The Different Mechanisms Between Late and Very Late
Recurrences of Atrial Fibrillation in Patients Undergoing
a Repeated Catheter Ablation
MING-HSIUNG HSIEH, M.D.,
∗,
‡ CHING-TAI TAI, M.D.,† SHIH-HUANG LEE, M.D.,†
YUNG-KUO LIN, M.D.,
† HSUAN-MING TSAO, M.D.,† SHIH-LIN CHANG, M.D.,†
YENN-JIANG LIN, M.D.,
† WANWARANG WONGCHAOEN, M.D.,† KUN-TAI LEE, M.D.,†
and SHIH-ANN CHEN, M.D.,
†
From the∗Division of Cardiovascular Medicine, Department of Medicine, Taipei Medical University School of Medicine, and Taipei Wan-Fang Hospital,†Division of Cardiology, Department of Medicine, Cardiovascular Research Institute, National Yang-Ming University
School of Medicine, and Taipei Veterans General Hospital, and‡Institute of Clinical Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan
Late and Very Late Recurrence of AF.
Introduction: The mechanisms of late (<1 year after the ablation) and very late (>1 year after the ablation) recurrences of paroxysmal atrial fibrillation (AF) after catheter ablation have not been reported.Methods and Results: Fifty consecutive patients undergoing a repeated electrophysiologic study to investi-gate the recurrence of paroxysmal AF after the first ablation were included. Group 1 consisted of 12 patients with very late (26± 13 months) and group 2 consisted of 38 patients with late (3 ± 3 months) recurrence of paroxysmal AF. In the baseline study, group 1 had a lower incidence of AF foci from the pulmonary veins (PVs) (67% vs 92%, P = 0.048) and a higher incidence of AF foci from the right atrium (50% vs 13%, P = 0.014) than group 2. In the repeated study, group 1 had a higher incidence of AF foci from the right atrium (67% vs 3%, P < 0.001) and a lower incidence of AF foci from the left atrium (50% vs 97%, P < 0.001), including a lower incidence of AF foci from the PVs (50% vs 79%, P = 0.07) and from the left atrial free wall (0% vs 29%, P = 0.046) than group 2. Furthermore, most of these AF foci (64% of group 1, 65% of group 2) were from the previously targeted foci.
Conclusion: The right atrial foci played an important role in the very late recurrence of AF, whereas the left atrial foci (the majority were PVs) were the major origin of the late recurrence of AF after the catheter ablation of paroxysmal AF. (J Cardiovasc Electrophysiol, Vol. 17, pp. 231-235, March 2006)
atrial fibrillation, catheter ablation, recurrence
Introduction
The high recurrence rate of atrial fibrillation (AF) is the major problem after isolation of the pulmonary veins (PVs).1-4 Although the recurrence rate was decreased by 10–20% after a follow-up of 6–12 months after perform-ing additional left atrial linear ablation, the recurrence rate might be higher after a longer follow-up period.5,6Previous studies have reported that most of the recurrences of AF oc-curred within 6 months after the catheter ablation, and the re-covery of left atrium-PV conduction has been demonstrated to be the major mechanism of the recurrences of AF after successful isolation of the PVs.1-7 Our previous study re-ported the characteristics of very late recurrence (more than 1 year) of paroxysmal AF after the catheter ablation; however, the electrophysiological mechanisms of the very late recur-rence of paroxysmal AF have not been reported.7In addition, there have been no studies that have investigated the differ-ence in the mechanisms between late (less than 1 year) and Address for correspondence: Shih-Ann Chen, M.D., Division of Cardiology, Taipei Veterans General Hospital, 201 Sec 2, Shih-Pai Road, Taipei, Taiwan. Fax: 886-2-28735656, E-mail: epsachen@ms41.hinet.net
Manuscript received 23 May 2005; Revised manuscript received 25 July 2005; Accepted for publication 24 August 2005.
doi: 10.1111/j.1540-8167.2005.00323.x
very late recurrences of paroxysmal AF after the catheter ab-lation. Therefore, the purpose of this study was to define the mechanisms of late and very late recurrence of paroxysmal AF after catheter ablation.
