial scintigrams must be acquired over 24 h. However, protein losses that develop over more than 24 h might escape detection and led to false-negative results [13]. In this study, the area of loss was not apparent in one patient during the first examination. The pro-tein loss was demonstrated on repeat evaluation 3 days later.
Although most causes of hypoalbuminemia can be assessed effectively with current routine investigations, they fail to reveal some obscure conditions. A negative result from 99m
Tc-HSA scintigraphy provides a reminder to re-eval-uate patients in certain medical circumstances [14]. False-positive results might be caused by free 99m
Tc-pertechnetate from the process of radiolabeling. Secreted from the salivary glands and gastric mucosa, free 99m
Tc-pertechnetate abnor-mally accumulates in the intestine. In this study, we eliminat-ed the influence of excess 99mTc-pertechnetate by conducting
labeling quality control before we administered the radio-pharmaceutical.
Conclusion
99mTc-HSA scintigraphy is noninvasive and can be used
to detect, localize, and follow up occult or intermittent PLE. On-site radiolabeling by using native serum products offers advantages such as ease of completion, and good labeling efficiency and bioavailability. It might also reduce transfu-sion-induced infections. Our pilot study demonstrated that on-site preparation of 99m
Tc-HSA was appropriate for clinical application. It provided good image quality and improved patient safety.
References
1. Zheng WJ, Tian XP, Li L, et al. Protein-losing enteropa-thy in systemic lupus erythematosus: analysis of the clin-ical features of fifteen patients. J Clin Rheumatol 2007; 13:313-316.
2. Miyata M, Yoshida M, Saka M, Kasukawa R. Protein-losing gastroenteropathy in system lupus erythematosus: diagnosis with 99m
Tc-human serum albumin scintigraphy. Arthritis Rheum 2000;43:1900.
3. Takeda H, Takahashi T, Ajitsu S, et al. Protein-losing gastroenteropathy detected by technetium-99m-labeled human serum albumin. Am J Gastroenterol 1991;86:450-453.
4. Wang YF, Chuang MH, Chiu JS, Cham TM, Chung MI. On-site preparation of technetium-99m labeled human serum albumin for clinical application. Tohoku J Exp Med 2007;211:379-385.
5. Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine acti-vation. Chest 1999;115:836-847.
6. Johnson AM. Low levels of plasma proteins: malnutri-tion or inflammamalnutri-tion? Clin Chem Lab Med 1999;37:91-96.
7. Takeda H, Nishise S, Furukawa M, Nagashima R, Shinzawa H, Takahashi T. Fecal clearance of alpha1-antitrypsin with lansoprazole can detect protein-losing gastropathy. Dig Dis Sci 1999;44:2313-2318.
8. Benner KG, Montanaro A. Protein-losing enteropathy in systemic lupus erythematosus. Diagnosis and monitoring
immunosuppressive therapy by alpha-1-antitrypsin clear-ance in stool. Dig Dis Sci 1989;34:132-135.
9. Lindgren A, Engström CP, Nilsson O, Abrahamsson H. Protein-losing enteropathy in an unusual form of sar-coidosis. Eur J Gastroenterol Hepatol 1995;7:1005-1007. 10. Divgi CR, Lisann NM, Yeh SD, Benua RS. Technitium-99m albumin scintigraphy in the diagnosis of protein-losing enteropathy. J Nucl Med 1986;27:1710-1712. 11. Chen YC, Chiu JS, Chuang MH, Chung MI, Wang YF.
Scintigraphic evidence of chronic edema easily neglected by nephrologists: protein losing enteropathy. Kidney Int (In press)
12. Seto H, Kageyama M, Wu YW, et al. Sequential subtrac-tion scintigraphy with 99m
Tc-RBC for the early detection of gastrointestinal bleeding. Ann Nucl Med 1995;9:203-208.
13. Wang CS, Tzen KY, Huang MJ, Wang JY, Chen MF. Localization of obscure gastrointestinal bleeding by tech-netium 99m-labeled red blood cell scintigraphy. J Formos Med Assoc 1992;91:63-68.
14. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432-437.
(protein-losing enteropathy; PLE) -99m (human serum albumin; HSA) -99m-HSA -99m-HSA PLE -99m-HSA PLE PLE PLE -99m 2009;22:19-24