Methods Study Population
This study included 50 consecutive patients who under-went a repeated electrophysiologic study and catheter ab-lation for the recurrence of paroxysmal AF. They had fre-quent episodes of symptomatic paroxysmal AF (more than one episode per month) even while using 2± 1 antiarrhyth-mic drugs. Group 1 consisted of 12 patients with very late recurrence of paroxysmal AF (greater than 1 year after the first ablation). Group 2 consisted of 38 patients with late re-currence of paroxysmal AF (between 1 month and 1 year after the first ablation). In this study population, 293 pa-tients with the same ablation protocol were included, and 104 patients (35%) had recurrences of paroxysmal AF af-ter the first ablation procedure. Twenty-three patients had very late recurrence of paroxysmal AF and 12 (52%) of them underwent a repeated ablation because of frequent episodes of symptomatic AF. The other patients who did not un-dergo a repeated ablation had a benign clinical outcome (which we previously reported).7Eighty-one patients had late
recurrence of paroxysmal AF, and 38 (47%) of those patients underwent a repeated ablation because of frequent episodes of symptomatic AF.
Echocardiography was performed before each procedure to evaluate the atrial enlargement, left atrial dimension, and left ventricular hypertrophy (defined as a septal thickness of
>12 mm) in the parasternal long-axis view and left
ventric-ular ejection fraction. All antiarrhythmic drugs except amio-darone were discontinued for at least five half-lives before the repeated electrophysiologic study and catheter ablation. Pretreatment with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers was defined as the use of two kinds of drugs before each electrophysiologic study and catheter ablation. All those drugs were used to control the blood pressure.
Electrophysiologic Study and Catheter Ablation
Each patient underwent an electrophysiologic study and catheter ablation in the fasting, nonsedative state after written informed consent was obtained.
The details have been described previously.7-10 We at-tempted to find the spontaneous onset of atrial ectopic beats or repetitive episodes of short-runs or sustained AF before or after the infusion of isoproterenol, or following a designed algorithm for facilitating the initiation of paroxysmal AF.7-10 If a consistent ectopic focus and atrial activation pattern were demonstrated, which induced paroxysmal AF, the site with the earliest and most consistent atrial activation was consid-ered to be the initiating focus of paroxysmal AF. The methods used to provoke paroxysmal AF were attempted at least twice to ensure the reproducibility.7-10
In the first ablation session, only the arrhythmogenic PVs were targeted for isolation. Isolation of the PVs was performed segmentally from the PV ostium using the electrogram-guided approach (entrance block) in 42 pa-tients.7-10Isolation of the arrhythmogenic superior vena cava (SVC) was guided by a circular catheter or basket catheter recordings in 7 patients. In 12 patients with other non-PV ec-topy of AF, catheter ablation was performed in the area with the earliest bipolar activity or a local unipolar QS pattern of the ectopic beats preceding the AF.7-10 A temperature con-trol protocol with a maximal temperature setting of 50–55◦C, maximal power of<50 W, and duration of 20–40 seconds was used. The endpoint of the ablation was the disconnec-tion between the PV and left atrium or between the SVC and right atrium, and elimination of other non-PV ectopy with noninducibility of AF.
In the second ablation procedure, the same provocation maneuvers were used again to find the ectopy (PV or non-PV) initiating the paroxysmal AF before performing isola-tion of all 4 PVs. In 13 patients, isolaisola-tion of all 4 PVs from the atrial side of the PV ostium was performed by using the electrogram-guided approach (entrance block) simultaneous with the creation of the PV-left atrial geometry using a NavX system. In 6 patients, isolation of the arrhythmogenic SVC or ablation of the ectopy from the crista terminalis was facil-itated by the use of a three-dimensional noncontact mapping (EnSite) or NavX system (EnSite 3000, Endocardial Solu-tions, St. Paul, MN, USA). For the other patients with ar-rhythmogenic PV, SVC or other non-PV ectopy initiating the paroxysmal AF, catheter ablation was performed by using the same techniques as in the first ablation procedure.
Postablation Follow-Up of AF Recurrence
After discharge, the patients underwent follow-up (2 weeks after the catheter ablation, then every 1–3 months) at our cardiology clinic or with the referring physicians, and an-tiarrhythmic drugs were prescribed for 4–6 weeks to prevent the early recurrence of paroxysmal AF (defined as<1 month after the ablation). When the patients experienced symptoms suggestive of a tachycardia after the ablation, 24-hour Holter monitoring or cardiac event recording was performed to de-fine the cause of the clinical symptoms. If more than one episode of recurrent symptomatic AF was documented, the patients were encouraged to receive a second ablation pro-cedure, or antiarrhythmic drugs were prescribed to control the recurrent AF. Late recurrent AF was defined as episodes of recurrent AF occurring within the second month to 1 year after the ablation, and very late recurrent AF was defined as episodes of recurrent AF occurring more than 1 year after the ablation.7
Statistical Analysis
All the parametric data were expressed as mean± SD. The Student’s t-test was used to analyze the parametric data. The chi-square test with Yates correction or Fisher’s exact test was used to analyze the nonparametric data. The McNemar test was used to exam the difference between the follow-up and baseline clinical or electrophysiologic characteristics in the same group. For comparing any change at the follow-up to the preablation values, generalized estimation equation (GEE) models were used to estimate the difference between the two groups. Univariate analysis of the factors associated with late and very late recurrence of AF was performed using the log-rank test. Multivariate analysis was performed with the Cox proportional hazards model to determine the inde-pendent predictors of late and very late recurrence of AF. The variables selected to be tested in the multivariate anal-ysis were those P< 0.1 in the univariate model. A P value
<0.05 was considered statistically significant. Results
Clinical Characteristics
The baseline clinical characteristics were similar between the two groups (Table 1). At the repeated study or end of the follow-up, the clinical characteristics were also similar between the two groups (Table 2). Compared to the preabla-tion values, there was no significant change in those clinical characteristics in the two groups. However, a trend toward a regression change in the atrial enlargement was noted in group 1 (right atrial enlargement change from 25% to 0% and left atrial enlargement change from 33% to 17%) but not in the group 2 patients (right atrial enlargement change from 16% to 13% and left atrial enlargement change from 26% to 37%).
The mean time period for the recurrence of AF after the first ablation procedure was 26 ± 13 months in group 1 (range: 13–55 months) and 3± 3 months in group 2 (range: 1–12 months). In the group 1 patients, 6 (50%) patients had recurrent AF during the second year of follow-up, 4 (33%) during the third year of follow-up, and 2 (17%) more than 3 years after the first ablation. In group 2, 33 (87%) patients had
TABLE 1
Baseline Clinical and Electrophysiologic Characteristics of the Study Patients Group 1 Group 2 (N = 12) (N = 38) P Value Age (years) 57± 12 63± 11 0.08 Male (%) 58 74 0.47 Duration of AF (years) 3± 3 5± 4 0.18 Cardiovascular disease (%) 50 62 0.51
Right atrial enlargement (%) 25 16 0.64
Left atrial enlargement (%) 33 26 0.69
Right or left atrial enlargement (%) 50 32 0.43 Left ventricular hypertrophy (%) 8 8 1.0 Left atrial dimension (mm) 38± 4 38± 6 0.82
Left ventricular 47± 3 50± 6 0.07
end-diastolic dimension (mm)
Left ventricular 71± 6 65± 10 0.29
ejection fraction (%)
Pretreatment with ACE 8 13 1.0
inhibitors or ARBs
Number of antiarrhythmic drugs 2± 1 3± 1 0.51 used before ablation
AF foci
Pulmonary vein (%) 67 92 0.048
Nonpulmonary vein (%) 58 32 0.171
Right atrium (%) 50 13 0.014
Left atrial free wall (%) 8 21 0.425
Left atrium (%) 67 95 0.024
Multiple (≥2) foci (%) 33 53 0.404
Data are presented as mean± 1 SD or number (percent).
ACE= angiotensin-converting enzyme; ARB = angiotensin II receptor blocker.
recurrent AF within 6 months and the other 5 (13%) patients between 7 and 12 months after the first ablation.
Electrophysiological Characteristics
At the baseline electrophysiologic study, the group 1 pa-tients had a lower incidence of AF foci originating from the PVs (67% vs 92%, P= 0.048) and left atrium (67% vs 95%, P= 0.024) and a higher incidence of AF foci from the right atrium (50% vs 13%, P= 0.014) than the group 2 patients (Table 1). The incidence of multiple AF foci was similar between the two groups. At the repeated electrophysiologic study, the group 1 patients had a higher incidence of AF foci originating from the right atrium (67% vs 3%, P< 0.001) than the group 2 patients. However, the group 1 patients had a lower incidence of AF foci from the left atrium (50% vs 97%, P< 0.001), including a lower incidence of AF foci from the PVs (50% vs 79%, P= 0.07) and left atrial free wall (0% vs 29%, P= 0.046) than the group 2 patients. The incidence of multiple AF foci was also similar between the two groups (Table 2).
Mechanisms of Late and Very Late Recurrence of Paroxysmal AF
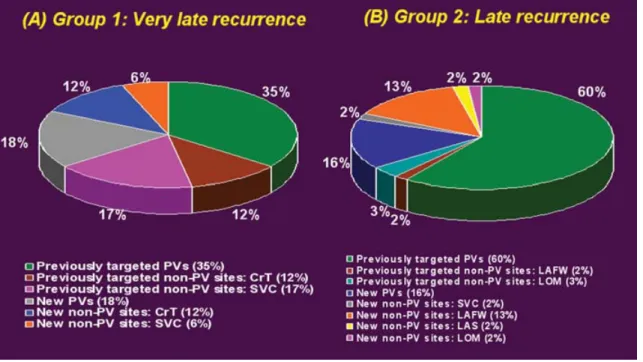
In the group 1 patients with very late recurrence of parox-ysmal AF, a total of 17 AF foci were identified; 64% of the AF foci were from the previously targeted arrhythmogenic foci (including 35% from the PVs and 29% from non-PV sites) and 36% of the AF foci were from new (previously nonar-rhythmogenic) foci (including 18% from the PVs and 18% from non-PV sites) (Fig. 1A). All the recurrent foci from the previously targeted PVs showed recovery of PV conduction.
TABLE 2
Clinical and Electrophysiological Characteristics of the Study Patients During the Repeated Electrophysiologic Study or Follow-Up
Group 1 Group 2 (N = 12) (N = 38) P Value
Recurrent time of AF (months) 26± 13 3± 3
Right atrial enlargement (%) 0 13 0.55
Left atrial enlargement (%) 17 37 0.35
Right or left atrial enlargement (%) 17 37 0.35 Left ventricular hypertrophy (%) 17 21 1.0 Left atrial dimension (mm) 37± 4 40± 5 0.30
Left ventricular 47± 3 50± 6 0.40
end-diastolic dimension (mm)
Left ventricular ejection fraction (%) 65± 3 68± 7 0.27 Recurrent foci of AF
Pulmonary vein (%) 50 79 0.071
Nonpulmonary vein (%) 67 40 0.19
Right atrium (%) 67 3 <0.001
Left atrial free wall (%) 0 29 0.046
Left atrium (%) 50 97 <0.001
Multiple foci (%) 42 42 1.0
Follow-up (months) 21± 14 37 ± 24
Use of antiarrhythmic drugs (%) 17 26 0.71
Use of amiodarone (%) 8 16 1.0
Pretreatment of ACE inhibitors or ARB (%) 25 37 0.72
Free of AF (%) 83 74 0.71
Data are presented as mean± 1 SD or number (percent).
ACE= angiotensin-converting enzyme; ARB = angiotensin II receptor blocker.
All the recurrent foci from the previously targeted non-PV sites were from the right atrium, including 12% from the crista terminalis (all the foci were nearby the previous abla-tion locaabla-tion with a similar P wave configuraabla-tion compared as the first ablation session) and 17% were from the SVC (exhibiting recovery of the conduction between the SVC and right atrium). All the new non-PV foci were also from the right atrium, including 12% from the crista terminalis and 6% from the SVC.
The group 2 patients with late recurrence of the parox-ysmal AF had a total of 57 AF foci; 65% of the AF foci were from the previously targeted arrhythmogenic foci (in-cluding 60% from the PVs and 5% from non-PV sites) and 35% were from new (previously nonarrhythmogenic) foci (including 16% from the PVs and 19% from non-PV sites) (Fig. 1B). All the recurrent foci from the previously targeted PVs exhibited recovery of the PV conduction. The recurrent foci from the previously targeted non-PV sites were from the left atrium, including 2% from the left atrial free wall and 3% from the ligament of Marshall. The new non-PV foci in-cluded 2% from the right atrium (SVC) and 17% from the left atrium (13% from the left atrial free wall, 2% from the ligament of Marshall, and 2% from the left atrial septum). Predictors of Late or Very Late Recurrence
of Paroxysmal AF
Univariate analysis of the clinical variables including age, sex, duration of AF, incidence of cardiovascular disease, right or left atrial enlargement and left ventricular hyper-trophy, left atrial dimension, left ventricular end-diastolic di-mension, left ventricular ejection fraction, pretreatment with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers and incidence of original AF foci from the
Figure 1. The distribution of right and left atrial foci initiating recurrent atrial fibrillation in group 1 (Panel A) and group 2 (Panel B) patients. CrT= crista terminalis; LAFW= left atrial free wall; LAS = left atrial septum; LOM = ligament of Marshall; PV = pulmonary vein; SVC = superior vena cava.
PVs, non-PV sites, right atrium or left atrial free wall, and multiple AF foci showed that the right atrial foci were asso-ciated with a very late recurrence of paroxysmal AF and PV foci were related to a late recurrence of paroxysmal AF after the catheter ablation. However, multivariate analysis did not show any predictor of late or very late recurrence of parox-ysmal AF.
Clinical Outcomes
In the group 1 patients, 10 (83%) patients were free of recurrent AF without the use of antiarrhythmic drugs and 2 (17%) patients were controlled by previously ineffective an-tiarrhythmic drugs after the repeated ablation during a mean follow-up period of 21 ± 14 months. In the group 2 pa-tients, 28 (74%) patients were free of recurrent AF without the use of antiarrhythmic drugs and 10 patients (26%) had taken previously ineffective antiarrhythmic drugs to control the paroxysmal AF after the repeated ablation during a mean follow-up period of 37± 24 months. The incidence of be-ing free of recurrent paroxysmal AF and incidence of usbe-ing antiarrhythmic drugs were similar between the two groups (Table 2).
Discussion Major Findings
In the 50 patients with recurrent paroxysmal AF who un-derwent a repeated electrophysiologic study, 12 patients had very late (>1 year after ablation) recurrence of paroxysmal AF and a higher incidence of recurrent AF originating from the right atrium and lower incidence of recurrent AF from the left atrium as compared to the 38 patients with late (be-tween 1 month and 1 year after ablation) recurrence of AF. In the 38 patients with late recurrence of paroxysmal AF, PV
ectopy initiating AF was the major mechanism initiating the recurrent AF.
Mechanisms of Late and Very Late Recurrence of Paroxysmal AF
Previous studies investigated the mechanism of recurrent AF after isolation of the PVs and recovery of the left atrium-PV conduction was the major etiology for the recurrence of AF.1-4However, little is known about the mechanism of very late (>1 year after the ablation) recurrence of paroxysmal AF. In the present study, we found that the patients with very late recurrence of paroxysmal AF had a higher incidence of recurrent AF initiated by right atrial ectopy. In contrast, the patients with late recurrence (<1 year after the ablation) of paroxysmal AF had a higher incidence of recurrent AF initiated by left atrial ectopy (including the PVs and left atrial non-PV sites). Therefore, right atrial foci played a critical role in the very late recurrence of paroxysmal AF and left atrial foci were the major origin of the late recurrence of paroxysmal AF after the catheter ablation.
Why do the right atrium and left atrium play different roles in the late and very late recurrence of paroxysmal AF? Previous studies on cellular electrophysiology have demon-strated different characteristics of the ionic currents or mRNA levels between the PVs and SVC cardiomyocytes or the right atrium and left atrium.11-14Previous clinical studies also have demonstrated that PV and SVC ectopies had different elec-trophysiological characteristics.8-10,15,16In addition, PV ec-topy could more easily initiate AF than SVC ecec-topy if a left atrium-PV or right atrium-SVC reconnection appeared after the isolation procedure. From the clinical studies, we found that most of the recurrences of AF originating from the PVs or left atrium after PV isolation were reported within 1 year after ablation.1-6 Therefore, even when isolation of all four PVs was performed, non-PV ectopy from the left atrium might be
easier to appear and initiate AF, and this phenomenon may happen in the early stages after the catheter ablation. In con-trast, non-PV ectopy from the right atrium may appear and initiate AF in the late stages.
Another possibility is that the PV-left atrial junction is thicker than the SVC-right atrial junction and would be easier for a PV-left atrium reconnection to occur compared to that for an SVC-right atrium reconnection in the early stages after isolation.17-19
Study Limitations
First, it is difficult to evaluate the asymptomatic episodes of paroxysmal AF, even when using regular Holter moni-toring or cardiac event recorders. In this study, we only in-vestigated the symptomatic episodes of recurrent paroxys-mal AF. In addition, a previous study reported infrequent asymptomatic recurrences of AF after successful catheter ab-lation.20Second, not all patients with very late recurrence of AF after the first ablation procedure underwent a repeated electrophysiologic study. In our previous study, 11 of 13 pa-tients with very late recurrence of paroxysmal AF did not need long-term antiarrhythmic drugs to prevent further AF attacks, and they refused a repeated catheter ablation.7Only 2 patients had frequent episodes of symptomatic AF and un-derwent a repeated electrophysiologic study. Therefore, the electrophysiological characteristics in this study did not rep-resent all the patients with very late recurrence of paroxys-mal AF. Third, the results of this study may not be strictly comparable to other ablation procedures. In particular, some electrophysiologists performed a more extensive ablation of the left atrium during the initial procedure if they found less right atrial foci at baseline. The distribution and temporal pattern of the recurrences may differ from those presented here.
Clinical Implications
The present study demonstrated that non-PV foci were very important in the very late recurrence of paroxysmal AF after catheter ablation. Therefore, provocation of ectopy-initiating AF before starting the ablation procedure and testing the AF inducibility as successful endpoints were important for the ablation of paroxysmal AF, especially in the repeated ablation procedures for recurrence of the paroxysmal AF.3,7-11
Conclusions
The right atrial foci played an important role in the very late recurrence of paroxysmal AF, whereas the left atrial foci (the majority were from the PVs) were the major origin of the late recurrence of paroxysmal AF after the catheter ablation.
References
1. Gerstenfeld EP, Callans DJ, Dixit S, Zado E, Marchlinski FE: Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: Implications for ablation strategies. J Cardiovasc Electrophysiol 2003;14:685-690.
2. Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, Es-posito C, Furlanello F, De Ambroggi L: Prospective assessment of late conduction recurrence across radiofrequency lesions producing
electri-cal disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation 2003;108:1599-1604.
3. Nanthakumar K, Plumb VJ, Epstein AE, Veenhuyzen GD, Link D, Kay GN: Resumption of electrical conduction in previously iso-lated pulmonary veins: Rationale for a different strategy? Circulation 2004;109:1226-1229.
4. Callans DJ, Gerstenfeld EP, Dixit S, Zado E, Vanderhoff M, Ren JF, Marchlinski FE: Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:1050-1055.
5. Jais P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clementy J, Haissa-guerre M: Technique and results of linear ablation at the mitral isthmus. Circulation 2004;110:2996-3002.
6. Lemola K, Hall B, Cheung P, Good E, Han J, Tamirisa K, Chugh A, Bogun F, Pelosi F, Morady F, Oral H: Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm 2004;1:197-202.
7. Hsieh MH, Tai CT, Tsai CF, Lin WS, Lin YK, Tsao HM, Huang JL, Ueng KC, Yu WC, Chan P, Ding YA, Chang MS, Chen SA: Clini-cal outcome of very late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2003;14:598-601.
8. Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS, Chen SA: Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003;107:3176-3183.
9. Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS: Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiologic characteris-tics, pharmacologic responses, and effects of radiofrequency ablation. Circulation 1999;100:1879-1886.
10. Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA: Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: Electrophysiological character-istics and results of radiofrequency ablation. Circulation 2000;102:67-74.
11. Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, Lin CI: Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation 2001;104:2849-2854.
12. Chen YJ, Chen YC, Yeh HI, Lin CI, Chen SA: Electrophysiology and arrhythmogenic activity of single cardiomyocytes from canine superior vena cava. Circulation 2002;105:2679-2685.
13. Li D, Zhang L, Kneller J, Nattel S: Potential ionic mechanism for re-polarization differences between canine right and left atrium. Circ Res 2001;88:1168-1175.
14. Lai LP, Su MJ, Lin JL, Tsai CH, Lin FY, Chen YS, Hwang JJ, Huang SK, Tseng YZ, Lien WP: Measurement of funny current (I(f)) channel mRNA in human atrial tissue: Correlation with left atrial filling pressure and atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:947-953. 15. Tai CT, Chiou CW, Wen ZC, Hsieh MH, Tsai CF, Lin WS, Chen CC, Lin
YK, Yu WC, Ding YA, Chang MS, Chen SA: Effect of phenylephrine on focal atrial fibrillation originating in the pulmonary veins and superior vena cava. J Am Coll Cardiol 2000;36:788-793.
16. Lu TM, Tai CT, Hsieh MH, Tsai CF, Lin YK, Yu WC, Tsao HM, Lee SH, Ding YA, Chang MS, Chen SA: Electrophysiologic characteristics in initiation of paroxysmal atrial fibrillation from a focal area. J Am Coll Cardiol 2001;37:1658-1664.
17. Saito T, Waki K, Becker AE: Left atrial myocardial extension onto pulmonary veins in humans: Anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol 2000;11:888-894.
18. Hassink RJ, Aretz HT, Ruskin J, Keane D: Morphology of atrial my-ocardium in human pulmonary veins: A postmortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol 2003;42:1108-1114.
19. Kholova I, Kautzner J: Morphology of atrial myocardial extensions into human caval veins: A postmortem study in patients with and without atrial fibrillation. Circulation 2004;110:483-488.
20. Oral H, Veerareddy S, Good E, Hall B, Cheung P, Tamirisa K, Han J, Fortino J, Chugh A, Bogun F, Pelosi F Jr, Morady F: Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofre-quency catheter ablation. J Cardiovasc Electrophysiol 2004;15:920-924.